ITAC and Non-ITAC Sinonasal Adenocarcinoma: Classification, Etiopathogenesis, Diagnosis and Therapy Focusing on Interdisciplinarity
Abstract
1. Introduction
2. Diagnosis
2.1. Clinical Presentation and Epidemiology
2.2. Imaging (CT and MRI)
2.3. PET/CT
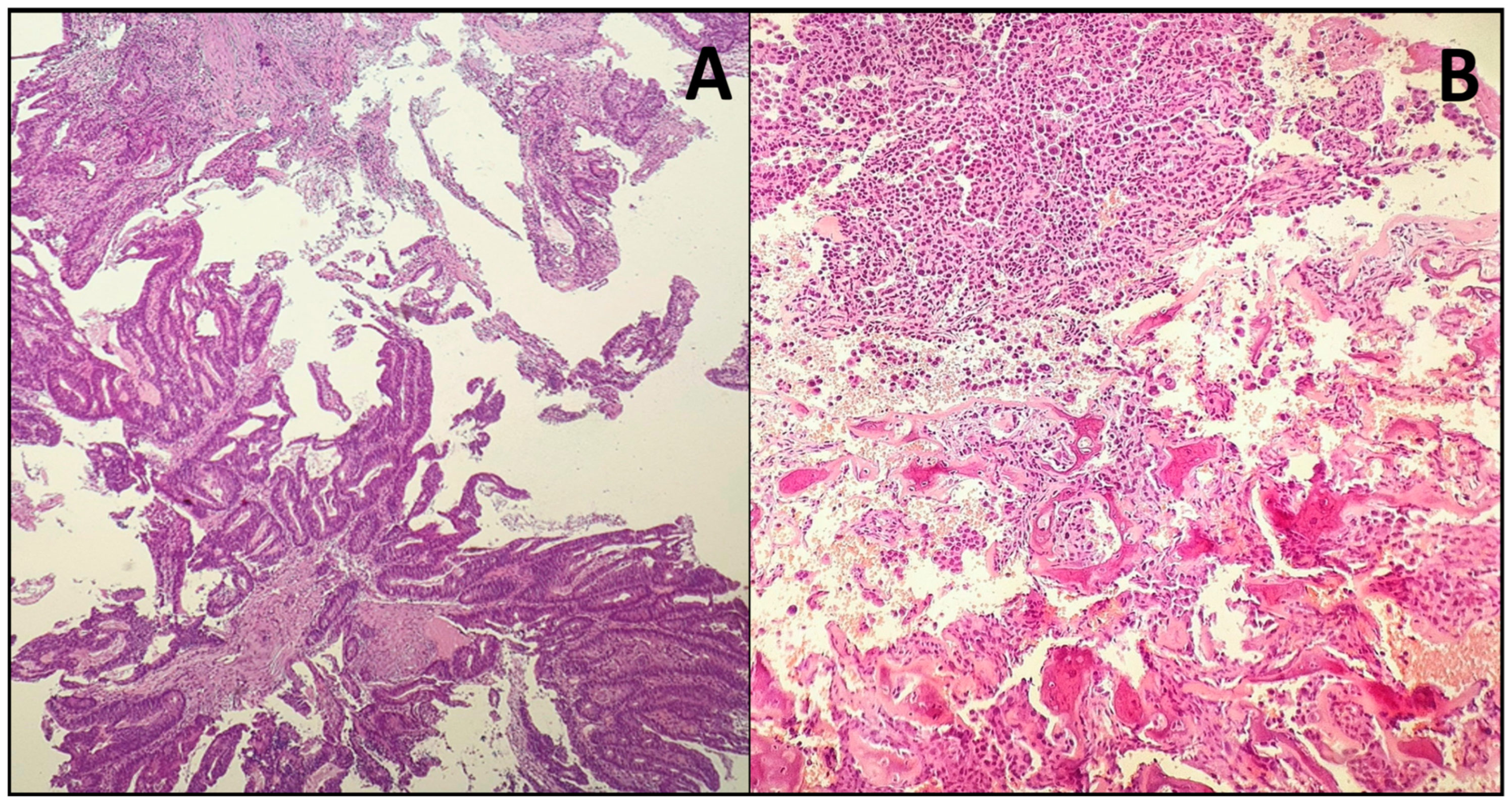
2.4. Histopathology
2.5. Tumour Staging
3. Therapy
3.1. Surgery
3.1.1. Open (External) Surgery
- The trans-facial approach can be used for cancer in all sinonasal regions. There are two types of trans-facial approaches: the lateral rhinotomy approach and the sublabial approach.
- ○
- Lateral Rhinotomy: The skin incision starts from the medial can, thus, and continues to the nasolabial sulcus and the alar-facial sulcus. This exposes the maxillary sinus, the orbital rim, and the piriform aperture. The zygomatic bone and the maxillary tuberosity can also be exposed through this incision. Depending on the involvement of the infraorbital nerve, it can be preserved or sacrificed.
- ○
- Sublabial Approach: The incision is made on the mucosa of the upper vestibule, down to the bone. This approach provides access to the midface skeleton without a skin incision but offers less exposure. There are two types of sublabial approaches: Rouge Denker and degloving.
- ▪
- Rouge Denker Approach: The incision is made in the upper vestibular mucosa, exposing the anterior part of the maxilla.
- ▪
- Degloving Approach: This involves a bilateral incision from one maxillary tuberosity to the other, providing greater exposure.
- Craniofacial resection is reserved for tumors extending to the anterior skull base, allowing resection of both the lower intrasinusal part of the tumor and any intracranial extension, including orbital invasion. A coronal incision is performed, followed by a bifrontal craniotomy. The frontal lobes are reclined to expose the intracranial tumor. If the cancer has invaded the dura, dissection can be performed intradurally or extradurally. The use of these approaches has decreased in favour of endoscopic surgery [46,47,48].
3.1.2. Endoscopic Surgery
- Tumor debulking;
- Identification of the tumor’s adhesion site;
- Tumor removal;
- Expansion of an additional safety plane due to tumor invasion;
- Multiple biopsies for final histological analysis;
- Reconstruction, if necessary.
3.1.3. Combined Open and Endoscopic Approaches
3.1.4. Neck Dissection
3.2. Radiotherapy
- For tumors originating in the maxillary sinus, the following areas should be included:
- ○
- Anteriorly: ipsilateral nasolacrimal duct, anterior wall of the ipsilateral maxillary sinus, labial gingival sulcus, and the maxillary nerve.
- ○
- Posteriorly: posterior wall of the infratemporal and pterygopalatine fissures with the ipsilateral process and the foramen ovale, ipsilateral sphenoid sinus, and the foramen rotundum.
- ○
- Superiorly: inferior orbital wall, ipsilateral Gasser ganglion; in case of orbital invasion, also the superior orbital fissure and the optic canal.
- ○
- Inferiorly: hard palate and the alveolar border.
- ○
- Medially: ipsilateral nasal cavity, including the nasal septum.
- ○
- Laterally: perijugal fat and the infratemporal fossa.
- For tumors originating in the nasal cavities, these include the following:
- ○
- Anteriorly: nasal vestibule, cheek, follow the margin of the nasal bones and the anterior portion of the maxillary nerve.
- ○
- Posteriorly: nasopharynx, including the clivus; in large tumors, also the sphenoid sinus and the pterygoid processes.
- ○
- Superiorly: ethmoid, pterygopalatine fossa, sphenopalatine foramen, foramen rotundum, inferior orbital wall, and the maxillary sinus.
- ○
- Inferiorly: same expansions as for maxillary sinus tumors.
- ○
- Laterally: nasolacrimal ducts, ipsilateral maxillary sinus, middle meatus, and pterygoid processes.
3.3. Systemic Therapy
3.4. Post-Treatment Surveillance
- Clinical Examination with Nasal Endoscopy: Performed every 3–4 months for the first two years, every 6 months for years three to five, and annually thereafter. This allows for direct visualization of the surgical cavity.
- Cross-Sectional Imaging: Contrast-enhanced MRI or CT scans of the primary site and neck are recommended every 6–12 months for the first five years to detect deep or submucosal recurrences not visible on endoscopy.
- Systemic Imaging: Chest imaging (e.g., CT scan) should be considered annually to screen for distant metastases, particularly in patients with high-grade or advanced-stage tumors.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ITAC | Intestinal-type adenocarcinoma |
| SNCs | Sinonasal cancers |
| SNAC | Sinonasal adenocarcinoma |
| SCC | Squamous cell carcinoma |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| 18F-FDG | Fluorine-18-fluorodeoxyglucose |
| 68Ga-FAPI | Gallium-68 Fibroblast Activation Protein Inhibitor |
| PET | Positron emission tomography |
| AdCC | Adenoid cystic carcinoma |
| HPV | Human papilloma virus |
| PFS | Progression free survival |
| PND | Prophylactic neck dissection |
| BT | Brachytherapy |
| ICRU | International Commission on Radiation Units and Measurements |
| CTV-HR | Clinical target volume-high risk |
| CTV-LR | Clinical target volume-low risk |
| PLF | Platinum-based chemotherapy, leucovorin, fluorouracil |
| OS | Overall survival |
| DFS | Disease free survival |
References
- Agarwal, A.; Bhatt, A.A.; Bathla, G.; Kanekar, S.; Soni, N.; Murray, J.; Vijay, K.; Vibhute, P.; Rhyner, P.H. Update from the 5th Edition of the WHO Classification of Nasal, Paranasal, and Skull Base Tumors: Imaging Overview with Histopathologic and Genetic Correlation. Am. J. Neuroradiol. 2023, 44, 1116–1125. [Google Scholar] [CrossRef]
- Binazzi, A.; Corfiati, M.; Di Marzio, D.; Cacciatore, A.M.; Zajacovà, J.; Mensi, C.; Galli, P.; Miligi, L.; Calisti, R.; Romeo, E.; et al. Sinonasal Cancer in the Italian National Surveillance System: Epidemiology, Occupation, and Public Health Implications. Am. J. Ind. Med. 2018, 61, 239–250. [Google Scholar] [CrossRef]
- Kilic, S.; Shukla, P.A.; Marchiano, E.J.; Patel, R.H.; Baredes, S.; Liu, J.K.; Eloy, J.A. Malignant Primary Neoplasms of the Nasal Cavity and Paranasal Sinus. Curr. Otorhinolaryngol. Rep. 2016, 4, 249–258. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours. In WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2024; Volume 9, Available online: https://tumourclassification.iarc.who.int/chapters/52 (accessed on 6 October 2025).
- Rampinelli, V.; Ferrari, M.; Nicolai, P. Intestinal-Type Adenocarcinoma of the Sinonasal Tract: An Update. Curr. Opin. Otolaryngol. Head Neck Surg. 2018, 26, 115–121. [Google Scholar] [CrossRef]
- Fiorentino, V.; Straccia, P.; Tralongo, P.; Musarra, T.; Pierconti, F.; Martini, M.; Fadda, G.; Rossi, E.D.; Larocca, L.M. DOG1 as an Immunohistochemical Marker of Acinic Cell Carcinoma: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 9711. [Google Scholar] [CrossRef]
- Veuger, J.; Kuipers, N.C.; Willems, S.M.; Halmos, G.B. Tumor Markers and Their Prognostic Value in Sinonasal ITAC/Non-ITAC. Cancers 2023, 15, 3201. [Google Scholar] [CrossRef] [PubMed]
- Riobello, C.; Vivanco, B.; Reda, S.; López-Hernández, A.; García-Inclán, C.; Potes-Ares, S.; Cabal, V.N.; López, F.; Llorente, J.L.; Hermsen, M.A. Programmed Death Ligand-1 Expression as Immunotherapeutic Target in Sinonasal Cancer. Head Neck 2018, 40, 818–827. [Google Scholar] [CrossRef]
- Villanueva-Fernández, E.; Hermsen, M.A.; Suárez-Fernández, L.; Vivanco, B.; Franchi, A.; García-Marín, R.; Cabal, V.N.; Codina-Martínez, H.; Lorenzo-Guerra, S.L.; Llorente, J.L.; et al. Biomarkers for Immunotherapy in Poorly Differentiated Sinonasal Tumors. Biomedicines 2022, 10, 2205. [Google Scholar] [CrossRef]
- Park, J.C.; Faquin, W.C.; Durbeck, J.; Faden, D.L. Immune Checkpoint Inhibitors in Sinonasal Squamous Cell Carcinoma. Oral Oncol. 2020, 109, 104776. [Google Scholar] [CrossRef] [PubMed]
- Girolami, I.; Marletta, S.; Fiorentino, V.; Battocchio, S.; Cerbelli, B.; Fiamengo, B.; Gerosa, C.; Gianatti, A.; Morelli, L.; Riva, G.; et al. Effect of Radio-Chemotherapy on PD-L1 Immunohistochemical Expression in Head and Neck Squamous Cell Carcinoma. J. Pers. Med. 2023, 13, 363. [Google Scholar] [CrossRef]
- Fiorentino, V.; Pizzimenti, C.; Franchina, M.; Pepe, L.; Russotto, F.; Tralongo, P.; Micali, M.G.; Militi, G.B.; Lentini, M. Programmed Cell Death Ligand 1 Immunohistochemical Expression and Cutaneous Melanoma: A Controversial Relationship. Int. J. Mol. Sci. 2024, 25, 676. [Google Scholar] [CrossRef] [PubMed]
- Tabaee, A.; Hsu, A.K.; Kacker, A. Indications, Technique, Safety, and Accuracy of Office-based Nasal Endoscopy with Biopsy for Sinonasal Neoplasm. Int. Forum Allergy Rhinol. 2011, 1, 225–228. [Google Scholar] [CrossRef]
- Bliss, M.; Muntz, H. Nasal Endoscopy: New Tools and Technology for Accurate Assessment. In Advances in Oto-Rhino-Laryngology; Raol, N., Hartnick, C.J., Eds.; S. Karger AG: Basel, Switzerland, 2015; Volume 76, pp. 18–26. ISBN 978-3-318-02786-0. [Google Scholar]
- Veloso-Teles, R.; Ribeiro, I.; Castro-Silva, J.; Monteiro, E. Adenocarcinomas of the Sinonasal Tract: A Case Series from an Oncology Centre in Northern Portugal. Eur. Arch. Otorhinolaryngol. 2015, 272, 1913–1921. [Google Scholar] [CrossRef]
- Leivo, I. Sinonasal Adenocarcinoma: Update on Classification, Immunophenotype and Molecular Features. Head Neck Pathol. 2016, 10, 68–74. [Google Scholar] [CrossRef]
- Alonso-Sardón, M.; Chamorro, A.-J.; Hernández-García, I.; Iglesias-de-Sena, H.; Martín-Rodero, H.; Herrera, C.; Marcos, M.; Mirón-Canelo, J.A. Association between Occupational Exposure to Wood Dust and Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0133024. [Google Scholar] [CrossRef]
- Andersson, M.; Selin, F.; Järvholm, B. Asbestos Exposure and the Risk of Sinonasal Cancer. Occup. Med. 2016, 66, 326–331. [Google Scholar] [CrossRef]
- Ud Din, N.; Akram, S.; Raza, M.; Ahmad, Z. Intestinal Type Sinonasal Adenocarcinoma: A Clinicopathological Study of 48 Patients with Review of Literature. Int. J. Surg. Pathol. 2025, 33, 1321–1333. [Google Scholar] [CrossRef]
- Abi-Saab, T.; Lozar, T.; Chen, Y.; Tannenbaum, A.P.; Geye, H.; Yu, M.; Weisman, P.; Harari, P.M.; Kimple, R.J.; Lambert, P.F.; et al. Morphologic Spectrum of HPV-Associated Sinonasal Carcinomas. Head Neck Pathol. 2024, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Sklar, E.M.L.; Pizarro, J.A. Sinonasal Intestinal-Type Adenocarcinoma Involvement of the Paranasal Sinuses. AJNR Am. J. Neuroradiol. 2003, 24, 1152–1155. [Google Scholar] [PubMed]
- Ali, E.H.; Mengesha, M.W. Sinonasal Adenocarcinoma Presented as a Giant Anterior Cranial Fossa Mass: A Case Report and Review of the Literature. J. Med. Case Rep. 2024, 18, 85. [Google Scholar] [CrossRef]
- Thawani, R.; Kim, M.S.; Arastu, A.; Feng, Z.; West, M.T.; Taflin, N.F.; Thein, K.Z.; Li, R.; Geltzeiler, M.; Lee, N.; et al. The Contemporary Management of Cancers of the Sinonasal Tract in Adults. CA Cancer J. Clin. 2023, 73, 72–112. [Google Scholar] [CrossRef] [PubMed]
- Reghunath, A.; Mittal, M.K.; Thukral, B.B.; Sinha, M. Approach to Sinonasal Masses: A Comprehensive Review. J. Head Neck Physicians Surg. 2022, 10, 14–25. [Google Scholar] [CrossRef]
- Georgel, T.; Jankowski, R.; Henrot, P.; Baumann, C.; Kacha, S.; Grignon, B.; Toussaint, B.; Graff, P.; Kaminsky, M.C.; Geoffrois, L.; et al. CT Assessment of Woodworkers’ Nasal Adenocarcinomas Confirms the Origin in the Olfactory Cleft. Am. J. Neuroradiol. 2009, 30, 1440–1444. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Kato, H.; Tomita, H.; Mizuta, K.; Aoki, M.; Hara, A.; Matsuo, M. Imaging Characteristics of Malignant Sinonasal Tumors. J. Clin. Med. 2017, 6, 116. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, J.; Liu, M.; Li, Z.; Li, Q. Diagnosis of Primary Intestinal-Type Adenocarcinoma in the Nasal Cavity by 18F-FDG PET/CT. Clin. Nucl. Med. 2016, 41, 888–889. [Google Scholar] [CrossRef]
- Felix-Ravelo, M.; Bey, A.; Arous, F.; Paris-Grandpierre, S.; Jankowski, R.; Nguyen, D.T. Relationship between 18FDG-PET and Different Types of Sinonasal Malignancies. Acta Otolaryngol. 2017, 137, 191–195. [Google Scholar] [CrossRef]
- Ding, H.; Wang, Y.; Liang, J.; Liu, Y.; Chen, Y. Significantly Higher 68 Ga-FAPI than 18 F-FDG Uptake by Hidradenocarcinoma of Head and Neck on PET/CT. Clin. Nucl. Med. 2024, 49, 466–467. [Google Scholar] [CrossRef]
- Villar, R.; Ramos, B.; Acosta, M.; Haro, J.J.; Gómez, A. Recurrent Adenocarcinoma of the Sinonasal Tract. Oral Maxillofac. Surg. 2013, 17, 155–158. [Google Scholar] [CrossRef]
- Önner, H.; Özer, H.; Gökmen, M.F.; Eren, O.Ö. 68Ga-PSMA-Avid Sinonasal Intestinal-Type Adenocarcinoma. Mol. Imaging Radionucl. Ther. 2025, 34, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Purgina, B.; Bastaki, J.M.; Duvvuri, U.; Seethala, R.R. A Subset of Sinonasal Non-Intestinal Type Adenocarcinomas Are Truly Seromucinous Adenocarcinomas: A Morphologic and Immunophenotypic Assessment and Description of a Novel Pitfall. Head Neck Pathol. 2015, 9, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Bignami, M.; Lepera, D.; Volpi, L.; Lambertoni, A.; Arosio, A.; Pistochini, A.; Nicolai, P.; Castelnuovo, P. Sinonasal Non-Intestinal-Type Adenocarcinoma: A Retrospective Review of 22 Patients. World Neurosurg. 2018, 120, e962–e969. [Google Scholar] [CrossRef]
- Haerle, S.K.; Gullane, P.J.; Witterick, I.J.; Zweifel, C.; Gentili, F. Sinonasal Carcinomas. Neurosurg. Clin. N. Am. 2013, 24, 39–49. [Google Scholar] [CrossRef]
- Kleinsasser, O.; Schroeder, H.G. [The pathology and clinical picture of adenocarcinoma of the nose after wood dust exposure]. Strahlenther. Onkol. Organ Dtsch. Rontgenges. 1989, 165, 437–440. [Google Scholar]
- Arcovito, G.; Franchi, A. Sinonasal Adenocarcinomas: An Update. Surg. Pathol. Clin. 2024, 17, 653–666. [Google Scholar] [CrossRef]
- Leivo, I. Intestinal-Type Adenocarcinoma: Classification, Immunophenotype, Molecular Features and Differential Diagnosis. Head Neck Pathol. 2017, 11, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Chang, H.; Jeong, E.C. Sinonasal Intestinal-Type Adenocarcinoma in the Frontal Sinus. Arch. Craniofacial Surg. 2018, 19, 210–213. [Google Scholar] [CrossRef]
- Leivo, I. Update on Sinonasal Adenocarcinoma: Classification and Advances in Immunophenotype and Molecular Genetic Make-Up. Head Neck Pathol. 2007, 1, 38–43. [Google Scholar] [CrossRef]
- Skalova, A.; Sar, A.; Laco, J.; Metelkova, A.; Miesbauerova, M.; Steiner, P.; Švajdler, M.; Michal, M. The Role of SATB2 as a Diagnostic Marker of Sinonasal Intestinal-Type Adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.T. Expression Pattern of CK7, CK20, CDX-2, and Villin in Intestinal-Type Sinonasal Adenocarcinoma. J. Clin. Pathol. 2004, 57, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ordonez, B. Hamartomas, Papillomas and Adenocarcinomas of the Sinonasal Tract and Nasopharynx. J. Clin. Pathol. 2009, 62, 1085–1095. [Google Scholar] [CrossRef]
- Jain, C.; Caulley, L.; Macdonald, K.I.; Purgina, B.; Lai, C.K.; Esche, B.; Johnson-Obaseki, S. Nasopharyngeal Non-Intestinal-Type Adenocarcinoma: A Case Report and Updated Review of the Literature. Curr. Oncol. 2017, 24, 55–60. [Google Scholar] [CrossRef]
- Tilson, M.P.; Gallia, G.L.; Bishop, J.A. Among Sinonasal Tumors, CDX-2 Immunoexpression Is Not Restricted to Intestinal-Type Adenocarcinomas. Head Neck Pathol. 2014, 8, 59–65. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge from a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Howard, D.J.; Lund, V.J. The Midfacial Degloving Approach to Sinonasal Disease. J. Laryngol. Otol. 1992, 106, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Blacklock, J.B.; Weber, R.S.; Lee, Y.-Y.; Goepfert, H. Transcranial Resection of Tumors of the Paranasal Sinuses and Nasal Cavity. J. Neurosurg. 1989, 71, 10–15. [Google Scholar] [CrossRef]
- McCutcheon, I.E.; Blacklock, J.B.; Weber, R.S.; DeMonte, F.; Moser, R.P.; Byers, M.; Goepfert, H. Anterior Transcranial (Craniofacial) Resection of Tumors of the Paranasal Sinuses: Surgical Technique and Results. Neurosurgery 1996, 38, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Castelnuovo, P.; Battaglia, P.; Locatelli, D.; Delù, G.; Sberze, F.; Bignami, M. Endonasal Micro-Endoscopic Treatment of Malignant Tumors of the Paranasal Sinuses and Anterior Skull Base. Oper. Tech. Otolaryngol.-Head Neck Surg. 2006, 17, 152–167. [Google Scholar] [CrossRef]
- Stavrakas, M.; Karkos, P.D.; Tsinaslanidou, Z.; Constantinidis, J. Endoscopic Denker’s Approach for the Treatment of Extensive Sinonasal Tumors: Our Experience. Laryngoscope 2021, 131, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Rotsides, J.M.; Franco, A.; Albader, A.; Casiano, R.R.; Lieberman, S.M. Nasolacrimal Duct Management During Endoscopic Sinus and Skull Base Surgery. Ann. Otol. Rhinol. Laryngol. 2019, 128, 932–937. [Google Scholar] [CrossRef]
- Bastier, P.L.; De Gabory, L. Design and Assessment of an Anatomical Diagram for Sinonasal Malignant Tumour Resection. Rhinol. J. 2016, 54, 361–367. [Google Scholar] [CrossRef]
- Sharp, S.; Gunda, D.; Gogos, A.; Wang, Y.Y.; Lyons, B.; Dixon, B. Open versus Endoscopic Craniofacial Resections in Sinonasal Adenocarcinoma: Long Term Follow up from Two Institutions. ANZ J. Surg. 2025, 95, 1725–1731. [Google Scholar] [CrossRef]
- Castelnuovo, P.G.; Belli, E.; Bignami, M.; Battaglia, P.; Sberze, F.; Tomei, G. Endoscopic Nasal and Anterior Craniotomy Resection for Malignant Nasoethmoid Tumors Involving the Anterior Skull Base. Skull Base 2006, 16, 015–018. [Google Scholar] [CrossRef]
- Chatelet, F.; Simon, F.; Bedarida, V.; Le Clerc, N.; Adle-Biassette, H.; Manivet, P.; Herman, P.; Verillaud, B. Surgical Management of Sinonasal Cancers: A Comprehensive Review. Cancers 2021, 13, 3995. [Google Scholar] [CrossRef]
- Galloni, C.; Locatello, L.G.; Bruno, C.; Cannavicci, A.; Maggiore, G.; Gallo, O. The Role of Elective Neck Treatment in the Management of Sinonasal Carcinomas: A Systematic Review of the Literature and a Meta-Analysis. Cancers 2021, 13, 1842. [Google Scholar] [CrossRef]
- Thariat, J.; Carsuzaa, F.; Marcy, P.Y.; Verillaud, B.; de Gabory, L.; Ferrand, F.R. Precision Postoperative Radiotherapy in Sinonasal Carcinomas after Endonasal Endoscopic Surgery. Cancers 2021, 13, 4802. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Xu, J.; Wang, H.; Gao, Q.; Ye, F.; Xu, Y.; Wu, S.; Cheng, S.; Lu, Y.; et al. A Retrospective Review of Non-intestinal-type Adenocarcinoma of Nasal Cavity and Paranasal Sinus. Oncol. Lett. 2023, 25, 132. [Google Scholar] [CrossRef]
- Burnet, N.G. Defining the Tumour and Target Volumes for Radiotherapy. Cancer Imaging 2004, 4, 153–161. [Google Scholar] [CrossRef]
- Brouwer, C.L.; Steenbakkers, R.J.H.M.; Bourhis, J.; Budach, W.; Grau, C.; Grégoire, V.; Van Herk, M.; Lee, A.; Maingon, P.; Nutting, C.; et al. CT-Based Delineation of Organs at Risk in the Head and Neck Region: DAHANCA, EORTC, GORTEC, HKNPCSG, NCIC CTG, NCRI, NRG Oncology and TROG Consensus Guidelines. Radiother. Oncol. 2015, 117, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Dagan, R.; Uezono, H.; Bryant, C.; Holtzman, A.L.; Morris, C.G.; Mendenhall, W.M. Long-Term Outcomes from Proton Therapy for Sinonasal Cancers. Int. J. Part Ther. 2021, 8, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Kadah, B.A.; Niewald, M.; Papaspyrou, G.; Dzierma, Y.; Schneider, M.; Schick, B. Customized Individual Applicators for Endocavitary Brachytherapy in Patients with Cancers of the Nasal Cavity, Sinonasal Region and Nasopharynx. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, V.; Mackie, T.R. State of the Art on Dose Prescription, Reporting and Recording in Intensity-Modulated Radiation Therapy (ICRU Report No. 83). Cancer/Radiothérapie 2011, 15, 555–559. [Google Scholar] [CrossRef]
- Guillemin, F.; Miroir, J.; Piram, L.; Bellini, R.; Saroul, N.; Pham Dang, N.; Boisselier, P.; Bourhis, J.; Calugaru, V.; Coutte, A.; et al. Proposal for the delineation of postoperative primary clinical target volumes in ethmoid cancers. Cancer Radiother. J. Soc. Francaise Radiother. Oncol. 2021, 25, 200–205. [Google Scholar] [CrossRef]
- Licitra, L.; Locati, L.D.; Cavina, R.; Garassino, I.; Mattavelli, F.; Pizzi, N.; Quattrone, P.; Valagussa, P.; Gianni, L.; Bonadonna, G.; et al. Primary Chemotherapy Followed by Anterior Craniofacial Resection and Radiotherapy for Paranasal Cancer. Ann. Oncol. 2003, 14, 367–372. [Google Scholar] [CrossRef]
- Bossi, P.; Perrone, F.; Miceli, R.; Cantù, G.; Mariani, L.; Orlandi, E.; Fallai, C.; Locati, L.D.; Cortelazzi, B.; Quattrone, P.; et al. Tp53 Status as Guide for the Management of Ethmoid Sinus Intestinal-Type Adenocarcinoma. Oral Oncol. 2013, 49, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Licitra, L.; Suardi, S.; Bossi, P.; Locati, L.D.; Mariani, L.; Quattrone, P.; Lo Vullo, S.; Oggionni, M.; Olmi, P.; Cantù, G.; et al. Prediction of TP53 Status for Primary Cisplatin, Fluorouracil, and Leucovorin Chemotherapy in Ethmoid Sinus Intestinal-Type Adenocarcinoma. J. Clin. Oncol. 2004, 22, 4901–4906. [Google Scholar] [CrossRef]
- Tachino, H.; Takakura, H.; Shojaku, H.; Fujisaka, M.; Akaogi, K.; Kawabe, H.; Naruto, N.; Shojaku, H.; Noguchi, K.; Miwa, S.; et al. Case Report: Response to Intra-Arterial Cisplatin and Concurrent Radiotherapy Followed by Salvage Surgery in a Patient with Advanced Primary Sinonasal Low-Grade Non-Intestinal Adenocarcinoma. Front. Surg. 2020, 7, 599392. [Google Scholar] [CrossRef] [PubMed]
- Claus, J.; Jorissen, M.; Vander Poorten, V.; Nuyts, S.; Hermans, R.; Casteels, I.; Clement, P.M.J. Clinically Relevant Response to Cisplatin-5-Fluorouracyl in Intestinal-Type Sinonasal Adenocarcinoma with Loss of Vision: A Case Report. Case Rep. Oncol. 2019, 12, 277–281. [Google Scholar] [CrossRef]
- Ferrari, M.; Bossi, P.; Mattavelli, D.; Ardighieri, L.; Nicolai, P. Management of Sinonasal Adenocarcinomas with Anterior Skull Base Extension. J. Neurooncol. 2020, 150, 405–417. [Google Scholar] [CrossRef]
- Almeyda, R.; Capper, J. Is Surgical Debridement and Topical 5 Fluorouracil the Optimum Treatment for Woodworkers’ Adenocarcinoma of the Ethmoid Sinuses? A Case-controlled Study of a 20-year Experience. Clin. Otolaryngol. 2008, 33, 435–441. [Google Scholar] [CrossRef]
- Singh, N.; Wong, E.; Huang, J.; Riffat, F. Trans-Frontal Five-Fluorouracil (TraFFF): A Novel Technique for the Application of Adjuvant Topical Chemotherapeutic Agents in Sinonasal Adenocarcinoma. BMJ Case Rep. 2018, 2018, bcr2018226234. [Google Scholar] [CrossRef]
- Revercomb, L.; Patel, A.M.; Filimonov, I.; Lerner, D.; Filimonov, A. Topical Application of 5-Fluorouracil for the Treatment of Sinonasal Adenocarcinoma and Inverted Papilloma. Am. J. Rhinol. Allergy 2024, 38, 354–360. [Google Scholar] [CrossRef]
- Riobello, C.; López-Hernández, A.; Cabal, V.N.; García-Marín, R.; Suárez-Fernández, L.; Sánchez-Fernández, P.; Vivanco, B.; Blanco, V.; López, F.; Franchi, A.; et al. IDH2 Mutation Analysis in Undifferentiated and Poorly Differentiated Sinonasal Carcinomas for Diagnosis and Clinical Management. Am. J. Surg. Pathol. 2020, 44, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, N.; Suhonen, J.; Rice, K.; Välimäki, E.; Toriseva, M.; Routila, J.; Halme, P.; Rahi, M.; Irjala, H.; Leivo, I.; et al. Assessment of Targeted Therapy Opportunities in Sinonasal Cancers Using Patient-Derived Functional Tumor Models. Transl. Oncol. 2024, 44, 101935. [Google Scholar] [CrossRef]
- Sipilä, L.J.; Katainen, R.; Aavikko, M.; Ravantti, J.; Donner, I.; Lehtonen, R.; Leivo, I.; Wolff, H.; Holmila, R.; Husgafvel-Pursiainen, K.; et al. Genome-Wide Somatic Mutation Analysis of Sinonasal Adenocarcinoma with and without Wood Dust Exposure. Genes Environ. 2024, 46, 12. [Google Scholar] [CrossRef] [PubMed]
- Thomas, Z.; Jambunathan, P.; Jibi, A.; John, A.O.; Singh, A. Low-Dose Nivolumab and Cabozantinib in Recurrent Intestinal-Type Papillary Adenocarcinoma of the Sinonasal Region. BMJ Case Rep. 2023, 16, e255021. [Google Scholar] [CrossRef] [PubMed]
- Stelow, E.B.; Bishop, J.A. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Tumors of the Nasal Cavity, Paranasal Sinuses and Skull Base. Head Neck Pathol. 2017, 11, 3–15. [Google Scholar] [CrossRef]
- Cavalieri, S.; Filippini, D.M.; Ottini, A.; Bergamini, C.; Resteghini, C.; Colombo, E.; Lombardo, R.; Nuzzolese, I.; Alfieri, S.; Licitra, L.; et al. Immunotherapy in Head and Neck Squamous Cell Carcinoma and Rare Head and Neck Malignancies. Explor. Target. Anti-Tumor Ther. 2021, 2, 522–542. [Google Scholar] [CrossRef]
- Bhojwani, A.; Unsal, A.; Dubal, P.M.; Echanique, K.A.; Baredes, S.; Liu, J.K.; Eloy, J.A. Frontal Sinus Malignancies: A Population-Based Analysis of Incidence and Survival. Otolaryngol. Head Neck Surg. 2016, 154, 735–741. [Google Scholar] [CrossRef]
| Intestinal-Type Adenocarcinoma (ITAC) | Non-Intestinal-Type Adenocarcinoma (Non-ITAC) | |
|---|---|---|
| Epidemiology & Etiology | Strongly associated with occupational exposure to wood and leather dust; predominantly affects older males. | More heterogeneous; May be sporadic or linked to general carcinogens. Divided into Low-Grade and High-Grade. |
| Common Anatomic Site | Ethmoid sinus (most common), followed by the nasal cavity. | Nasal cavity, maxillary sinus. |
| Histopathology | Resembles colorectal adenocarcinoma; forms glandular, papillary, colonic, solid, or mucinous patterns. | Low-Grade: Papillary or glandular patterns with minimal atypia. <br> High-Grade: Solid growth patterns with significant nuclear pleomorphism and necrosis. |
| Key Immunophenotype | CK20+, CDX2+, MUC2+. Often CK7-. | CK7+, CK20-, CDX2-. (Respiratory-type profile). |
| Prognosis | Generally considered aggressive with a high risk of local recurrence. | Low-Grade: Relatively indolent course. High-Grade: Aggressive clinical behavior, similar to ITAC. |
| Primary Treatment | Surgical resection followed by adjuvant radiotherapy. | Low-Grade: Surgical resection; adjuvant radiotherapy may be omitted if margins are widely negative. High-Grade: Surgical resection and adjuvant radiotherapy. |
| Adjuvant Radiotherapy Dose | Typically 60 Gy, with a possible boost to 66 Gy for positive margins. | Typically escalated to 66–70 Gy due to higher perceived radioresistance, especially in high-grade subtypes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciacca, M.; Chillari, F.; Pergolizzi, S.; Venuti, V.; Iatì, G.; Parisi, S.; Ciappina, G.; Minutoli, F.; Fiorentino, V.; Fadda, G.; et al. ITAC and Non-ITAC Sinonasal Adenocarcinoma: Classification, Etiopathogenesis, Diagnosis and Therapy Focusing on Interdisciplinarity. Medicina 2025, 61, 1895. https://doi.org/10.3390/medicina61111895
Sciacca M, Chillari F, Pergolizzi S, Venuti V, Iatì G, Parisi S, Ciappina G, Minutoli F, Fiorentino V, Fadda G, et al. ITAC and Non-ITAC Sinonasal Adenocarcinoma: Classification, Etiopathogenesis, Diagnosis and Therapy Focusing on Interdisciplinarity. Medicina. 2025; 61(11):1895. https://doi.org/10.3390/medicina61111895
Chicago/Turabian StyleSciacca, Miriam, Federico Chillari, Stefano Pergolizzi, Valeria Venuti, Giuseppe Iatì, Silvana Parisi, Giuliana Ciappina, Fabio Minutoli, Vincenzo Fiorentino, Guido Fadda, and et al. 2025. "ITAC and Non-ITAC Sinonasal Adenocarcinoma: Classification, Etiopathogenesis, Diagnosis and Therapy Focusing on Interdisciplinarity" Medicina 61, no. 11: 1895. https://doi.org/10.3390/medicina61111895
APA StyleSciacca, M., Chillari, F., Pergolizzi, S., Venuti, V., Iatì, G., Parisi, S., Ciappina, G., Minutoli, F., Fiorentino, V., Fadda, G., Bottari, A., & Ferrantelli, G. (2025). ITAC and Non-ITAC Sinonasal Adenocarcinoma: Classification, Etiopathogenesis, Diagnosis and Therapy Focusing on Interdisciplinarity. Medicina, 61(11), 1895. https://doi.org/10.3390/medicina61111895








