1. Introduction
Worldwide, the prevalence and incidence of obesity is alarming, especially in countries facing financial difficulties [
1]. Most of the time, an individual struggling with obesity suffers from a range of other comorbidities, such as type 2 diabetes (T2DM), dyslipidemia and hypertension (HTN), obstructive sleep apnea (OSA), or metabolic-associated fatty liver disease (MAFLD) [
2]. The health complications brought on by obesity leads to an increased risk of premature death, cardiovascular disease, mental health concerns, reproductive and fertility issues, kidney disease, and musculoskeletal problems [
3].
In 1985 the concept of oxidative stress was introduced as an imbalance between production in the human body of the free radicals and its ability to trap them into stable compounds. This results either from an increased production of reactive species such as ROS (reactive oxygen species), RNS (reactive nitrogen species), or other free radicals centered on other atoms such as S, C, etc. [
4]. This imbalance between the production and elimination of free radicals and other reactive species—relatively stable, non-radicalic compounds that can be converted into free radicals under certain conditions—plays a critical role in the pathophysiology of oxidative stress [
5].
Oxidative stress arises from an imbalance between the production of reactive oxygen species (ROS) and the body’s antioxidant defenses. Glutathione (GSH), a tripeptide containing cysteine, glutamic acid, and glycine, is one of the most important antioxidants. It neutralizes ROS by donating electrons via its thiol group, becoming oxidized to GSSG in the process. Superoxide dismutases (SOD) convert superoxide anions into hydrogen peroxide, which may further generate highly reactive hydroxyl radicals (•OH). These radicals can trigger lipid peroxidation, cellular damage, and inflammation [
6]. Malondialdehyde (MDA) is widely used as a marker of oxidative stress caused by •OH radicals [
7]. Nitric oxide (NO), another key signaling molecule, regulates vascular tone, blood flow, oxygen delivery, and muscle function. Its bioavailability is often assessed by measuring nitrite (NO
2−), while dietary nitrate (NO
3−) from vegetables can also contribute to NO production through the nitrate–nitrite–NO pathway [
8].
In previous work, we have focused on elucidating the relationship between chronic systemic inflammation and weight-reduction outcomes following laparoscopic sleeve gastrectomy (LSG), emphasizing the utility of plasma cytokine profiling as a biomarker of inflammatory status [
9,
10]. Another study investigated the link between ROS, obesity, and metabolic syndrome to evaluate whether bariatric surgery-induced weight loss mitigates obesity-related inflammation and metabolic comorbidities [
11].
The purpose of this study was to evaluate if obesity is associated with oxidative stress biomarkers such as MDA, GSH, GSH/GSSG, total glutathione levels, NO2−, and NO3− in patients with obesity before and after being subjected to bariatric surgery and to follow the changes brought by such surgery. A group of patients without obesity served as reference for normal values.
2. Materials and Methods
2.1. Study Design
We established a prospective observational cohort at two centers in Târgu Mureș, Romania, enrolling 50 adults with morbid obesity undergoing bariatric surgery and 50 non-obese surgical patients as controls (enrollment between 1 February 2021–1 December 2023). Bariatric participants were sampled preoperatively (B1) and 12 months postoperatively (B2); thus, B1 and B2 represent the same individuals. Inclusion and exclusion criteria, comorbidities, and perioperative care followed established guidance. All participants provided written informed consent.
Sub-objectives of this study were to assess the evolution of inflammatory and oxidative stress biomarkers among severely obese subjects undergoing LSG. Thereby, we submit a comprehensive survey of some of the most important OS-status biomarkers in the morbidity of obese individuals, both at baseline and 1 year following bariatric surgery, in comparison with baseline values from patients undergoing non-obesity-related surgery procedures.
Inclusion Criteria
Adults with severe obesity eligible for LSG
Complete medical records available
Consent to participate in the study
Exclusion Criteria
Malignancies
Acute inflammatory diseases
Severe blood or autoimmune disorders
Major mental health conditions
Overweight or first-degree obesity patients
Chronic inflammatory conditions (e.g., Crohn’s disease, chronic pancreatitis)
Ongoing use of anti-inflammatory, antibiotic, antiviral, or other long-term drug therapies
Active alcohol or illicit drug abuse
Incomplete medical records
Laboratory-confirmed COVID-19 infection at the time of surgery (all patients were tested preoperatively)
Each patient received full information about the purpose of the study and the procedures to be followed and gave their consent. Approval for the study was obtained from the committees on ethics of the two hospitals involved, Mures County Emergency Hospital (protocol code 3570, on 19 February 2021) and Topmed Private Hospital Târgu Mureș (registration number 114, on 16 March 2021), to ensure that the principles set out in the Helsinki Declaration are strictly followed.
2.2. Clinical Assessments
Patients were assessed preoperatively by a multidisciplinary team, covering medical history gathering, physical examination and laboratory tests. Anthropometric measurements were performed preoperatively which included body height (BH), precision on measurement +/− 0.5 cm, body weight (BW), precision on measurement +/− 0.1 kg, and waist circumference (WC) measured on the midline between the anterior superior iliac crest and the lowest rib. Their body mass index (BMI) levels were calculated based on weight (expressed in kilograms) divided by the square of the body height (measured in meters). Blood pressure and heart rate values were obtained with the use of a semi-automatic oscillometric device, following standard recommendations. In addition, we recorded relevant clinical data (comorbidities like hypertension, diabetes, dyslipidaemia) as well as laboratory data from routine patient assessment (for example, lipid profiles) [
12].
2.3. Oxidative Stress Markers and Antioxidants Assessment
The following markers of oxidative stress were measured before surgical intervention and one year after: malondialdehyde, reduced and oxidized glutathione, nitrite, and nitrate anions.
2.3.1. Reduced and Oxidized Glutathione Measurement
Quantification of GSH and GSSG was carried out according to the method of Jîtcă G. et al., based on the reaction with Ellman’s reagent (DTNB). In this assay, reduced glutathione reacts with DTNB to produce a chromophore detected at 330 nm. Total glutathione was determined after chemical reduction of oxidized forms, while GSSG was calculated from the difference between total glutathione and GSH in
Table 1. Care was taken to avoid hemolysis, as erythrocytes contain markedly higher glutathione concentrations than plasma, which could bias results. Analyses were performed on a quaternary HPLC system equipped with a diode array detector (Merck, Darmstadt, Germany). Calibration curves were established using certified standards of GSH and GSSG [
12].
2.3.2. Malondialdehyde Measurement
MDA levels were assessed using the thiobarbituric acid-reactive substances (TBARS) assay coupled with HPLC, as previously described by Fogarasi E et al. This approach provides greater specificity than simple spectrophotometric detection. 1,1,3,3-Tetramethoxypropane (TMP) was used as a stable precursor for MDA calibration. Chromatographic detection was performed at 532 nm, with isocratic elution of acetonitrile and phosphate buffer (pH 6.0) [
13].
2.3.3. Nitrite and Nitrate Measurement
Nitrite and nitrate concentrations were determined simultaneously by HPLC with UV/VIS detection following the method of Croitoru et al. Nitrite was quantified through the Griess reaction, producing an azo dye detectable at 520 nm, while nitrate was measured by ion-pair chromatography with tetrabutylammonium hydroxide as the pairing agent in
Table 2. Calibration was performed with sodium nitrite and sodium nitrate standards [
14].
2.4. Surgical Intervention Protocol
Patients with obesity underwent two kinds of bariatric interventions: most of them underwent laparoscopic sleeve gastrectomy (LSG), with the steps of the procedure previously reported by us [
9], without significant technical differences. A minority of patients among this group (i.e., the bariatric group) underwent laparoscopic gastric bypass with a single anastomosis (OAGB), where a gastrojejunostomy was performed 180 cm below the duodenojejunal angle in a side-to-side antecolic manner with either a linear or manual stapler. The procedures carried out in the control group were the following: partial or total thyroidectomy, laparoscopic cholecystectomy, transabdominal preperitoneal or total extraperitoneal inguinal hernia repair.
2.5. Follow-Up
During the first year, we prospectively followed all our patients for 3 months post-surgery. Records were gathered during postoperative follow-up appointments; we recorded the weight-loss process, influence of the bariatric procedure over the main comorbidities of obesity and other clinical as well as technical matters linked to the procedure. All bariatric participants were sampled at 12 months for biomarker reassessment.
2.6. Analytical Validation and Reproducibility
All biomarker determinations were performed using HPLC-based techniques with UV or VIS detection, as detailed above. Reduced and oxidized glutathione (GSH and GSSG) were quantified using Ellman’s reagent with calibration against certified standards, while malondialdehyde (MDA) was measured via the thiobarbituric acid reaction using 1,1,3,3-tetramethoxypropane as a stable standard. Nitrite and nitrate were determined by the Griess reaction combined with ion-pair chromatography. The HPLC system consisted of a quaternary pump, column thermostat, diode array detector, and autosampler (Merck, Darmstadt, Germany). Calibration curves for all analytes showed excellent linearity (R
2 > 0.99). Samples were measured in duplicate, and intra-assay coefficients of variation were <10% for all assays. To ensure data reliability, hemolyzed samples were excluded due to the high intracellular glutathione content in erythrocytes. Analytical reproducibility and accuracy were confirmed by repeat analyses of randomly selected samples and through comparison with published methods [
13,
14,
15].
2.7. Statistical Analysis
Sample size justification: The study included 50 patients with obesity and 50 normoponderal controls. Although no formal a priori power calculation was performed, this sample size is comparable to or larger than those used in previous clinical studies investigating oxidative stress biomarkers in obesity and bariatric cohorts [
15]. Therefore, it was considered sufficient to detect clinically meaningful differences within the analyzed parameters.
All statistical analyses were performed using GraphPad Prism 9 (GraphPad Software, Inc.), Microsoft Excel, and OriginPro 2018. Continuous variables were expressed as mean ± standard deviation (SD). Data distribution was assessed using the Shapiro–Wilk test to determine normality. Depending on the data distribution, either parametric tests (e.g., paired or unpaired two-tailed Student’s t-test) or non-parametric tests (e.g., Wilcoxon signed-rank test, Mann–Whitney U test) were applied for within-group and between-group comparisons, respectively. One-tailed t-tests were used in cases where a directional hypothesis was justified based on prior literature or expected biological outcomes.
Correlation analyses were conducted using Pearson’s correlation coefficient (for parametric data) or Spearman’s rank correlation coefficient (for non-parametric data), with respective p-values reported. Repeated measures ANOVA was used where applicable to assess changes over time in the bariatric group.
Statistical significance was considered at p < 0.05, and all tests were two-tailed unless otherwise specified. Confidence intervals (95%) were calculated where appropriate. Statistical outputs were visualized using bar plots, scatter plots with regression lines, and box-and-whisker plots.
3. Results
In our test we measured the concentration of the selected biomarkers [malondialdehyde (MDA), reduced and oxidized glutathione (GSH and GSSG), nitrite (NO2−), and nitrate (NO3−)] to evaluate the effects of bariatric surgery on the oxidative parameters of patients with obesity.
Both the obese and normoponderal groups included 50 participants each. While no formal a priori power analysis was performed, the sample size is in line with or exceeds that reported in comparable studies on oxidative stress in obesity [
16] and was considered adequate for detecting relevant group differences.
ANOVA test was used to compare the means of a continuous outcome across more than two groups and Chi-square test was used to compare the distribution of a categorical variable across multiple groups. Data are presented as mean ± standard deviation (SD). All abbreviations used in this
Table 3 are defined at the end of the article in the Abbreviations section.
Laboratory parameters such as glucose, liver enzymes (GOT, GPT, GGT), hemoglobin, hematocrit, leukocytes and their subpopulations (neutrophils, lymphocytes, eosinophils), as well as platelet count, were included to provide a comprehensive metabolic and clinical profile of the patients. These markers reflect systemic inflammation, hepatic function, and metabolic status, all of which are commonly altered in obesity and can influence oxidative stress levels. Their inclusion allowed for a more accurate interpretation of redox biomarkers by accounting for potential confounding factors that could impact oxidative stress independently of the bariatric intervention.
As expected, the reduction in the intensity of the metabolic processes brought about by the low calorie intake that follows a bariatric surgery took place, and our measurements proved this through tests for the reduction in free radical formation.
We measured malondialdehyde (MDA) as the main free radical formed in the human body in both obese and normoponderal subjects. It is formed during HO reaction with polyunsaturated fatty acids—cell membrane being the most sensitive cell component.
The expected outcome of our study was the modification of all of our measured parameters: MDA, GSH, GSSG and their ratio (GSH/GSSG), nitrite, and nitrate and their ratio.
3.1. Glutathione Values
Free radical formation was indeed commonly reduced following bariatric surgical treatment.
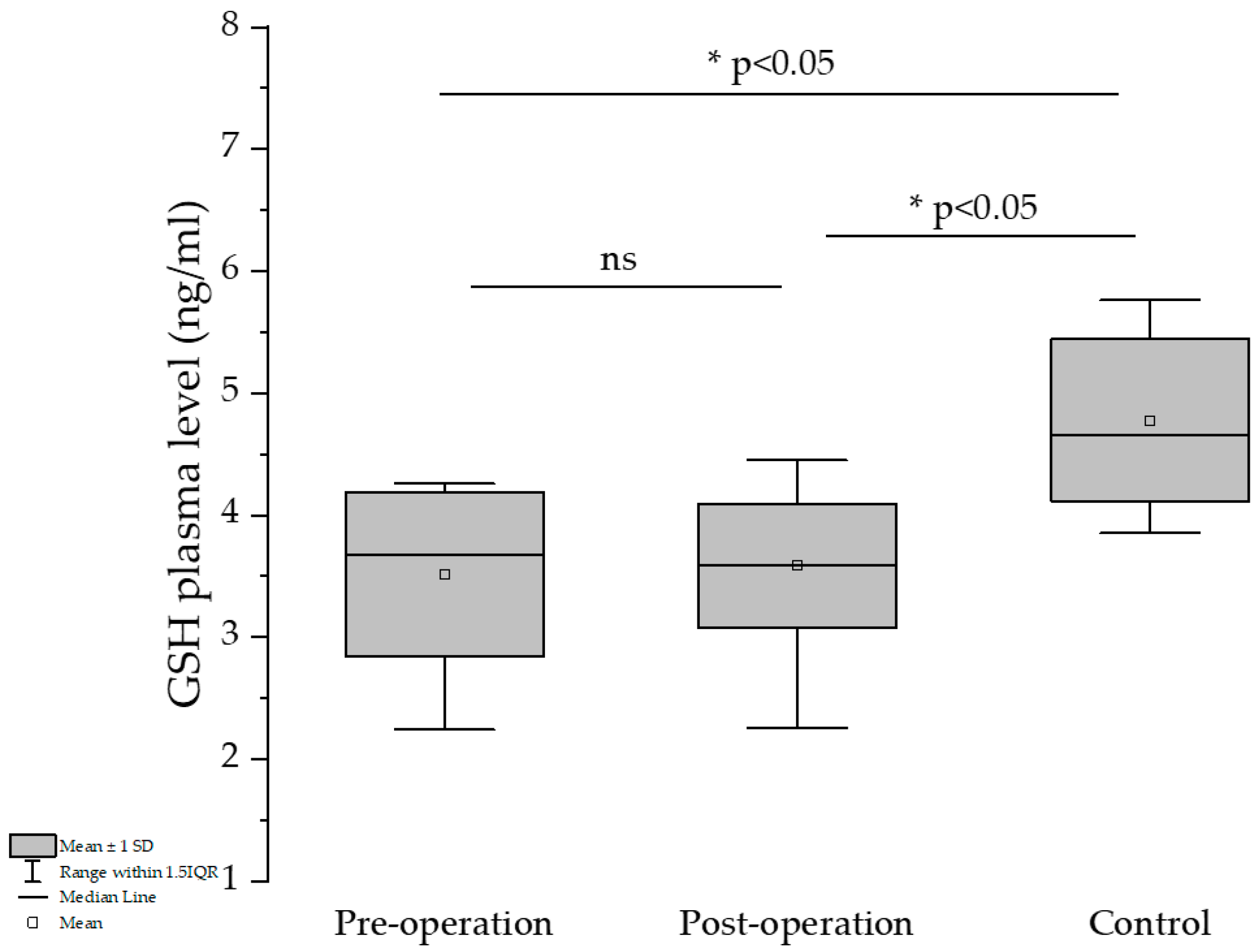
Comparing the pre- and post-operation groups, a slight GSH-level elevation could be observed for the latter, but the differences are not significant (
Figure 1).
The percentage of GSH was slightly increased in the post-surgery group compared to the pre-treatment group and almost attained the control group’s GSH-level percentage (42.7 and 43.7 vs. 47.4%). The GSH/GSSG ratio is below 1 in all three groups, which can be explained by the fact that all of them suffered under different pathological conditions.
As expected, increase in BMI reduction (ΔBMI) was inversely associated with the decrease in the Δ GSH values, which was revealed by a one-tailed t-test—this type of test was used because it was expected that an increase in body weight reduction would increase production of GSH, and only that hypothesis was tested. Furthermore, the reduction in BMI is inversely correlated with GSH values (r = −0.2526, p* = 0.0384, one-tailed t-test), showing the beneficial effects of bariatric surgery on the protective-antioxidant effect on the increase in GSH values. It is worth mentioning that preoperatory values of GSH in patients with obesity were significantly lower than those measured in the control group (3.513 vs. 4.778, unpaired two-tailed t-test, p < 0.0001 ****) but were almost the same as values recorded after surgery, which were still statistically lower than values recorded for the control group (3.586 vs. 4.778, unpaired two-tailed test, p < 0.0001 ***).
Additionally, our measurements showed that decreases in BDW induce less increases in GSH values (r = −0.256, one-tailed t-test, p* = 0.0384).
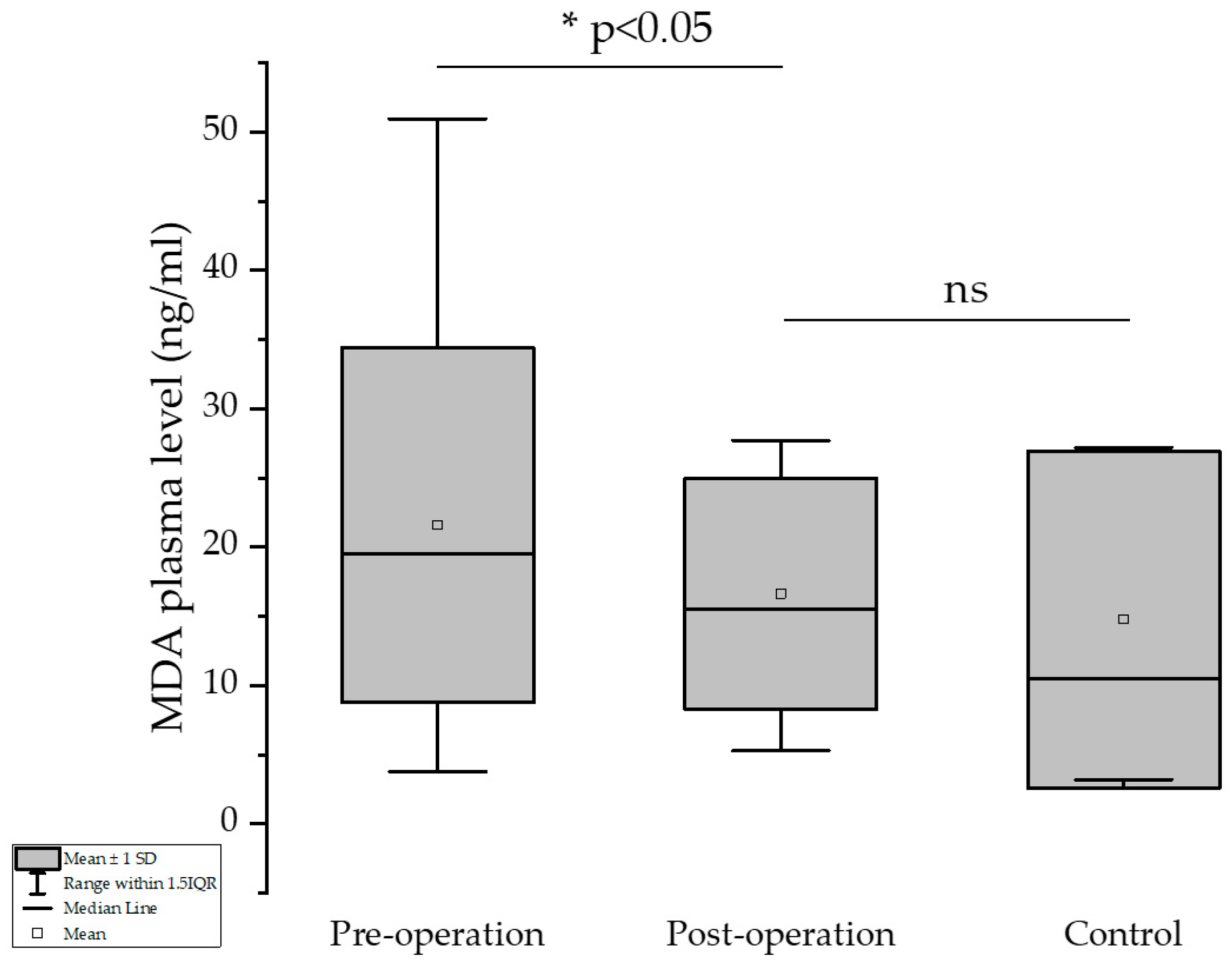
3.2. Malondialdehyde Values
The results showed that excessive formation of MDA due to increased metabolism and excessive formation of its precursors (mainly H
2O
2) was significantly reduced after the bariatric surgery procedure. The higher the loss of body weight (expressed as difference in BMI pre- and one-year post-surgical intervention), the higher the decrease in the MDA values (Pearson’s test, −0.25588,
p* = 0.0348). Mean of the MDA values pre- and post-operation were 21.58 and 16.62 ng/mL, respectively, showing a significant decrease in this significant marker of the oxidative stress (
p = 0.013, one-tailed paired
t-test). There was no significant difference between the post-operation and control groups (
p > 0.05, one-sample
t-test) in terms of MDA plasma level (
Figure 2).
3.3. Nitrite and Nitrate Values
Regarding the nitrate values, the mean values increased in the order of pre-, post-operation and control group, showing significant differences in all cases in
Figure 3A (
p < 0.05, using paired
t-test and two-sample
t-test, respectively).
In the case of nitrite, the mean value of the post-operation group was close to that obtained in the control group (9.40 and 8.01 ng/mL, respectively), the difference not being statistically significant (two-sample
t-test). Regarding the pre-operation group’s mean nitrite value, a significant decrease can be observed compared to that of the one-year post-operation group in
Figure 3B (19.22 vs. 9.40 ng/mL,
p < 0.05, paired
t-test).
As shown in
Figure 4A,B, age was significantly correlated with both the GSH/GSSG ratio and plasma GSSG levels. Specifically, the GSH/GSSG ratio decreased with increasing age (r = –0.34,
p = 0.017), while GSSG concentrations rose slightly with age (r = 0.30,
p = 0.037). These findings indicate an age-related decline in redox balance.
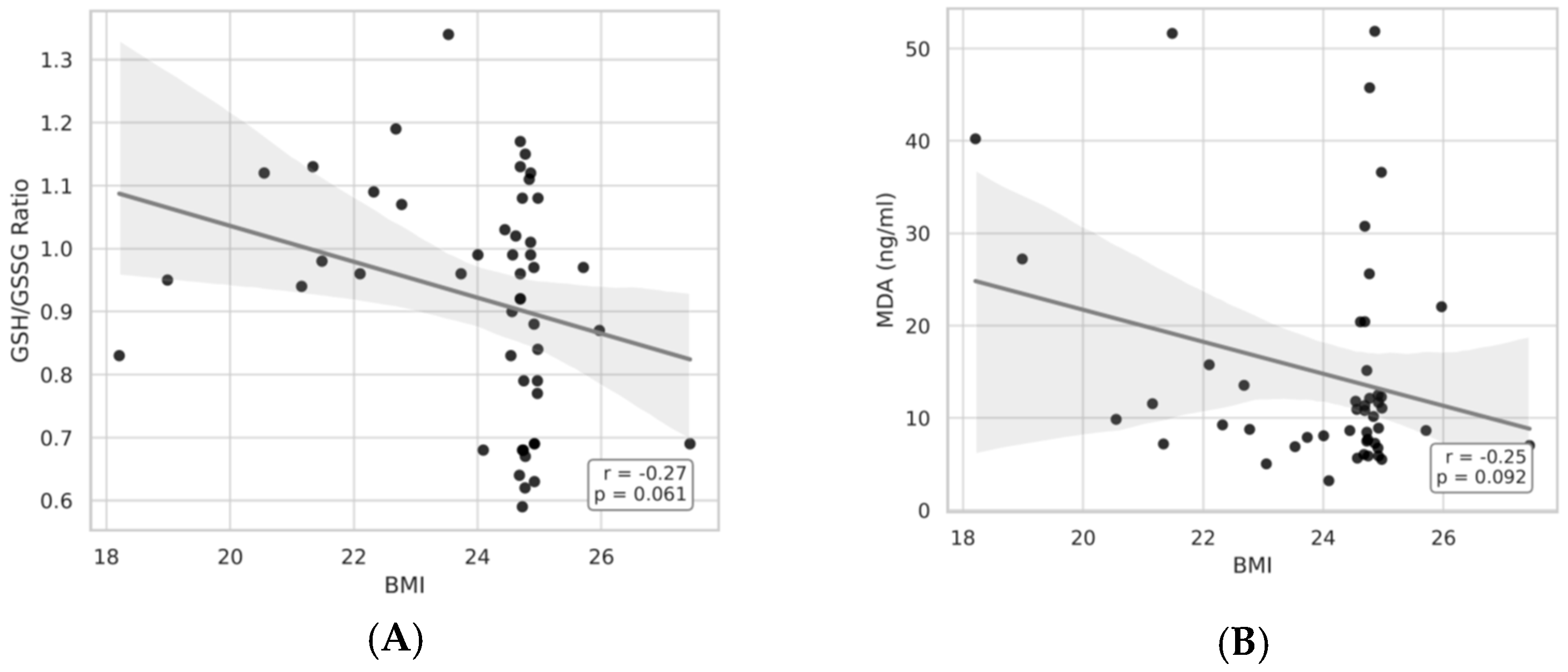
As illustrated in
Figure 5 and
Figure 6A,B, BMI showed significant correlations with both the GSH/GSSG ratio, plasma GSH and MDA concentrations. In particular, the GSH/GSSG ratio declined with advancing BMI (r = −0.27,
p = 0.061), whereas plasma GSH levels exhibited a modest decrease (r = −0.27,
p = 0.059). Together, these results suggest an bmi-associated reduction in redox homeostasis.
4. Discussion
Obesity and oxidative stress are correlated based on the data in the scientific literature [
16]. In our study, several markers of oxidative stress were measured in the blood of patients with obesity requiring bariatric surgery to control their weight, health, and quality of life. The markers of oxidative stress we measured were GSH (reduced glutathione), GSSG (oxidized glutathione), MDA (malondialdehyde), nitrite, and nitrate anions (and their ratio, also). These markers cover a large part of the process called oxidative stress, which describes the imbalance between the production and neutralization of molecules and atoms that contain a single electron in the valence orbitals (definition of free radicals). These reactive species can react with biomolecules, leading to destruction of important cellular structures, or can reduce the availability of antioxidant molecules (also known as spin traps) that are used to protect cells against free radicals that are produced even under the best biochemical and health circumstances [
17].
This study confirms previous findings that obesity is associated with increased oxidative stress, and that bariatric surgery can ameliorate several redox-related parameters. Specifically, we found that GSH levels increased, MDA levels decreased, and nitrite/nitrate concentrations were altered one year after surgery.
The explanation for why the following markers were chosen in order to obtain good insight into the oxidative damage suffered by our patients are presented hereunder. It can be seen that a good coverage of the oxidative damage to tissues and the ability of the patient to reduce such damage is achieved by the measurements we made.
4.1. Glutathione (GSH/GSSG)
Our observation of increases in reduced glutathione (GSH) postoperatively, alongside an improved GSH/GSSG ratio, suggests enhanced systemic antioxidant capacity following weight loss. This aligns with findings from Choromańska et al. (2020) and Abulmeaty et al. (2023), who also observed redox restoration after bariatric interventions. The observed inverse correlations between ΔBMI and ΔGSH further support the relationship between fat-mass reduction and antioxidant recovery [
16,
17].
Glutathione blood concentration shows the ability of the human organism to neutralize free radicals (by electron transfer process, but also by directly binding to the free radical), through processes by which glutathione, by enzymatic pathway, can be restored to its original form or the formation of covalent bonds when glutathione is lost and cannot be recovered. A reasonable explanation for this fact could be the increased consumption of glutathione used during massive body-weight loss, giving rise to increased lipid peroxidation processes. Reduced, oxidized, and total glutathione levels serve as markers of oxidative stress [
18].
4.2. Malondialdehyde
MDA is produced when unsaturated fatty acids, present in the cell membrane, are damaged by the hydroxy free radical process that leads to cell necrosis—a pluricellular event (not only is the affected cell subjected to destruction, but the surrounding cells could also be affected). MDA is an extremely important marker because it induces peroxidation of cell-membrane lipids, associated with cell necrosis. No antioxidant is able to directly reduce the activity of hydroxy free radical, but substances able to reduce its precursors are known. Therefore, reduction in the levels of MDA serves as an evaluation of the antioxidant ability of certain molecules [
19].
As a well-established lipid peroxidation marker, MDA’s significant decrease post-surgery supports reduced free radical burden. The improvement in MDA aligns with better mitochondrial efficiency and decreased inflammatory response, typical after sustained weight loss [
7].
4.3. Nitrite and Nitrate (NO2−/NO3−)
Nitrite anion is a marker of nitric oxide formation, a molecule which can have both antioxidant and pro-oxidant activity in the human body. The nitrite/nitrate ratio was recently included in the markers of oxidative stress.
Nitrite is the first decomposition product of nitric oxide (NO). NO serves as an antioxidant in normal circumstances (preventing the cholesterol oxidation process = formation of the pro-atherosclerotic moiety, 25-OH cholesterol, by preventing the superoxide anion reaction with cholesterol). It also serves as coronary dilatator, neurotransmitter, and supports other biological functions. But as a free radical, when archives are higher than physiological (picomolar; pM) concentrations it transforms into nitrogen dioxide (NO
2), a substance able to nitrate tyrosine residues in proteins, a process known to negatively affect the cardiac muscle. The nitrite/nitrate ratio is also accepted as an indicator of oxidative stress. However, due to the large amounts of nitrate in some types of vegetable foods, such a ratio should be treated with care and never used alone in estimation of oxidative stress [
8].
Despite reductions in nitrite and nitrate levels postoperatively, their interpretation is complex. Nitrite serves as a proxy for NO bioavailability, and reductions may reflect diminished systemic NO production or altered endothelial function. However, this change does not necessarily indicate a harmful shift. Similar trends were reported by Wei et al., who emphasized the contextual nature of NO metabolism. Our findings suggest that although oxidative damage is reduced, the balance of NO-related species warrants further exploration. Future work should include endothelial function markers (e.g., flow-mediated dilation or cGMP) to better understand this dynamic [
8,
15].
As expected, the weight loss achived by the patients improved reduced plasma glutathione concentrations (GSSH), probably due to the reduction in body weight, and the consecutive reduction in the excessive burn of calories due to the presence of excessive body mass. In such situations (excessive body mass = obesity), the metabolic processes will be accelerated when compared with that of an individual with so-called “normal body mass”. Overproduction of free radicals during increased physical exercise is usually counteracted by the so-called “antioxidant” mechanisms. However, studies have shown that low- and moderate-intensity exercises are associated with positive effects on the heart (other effects are not considered here) and excessive training could have similar effects on the heart infarction risk as sedentarism has [
20].
The decreased caloric intake that follows bariatric surgery leads to a reduction in the caloric usage of the organism and therefore, a reduction in formation of free radicals. Of course, the reduction in the caloric intake outweighs the reduction in the caloric consumption, therefore an advantage for the reduction in body mass can be seen for a long period of time [
21].
Before the surgery, GSH concentrations, as expected, inversely correlated with the BMI of the patient, showing that BMI has an impact either on the production or the consumption of the GSH (lower production associated or not with higher consumption). This is due to the fact that, in such patients with obesity, even a slight effort will require intensification of the metabolic processes at levels that are far beyond those that are acceptable as a physiological response to an increased physical activity. Post-surgery, the changes in the GSH values (ΔGSH) inversely correlated with the changes in the BMI (ΔBMI), showing that improvement in this oxidative stress marker is correlated with therapeutical success (reduction in the BMI—ΔBMI) [
22].
The biochemical improvements observed in oxidative stress markers postoperatively likely reflect complex molecular and metabolic adaptations following significant weight loss. The increase in reduced glutathione (GSH) may be attributed to enhanced activity of the Nrf2–ARE (antioxidant response element) signaling pathway, which regulates the transcription of genes responsible for antioxidant defenses such as GCLC and GCLM—enzymes essential for GSH synthesis [
23,
24]. Weight loss also diminishes systemic inflammation and pro-oxidant cytokines (e.g., TNF-α, IL-6), which are known to suppress Nrf2 activity [
25]. In parallel, the reduction in malondialdehyde (MDA), a marker of lipid peroxidation, may result from decreased mitochondrial-ROS production due to improved insulin sensitivity, reduced adipocyte dysfunction, and lower oxidative-metabolism stress [
26]. Furthermore, the observed decline in nitrite and nitrate levels suggests a downregulation of inducible nitric oxide synthase (iNOS) activity, a common consequence of resolving chronic low-grade inflammation. This reduction in NO metabolites might also reflect improved endothelial function, favoring eNOS-mediated NO production at physiologic levels rather than pathological overproduction. Together, these findings suggest that bariatric surgery not only reduces oxidative stress by limiting ROS production but also restores regulatory pathways that govern redox homeostasis [
27].
Our observation that bariatric surgery leads to reductions in BMI, venous plasma glucose, and oxidative stress markers aligns with existing literature not only in magnitude but in mechanistic interpretation. In particular, Jakubiak et al. used principal-component analysis in a large cohort to show that among the components of metabolic syndrome, the cluster defined by ‘obesity and insulin resistance’ exhibited the strongest association with oxidative stress parameters. Thus, our results support the hypothesis that the interplay of adiposity and insulin resistance is a principal driver of oxidative imbalance—and that surgical weight-loss mitigates oxidative stress largely through this axis [
28].
While our biomarker results are not novel per se, this study provides valuable regional insights by presenting data from a Romanian cohort—a population underrepresented in bariatric redox studies. Thus, the findings may inform region-specific health strategies or dietary interventions. To our knowledge, few studies have simultaneously assessed this panel of oxidative stress markers in this demographic context [
11,
17].
4.4. Strengths and Limitations
This study has several strengths. First, it is based on a relatively large and well-defined cohort, including both patients with obesity undergoing bariatric surgery and a matched control group of normoponderal surgical patients. The prospective design, with a one-year follow-up, allowed us to evaluate longitudinal changes in oxidative stress biomarkers within the same individuals, strengthening causal inference. Furthermore, we applied validated HPLC-based analytical methods for quantification of glutathione, malondialdehyde, nitrite, and nitrate, providing sensitive and specific biochemical measurements.
However, some limitations should also be acknowledged. The study was conducted at two centers in a single region of Romania, which may limit generalizability to other populations. Although we included 50 patients per group, larger multicenter cohorts would provide greater statistical power, especially for subgroup analyses. We did not perform additional mechanistic assessments (e.g., measurement of antioxidant enzyme activity, endothelial function, or inflammatory signaling pathways), which could have provided deeper insights into the observed biochemical changes. Moreover, while follow-up was limited to one year, longer-term studies are needed to determine the durability of redox-homeostasis improvements after bariatric surgery. Finally, dietary factors, physical activity, and medication use were not systematically controlled postoperatively, and these may have influenced oxidative-stress status.
Finally, translational studies exploring how improvements in oxidative stress relate to long-term clinical outcomes—such as reduced cardiovascular risk, better metabolic control, and improved quality of life—are needed to clarify the broader health benefits of bariatric surgery.
5. Conclusions
Our study, conducted with a significant number of patients, shows that weight loss could bring significant improvements in certain biomarkers of oxidative stress (glutathione), but could also negatively affect other parameters such as nitrite (a good biomarker of NO formation). Further studies are required to establish if weight loss after bariatric surgery has desirable or undesirable effects on the patients and what measures could be undertaken to ensure only positive effects are experienced.
Author Contributions
R.M.I. was responsible for conceptualization, formal analysis, data curation, and writing. R.M.N. was responsible for conceptualization, validation, and supervision. E.M. was responsible for validation, and data curation. O.A.R. was responsible for formal analysis. G.B. was responsible for resources. M.D.C. was responsible for visualization, data curation, and writing. I.F. was responsible for software, and statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Targu Mures, Romania, Research Grant Number 294/4/14.01.2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Targu Mures Emergency County Hospital, Romania (protocol code 3570, on 19 February 2021).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This paper was published with the support of the George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Targu Mures and is part of a Ph.D. thesis from the Doctoral School of Medicine and Pharmacy within the George Emil Palade University of Medicine, Pharmacy, Science, and Technology of Targu Mures with the title “Changes in the markers of systemic inflammation and oxidative stress in patients with morbid obesity”, which will be presented by Ion Razvan-Marius, having the approval of all authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| 25-OH | 25-hydroxy |
| BDW | Body dry weight |
| BH | Body height |
| BMI | Body mass index |
| BW | Body weight |
| EDTA | Etilendiaminotetraacetic acid |
| Fe2+ | Iron two |
| Gtot | Total glutathione |
| GGT | Gamma-glutamyl transferase |
| GOT | Aspartate aminotransferase |
| GPT | Alanine aminotransferase |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| H2O2 | Hydrogen peroxide |
| H2SO4 | Sulfuric acid |
| HDL | High density lipoprotein |
| HGB | Hemoglobin |
| •OH | Hydroxide ion |
| HPLC | High-performance liquid chromatography |
| HTC | Hematocrit |
| HTN | Hypertension |
| LDL | Low-density lipoprotein |
| LSG | Sleeve gastrectomy |
| MDA | Malondialdehyde |
| MAFLD | Metabolic-associated fatty liver disease |
| NO | Nitric oxide |
| NO2 | Nitrogen dioxide |
| NO2− | Nitrite |
| NO3− | Nitrate |
| OAGB | One-anastomosis gastric bypass |
| OS | Oxidative stress |
| OSA | Obstructive sleep apnea |
| ROS | Reactive oxygen species |
| RP | Reverse phase |
| RNS | Reactive nitrogen species |
| SOD | Superoxide dismutases |
| T2DM | Type 2 diabetes |
| TG | Triglyceride |
| WC | Waist circumference |
References
- Eurostat. Overweight and Obesity—BMI Statistics. 2024. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Overweight_and_obesity_-_BMI_statistics#:~:text=This%20article%20presents%20statistics%20on%20the%20proportion%20of%20the%20overweight (accessed on 23 September 2024).
- Garaulet, M.; Madrid, J.A. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv. Drug Deliv. Rev. 2010, 62, 967–978. [Google Scholar] [CrossRef]
- Adams, K.F.; Schatzkin, A.; Harris, T.B. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N. Engl. J. Med. 2006, 355, 763–778. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Lopez-Domenech, S.; Bañuls, C.; Diaz-Morales, N.; Escribano-Lopez, I.; Morillas, C.; Veses, S.; Orden, S.; Alvarez, A.; Victor, V.M.; Hernandez-Mijares, A.; et al. Obesity impairs leukocyte-endothelium cell interactions and oxidative stress in humans. Eur. J. Clin. Investig. 2018, 48, e12985. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Khoubnasabjafari, M.; Ansarin, K.; Jouyban, A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts 2015, 5, 123–127. [Google Scholar] [PubMed]
- Wei, C.; Vanhatalo, A.; Black, M.I.; Blackwell, J.R.; Rajaram, R.; Kadach, S.; Jones, A.M. Relationships between nitric oxide biomarkers and physiological outcomes following dietary nitrate supplementation. Nitric Oxide 2024, 148, 23–33. [Google Scholar] [CrossRef]
- Ion, R.M.; Hutanu, A.; Sala, D.T.; Muresan, M.G.; Fodor, S.R.; Voidazan, S.; Beresescu, G.; Neagoe, R.M. Short-Term Changes in TNF-Alpha, IL-6 and Adiponectin Following Bariatric Surgery in Caucasian Obese Adults: An Observational Case-Control Study. Medicina 2024, 60, 1789. [Google Scholar]
- Ion, R.M.; Sibianu, M.; Hutanu, A.; Beresescu, F.G.; Sala, D.T.; Flavius, M.; Rosca, A.; Constantin, C.; Scurtu, A.; Moriczi, R.; et al. A Comprehensive Summary of the Current Understanding of the Relationship between Severe Obesity, Metabolic Syndrome, and Inflammatory Status. J. Clin. Med. 2023, 12, 3818. [Google Scholar] [CrossRef]
- Ion, R.M.; Sibianu, M.; Neagoe, R.M.; Sala, D.; Beresescu, F.; Daniealopol, V.; Daniealopol, R.; Muresan, M. The role of nitro oxdative factors in metabolic dysfunctions: A link between severe obesity and weight-loss treatment—A narrative review. Rev. Romana Med. Lab. 2024, 32, 135–142. [Google Scholar]
- WHO Expert Committee. Physical Status: The Use and Interpretation of Anthropometry. WHO Technical Report Series, No. 854; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- JÎtcă, G.; Fogarasi, E.; Ősz, B.E.; Vari, C.E.; Fülöp, I.; Croitoru, M.D.; Rusz, C.M.; Dogaru, M.T. Profiling the Concentration of Reduced and Oxidized Glutathione in Rat Brain Using HPLC/DAD Chromatographic System. Molecules 2021, 26, 6590. [Google Scholar] [CrossRef]
- Fogarasi, E.; Croitoru, M.D.; Fülöp, I.; Nemes-Nagy, E.; Tripon, R.G.; Simon-Szabo, Z.; Muntean, D.-L. Malondialdehyde levels can be measured in serum and saliva by using a fast HPLC method with visible detection. Rev. Romana Med. Lab. 2016, 24, 319–326. [Google Scholar]
- Croitoru, M.D. Nitrite and nitrate can be accurately measured in samples of vegetal and animal origin using an HPLC-UV/VIS technique. J. Chromatogr. B 2012, 911, 154–161. [Google Scholar] [CrossRef]
- Choromańska, B.; Myśliwiec, P.; Łuba, M.; Wojskowicz, P.; Dadan, J.; Myśliwiec, H.; Choromańska, K.; Zalewska, A.; Maciejczyk, M. A Longitudinal Study of the Antioxidant Barrier and Oxidative Stress in Morbidly Obese Patients after Bariatric Surgery. Does the Metabolic Syndrome Affect the Redox Homeostasis of Obese People? J. Clin. Med. 2020, 9, 976. [Google Scholar] [CrossRef]
- Abulmeaty, M.M.A.; Ghneim, H.K.; Alkhathaami, A.; Alnumair, K.; Al Zaben, M.; Razak, S.; Al-Sheikh, Y.A. Inflammatory Cytokines, Redox Status, and Cardiovascular Diseases Risk after Weight Loss via Bariatric Surgery and Lifestyle Intervention. Medicina 2023, 59, 751. [Google Scholar] [CrossRef]
- Picu, A.; Petcu, L.; Ştefan, D.S.; Pîrcălăbioru, G.G.; Mitu, M.; Bajko, D.; Lixandru, D.; Guja, C.; Savu, O.; Stoian, A.P.; et al. Evolution of Inflammatory and Oxidative Stress Markers in Romanian Obese Male Patients with Type 2 Diabetes Mellitus after Laparoscopic Sleeve Gastrectomy: One Year Follow-Up. Metabolites 2020, 10, 308. [Google Scholar] [CrossRef]
- Cota-Magaña, A.I.; Vazquez-Moreno, M.; Rocha-Aguado, A.; Ángeles-Mejía, S.; Valladares-Salgado, A.; Díaz-Flores, M.; López-Díazguerrero, N.E.; Cruz, M. Obesity Is Associated with Oxidative Stress Markers and Antioxidant Enzyme Activity in Mexican Children. Antioxidants 2024, 13, 457. [Google Scholar] [CrossRef]
- Kazura, W.; Michalczyk, K.; Skrzep-Poloczek, B.; Chełmecka, E.; Zalejska-Fiolka, J.; Michalski, M.; Kukla, M.; Jochem, J.; Rutkowski, J.; Stygar, D. Liver Oxidative Status, Serum Lipids Levels after Bariatric Surgery and High-Fat, High-Sugar Diet in Animal Model of Induced Obesity. Int. J. Mol. Sci. 2023, 24, 16535. [Google Scholar] [CrossRef]
- Bawahab, M.A.; Assiri, A.S.; Maksoud, W.A.; Patel, A.; Kadoumi, O.; Zaman, G.S.; Alessih, R.M.K.; Haider, S.S. Effects of Weight Reduction After Sleeve Gastrectomy on Metabolic Variables in Saudi Obese Subjects in Aseer Province of Kingdom of Saudi Arabia. Obes. Surg. 2017, 27, 2005–2014. [Google Scholar] [CrossRef]
- Monzo-Beltran, L.; Vazquez-Tarragón, A.; Cerdà, C.; Garcia-Perez, P.; Iradi, A.; Sánchez, C.; Climent, B.; Tormos, C.; Vázquez-Prado, A.; Girbés, J.; et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 2017, 12, 389–402. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal. 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Miler, A.A.; Rotaru, M.; Ciobica, A.; Singeap, A.M.; Trifan, A.; Pînzariu, A.C.; Platon, R.L.; Timofte, D.V. The impact of bariatric surgery on oxidative stress in obese patients. Med.-Surg. J. 2025, 129, 391–399. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Jakubiak, G.K.; Osadnik, K.; Lejawa, M.; Osadnik, T.; Goławski, M.; Lewandowski, P.; Pawlas, N. “Obesity and Insulin Resistance” Is the Component of the Metabolic Syndrome Most Strongly Associated with Oxidative Stress. Antioxidants 2021, 11, 79. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).