A Multidisciplinary Perspective on Breast Phyllodes Tumors: A Literature Review
Abstract
1. Introduction
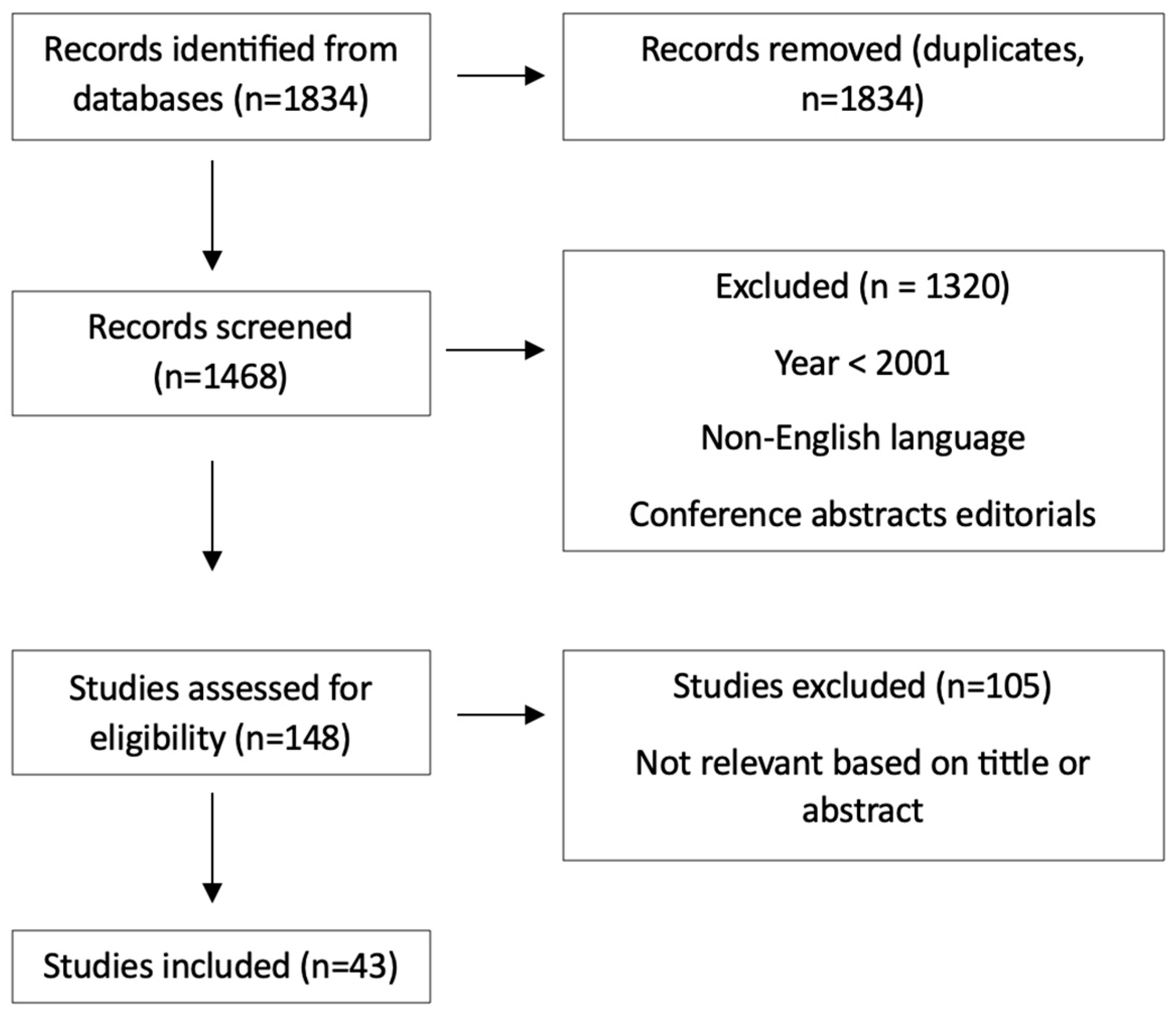
2. Methods
3. Clinical Presentation and Diagnosis
- Katiwada (USA) stated, studying the literature, that PTs occur in women with a median age of 45–49 years; age of appearance of PTs was earlier among people of Asian and Latino-White origin from USA, and more frequent recurrence was observed [8].
- Some of the Japanese authors stated that the patients diagnosed with PTs are younger in Japan, (23–35 years) and older in Western countries (40 years old), but Inoshita reported that the patient’s mean age was 43 years old. Patients with M PTs had a mean age of 43.9 years, with no M or BL tumors in case of teenagers in Japan [11]. Western countries reported teenagers with M PTs [4].
- Chin Chan Lin’s study of 33 Asian patients from Taiwan found the same mean age at diagnosis as other populations (49 years old), and confirmed a higher recurrence rate (27% compared to 21% in the WHO report) [16].
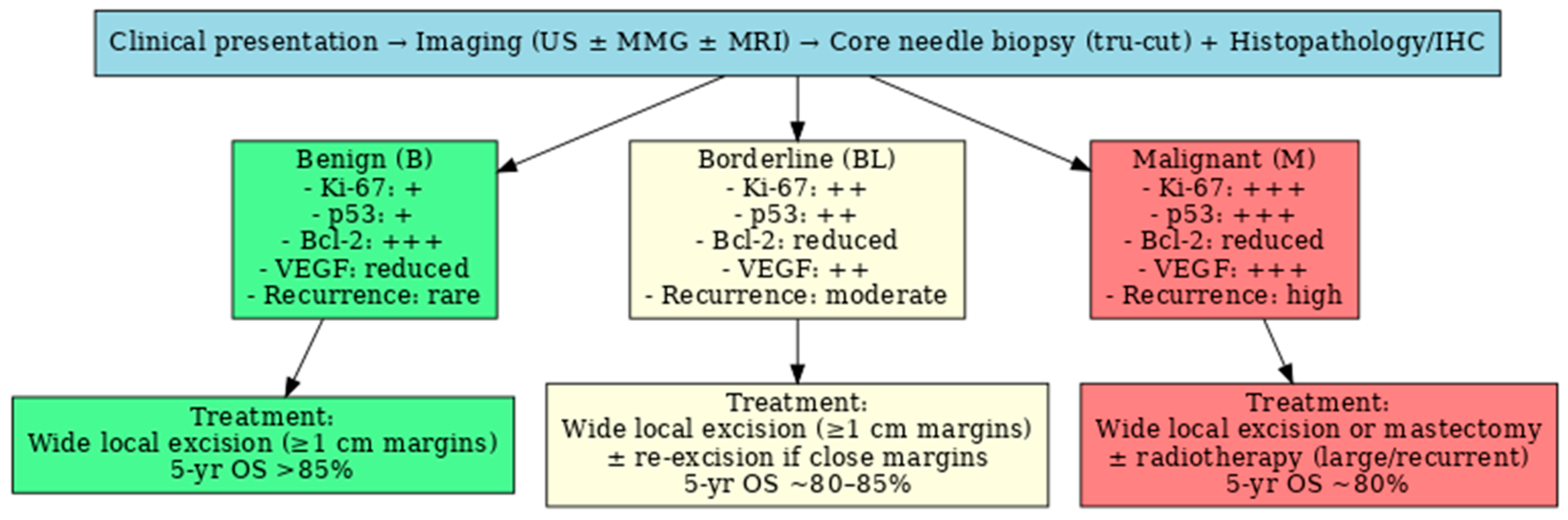
4. Histopathological Features
5. Immunohistochemical Markers (IHC)
6. Treatment Strategies
- reduces the recurrence rate in cases with a tumor size > 2 cm undergoing local excision and those with a tumor size > 10 cm undergoing mastectomy [40];
- a higher 5-year-survival rate in M PTs [41].;
- improved 10-year-local control rate of PT in BL and M groups without affecting overall survival, or without benefiting disease-free survival [34].
7. Prognostic Factors and Recurrence Risk
8. Future Perspectives and Research Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADC | Apparent diffusion coefficient |
| B | Benign |
| BC | Breast carcinoma |
| CT | Computed tomography |
| BL | Borderline |
| DWI | Diffusion-weighted imaging |
| ER | Estrogen receptors |
| FA | Fibroadenoma |
| HPF | High-power field |
| IHC | Immunohistochemical Markers |
| M | Malignant |
| MRI | Magnetic resonance imaging |
| MD | Mean dimensions |
| OS | Overall survival |
| PR | Progesterone receptor |
| R | Range |
| PT | Phyllodes tumor |
| SMA | Smooth muscle actin |
| US | Ultrasound |
| VEGF | Vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Tan, P.H. Fibroepithelial lesions revisited: Implications for diagnosis and management. Mod. Pathol. 2021, 34, 15–37. [Google Scholar] [CrossRef]
- Lissidini, G.; Mulè, A.; Santoro, A.; Papa, G.; Nicosia, L.; Cassano, E.; Ashoor, A.A.; Veronesi, P.; Pantanowitz, L.; Hornick, J.L.; et al. Malignant phyllodes tumor of the breast: A systematic review. Pathologica 2022, 114, 111–120. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhu, I.; Patel, A.; Krings, G.; Chen, Y.Y.; Yuen, F.; Mukhtar, R.A.; Melisko, M.; Singer, L.; Park, C.C.; et al. Management of Concurrent M Phyllodes Tumor and Invasive Breast Carcinoma. Adv. Radiat. Oncol. 2024, 9, 101448. [Google Scholar] [CrossRef]
- Di Liso, E.; Bottosso, M.; Lo Mele, M.; Tsvetkova, V.; Dieci, M.V.; Miglietta, F.; Falci, C.; Faggioni, G.; Tasca, G.; Giorgi, C.A.; et al. Prognostic factors in phyllodes tumours of the breast: Retrospective study on 166 consecutive cases. ESMO Open 2020, 5, e000843. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Jiang, N.; Zhang, C.; Luo, X.; Zhong, P.; Fang, J. Value of conventional magnetic resonance imaging texture analysis in the differential diagnosis of benign and borderline/malignant phyllodes tumors of the breast. Cancer Imaging 2021, 21, 29. [Google Scholar] [CrossRef]
- Lerwill, M.F.; Lee, A.H.S.; Tan, P.H. Fibroepithelial tumours of the breast—A review. Virchows Arch. 2022, 480, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.R.; Wang, C.C.; Sun, X.J.; Yang, Z.Z.; Chen, X.X.; Shao, Z.M.; Yu, X.L.; Guo, X.M. Prognostic factors in breast phyllodes tumors: A nomogram based on a retrospective cohort study of 404 patients. Cancer Med. 2018, 7, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Khatiwada, A.; Bastakoti, A.; Kc, S.; Sharma, U.; Rao, S.M. Transformation of recurrent benign phyllodes tumor: A case report and comprehensive review of literature. Ann. Med. Surg. 2024, 86, 7469–7473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamdy, O.; Saleh, G.A.; Raafat, S.; Shebl, A.M.; Denewer, A. Male Breast Huge Malignant Phyllodes. Chirurgia 2019, 114, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Yom, C.K. Malignant Phyllodes of Breast. Adv. Exp. Med. Biol. 2021, 1187, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, A.; Matsuura, K.; Hasebe, T.; Saeki, T. Phyllodes tumour arising in the ectopic axillary breast tissue, mimicking axillary lymphadenopathy. BMJ Case Rep. 2021, 14, e243341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maciulaitis, T.; Rimdeikaite, M.; Gudaviciene, D.; Jakutis, N. Giant juvenile phyllodes tumour: A case report. Front. Surg. 2025, 12, 1617716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, C.Y.; Huang, T.W.; Tam, K.W. Management of phyllodes tumor: A systematic review and meta-analysis of real-world evidence. Int. J. Surg. 2022, 107, 106969. [Google Scholar] [CrossRef] [PubMed]
- Jagdewsing, D.R.; Murtaza, G.; Jagdewsing, S.A.; Jagdewsing, S.A.; Fahmy, N.S.C.; Silva, F.A.; Koendjbiharie, T.; Djojomoenawi, S.; Kwakye, O.V.; Mahmud, N.M. Evaluation of the Clinicopathological Features Associated With Malignancy of Phyllodes Tumor of the Breast. Int. J. Clin. Oncol. 2024, 16, e76221. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Y.; Zhu, L.; Cartwright, P.; Song, E.; Jacobs, L.; Chen, K. Local Recurrence of Benign, Borderline, and Malignant Phyllodes Tumors of the Breast: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2019, 26, 1263–1275. [Google Scholar] [CrossRef]
- Lin, C.C.; Chang, H.W.; Lin, C.Y.; Chiu, C.F.; Yeh, S.P. The clinical features and prognosis of phyllodes tumors: A single institution experience in Taiwan. Int. J. Clin. Oncol. 2013, 18, 614–620. [Google Scholar] [CrossRef]
- Rajgopal, V.; Sammader, S.; Ranjan, P.; Mohammed, F. Phyllodes tumour presenting in a rare location: A case report and literature review. Int. Surg. J. 2022, 9, 1500. [Google Scholar] [CrossRef]
- Mustață, L.; Gică, N.; Botezatu, R.; Chirculescu, R.; Gică, C.; Peltecu, G.; Panaitescu, A.M. Malignant Phyllodes Tumor of the Breast and Pregnancy: A Rare Case Report and Literature Review. Medicina 2022, 58, 36. [Google Scholar] [CrossRef]
- Li, X.; Chai, W.; Sun, K.; Fu, C.; Yan, F. The value of whole-tumor histogram and texture analysis based on apparent diffusion coefficient (ADC) maps for the discrimination of breast fibroepithelial lesions: Corresponds to clinical management decisions. Jpn. J. Radiol. 2022, 40, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Torres, L.A.F.; Celso, D.S.G.; Defante, M.L.R.; Alzogaray, V.; Bearse, M.; de Melo Lopes, A.C.F.M. Ki-67 as a marker for differentiating BL and benign phyllodes tumors of the breast: A meta-analysis and systematic review. Ann. Diagn. Pathol. 2024, 18, 152429. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Tse, G.M. Core needle biopsy diagnosis of fibroepithelial lesions of the breast: A diagnostic challenge. Pathology 2020, 52, 627–634. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Pfeifer, J.D.; Caprioli, R.M.; Judd, A.M.; Patterson, N.H.; Reyzer, M.L.; Norris, J.L.; Maluf, H.M. Histopathologic, immunophenotypic, and proteomics characteristics of low-grade phyllodes tumor and fibroadenoma: More similarities than differences. Breast Cancer 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Mohammed, Y.; Basiony, M.; Hanbazazh, M.; Samman, A.; Abdelaleem, M.F.; Nasr, M.; Abozeid, H.; Mohamed, H.I.; Faisal, M.; et al. Clinico-pathological features and immunohistochemical comparison of p16, p53, and Ki-67 expression in muscle-invasive and non-muscle-invasive conventional urothelial bladder carcinoma. Clin. Pract. 2023, 13, 806–819. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.; Mihai, R.; Abbas, A.; Bennett, R.; Campora, M.; Morena, P.; Toss, M.; Ellis, I. Diagnostic concordance of phyllodes tumour of the breast. Histopathology 2021, 79, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Theparee, T.; Bean, G.R.; Rutland, C.D.; Schwartz, C.J.; Vohra, P.; Allard, G.; Wang, A.; Hosfield, E.M.; Peng, Y.; et al. Targeted DNA Sequencing in Diagnosis of M Phyllodes Tumors With Emphasis on Tumors With Keratin and p63 Expression. Mod. Pathol. 2024, 37, 100593. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.-Y.; Kim, D.H.; Jung, W.H.; Koo, J.S. Analysis of phyllodes tumor recurrence according to the histologic grade. Breast Cancer Res. Treat. 2013, 141, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Cervoni, G.E.; Quintana, L.; Erlinger, A.L.; Neo, D.T.; Recht, A.; Schnitt, S.J.; Hacker, M.R.; Sharma, R. Local recurrence after breast- conserving therapy for phyllodes tumors: A 15-year retrospective review. Breast J. 2019, 26, 988–990. [Google Scholar] [CrossRef]
- Lim, R.S.; Cordeiro, E.; Lau, J.; Lim, A.; Roberts, A.; Seely, J. Phyllodes tumors-the predictors and detection of recurrence. Can. Assoc. Radiol. J. 2021, 72, 251–257. [Google Scholar] [CrossRef]
- Tukenmez, M.; Mollavelioglu, B.; Onder, S.; Emiroglu, S.; Velidedeoglu, M.; Ergun, S.; Cabioglu, N.; Muslumanoglu, M. Surgery for phyllodes tumour of the breast. What should be surgical margins? ANZ J. Surg. 2023, 93, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Lalchandani, A.; Dausage, C. Recurrent phyllodes tumour of breast infiltrating the latissimus dorsi reconstruction flap. BMJ Case Rep. 2020, 13, e238306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gupta, K.; Puri, G.; Kataria, K.; Jayaram, J. Complex chest wall reconstruction after excision of malignant phyllodes tumour. BMJ Case Rep. 2022, 15, e247067. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chao, X.; Chen, K.; Zeng, J.; Bi, Z.; Guo, M.; Chen, Y.; Yao, Y.; Wu, W.; Shi, L.; Nie, Y. Adjuvant radiotherapy and chemotherapy for patients with breast phyllodes tumors: A systematic review and meta-analysis. BMC Cancer 2019, 19, 372. [Google Scholar] [CrossRef]
- Roberts, A.C.; Lunt, L.G.; Coogan, A.C.; Madrigrano, A. The Role of Radiation Therapy in Locally Advanced Breast Cancer in a Patient With Li-Fraumeni Syndrome. Am. Surg. 2023, 89, 4958–4960. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.A.; Rabah, R.S.; Sheikh, S.S. Malignant phyllodes tumor of the breast with metastasis to the pancreas: A case report and review of literature. Case Rep. Oncol. Med. 2018, 2018, 6491675. [Google Scholar] [CrossRef]
- Wakankar, R.; Dharmashaktu, Y.; Venugopal, A.; Kumar, R. Malignant Phyllodes Tumor With Sphenoid Bone Metastasis Detected on 99m Tc-MDP SPECT/CT and 18 F-FDG PET/CT. Clin. Nucl. Med. 2025, 50, e223–e224. [Google Scholar] [CrossRef] [PubMed]
- Ostapenko, E.; Burneckis, A.; Ostapenko, A.; Skaisgirytė, A.; Ostapenko, V. M phyllodes tumor of the breast with metastases to the lungs: A case report and literature review. Radiol. Case Rep. 2022, 17, 4006–4012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zieba, D.; Pories, S.; Thota, H.B.; Suster, D.I. Malignant Phyllodes Tumor of the Breast With Multiple Cutaneous Metastasis Resembling Pleomorphic Rhabdomyosarcoma. Am. J. Dermatopathol. 2025, 47, 217–219. [Google Scholar] [CrossRef]
- Charoenyothakun, A.; Shotelersuk, K.; Nantavithya, C.; Saksornchai, K. The impact of adjuvant radiotherapy on borderline and malignant phyllodes tumors of the breast. Breast Cancer 2025, 32, 1006–1012. [Google Scholar] [CrossRef]
- Pezner, R.D.; Schultheiss, T.E.; Paz, I.B. Malignant Phyllodes Tumor of the Breast: Local Control Rates With Surgery Alone. Int. J. Radiat. Oncol. 2008, 71, 710–713. [Google Scholar] [CrossRef]
- Pandey, M.; Mathew, A.; K, J.; Abraham, E.K.; Mathew, B.S.; Rajan, B.; Nair, K.M. Malignant Phyllodes Tumor. Breast J. 2001, 7, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Valenza, C.; Trapani, D.; Porta, F.M.; Olmeda, E.; Gaeta, A.; Boscolo Bielo, L.; Conversano, F.; De Pas, T.M.; Castellano, G.; Santoro, C.; et al. The pathologic and genomic evolution of primary malignant phyllodes tumors of the breast: Retrospective cohort study and case-control genomic analysis. Oncologist 2025, 30, oyaf012. [Google Scholar] [CrossRef] [PubMed]
- Slachmuylders, E.; Laenen, A.; Vernemmen, A.; Keupers, M.; Nevelsteen, I.; Han, S.N.; Neven, P.; Van Ongeval, C.; Wildiers, H.; Smeets, A.; et al. Expression patterns of H3K27me3 for differentiation of breast fibroadenomas and phyllodes tumors. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2025, 133, e13485. [Google Scholar] [CrossRef] [PubMed]
| Author | Yamada | Lin | Zhou | Di Liso | Jagdewsing | ||
|---|---|---|---|---|---|---|---|
| MD (R) (cm) | B | 4.9 | 6 (1.5–28.0) | <5 129 (77.7) | ≥5 37 (22.3) | 25.5 ± 18.5 (4–130) | 5.58 ± 2.29 |
| BL | 7.5 | 115 (65.7) | 60 (34.3) | 34.7 ± 24.4 (10–110) | 10.58 ± 6.79 | ||
| M | 7.5 | 21 (43.8) | 27 (56.3) | 57.4 ± 60.2 (18–250) | 14.90 ± 6.44 | ||
| Author | Yamada | Lin | Zhou | Di Liso | Jagdewsing | |
|---|---|---|---|---|---|---|
| Surgical treatment/ histology | Conservative surgery | 110/110B | 26/8B + 13BL + 5M | 428/B + BL | 77/54B + 23BL | 77/54B + 23BL |
| Mastectomy | 13/8BL + 5M | 7/7M | 26/26M | 24/13BL + 11M | 24/13BL + 11M | |
| Recurrence | B | 6 | 1 | 6 | 6 | 6 |
| BL | 0 | 3 | 26 | 2 | 2 | |
| M | 2 | 5 | 22 | 3 | 3 | |
| Overall outcome (5-year overall survival) | 47 month survival | 81.0% | 88.4% | 83.6% | Not evaluated | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naum, A.-G.; Ursu, A.-M.; Moisii, P.; Lupascu-Ursulescu, C.-V.; Gheorghe, L.; Jari, I. A Multidisciplinary Perspective on Breast Phyllodes Tumors: A Literature Review. Medicina 2025, 61, 1883. https://doi.org/10.3390/medicina61101883
Naum A-G, Ursu A-M, Moisii P, Lupascu-Ursulescu C-V, Gheorghe L, Jari I. A Multidisciplinary Perspective on Breast Phyllodes Tumors: A Literature Review. Medicina. 2025; 61(10):1883. https://doi.org/10.3390/medicina61101883
Chicago/Turabian StyleNaum, Alexandru-Gratian, Andra-Mara Ursu, Paloma Moisii, Corina-Veronica Lupascu-Ursulescu, Liliana Gheorghe, and Irina Jari. 2025. "A Multidisciplinary Perspective on Breast Phyllodes Tumors: A Literature Review" Medicina 61, no. 10: 1883. https://doi.org/10.3390/medicina61101883
APA StyleNaum, A.-G., Ursu, A.-M., Moisii, P., Lupascu-Ursulescu, C.-V., Gheorghe, L., & Jari, I. (2025). A Multidisciplinary Perspective on Breast Phyllodes Tumors: A Literature Review. Medicina, 61(10), 1883. https://doi.org/10.3390/medicina61101883





