Impact of Delivery Mode on Neonatal Outcomes in Extremely Preterm Infants Born at 22 + 0 to 25 + 6 Weeks of Gestation
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bradley, E.; Blencowe, H.; Moller, A.-B.; Okwaraji, Y.B.; Sadler, F.; Gruending, A.; Moran, A.C.; Requejo, J.; Ohuma, E.O.; Lawn, J.E. Born too soon: Global epidemiology of preterm birth and drivers for change. Reprod. Health 2025, 22, 105. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Walsh, M.C.; Carlo, W.A.; Shankaran, S.; Laptook, A.R.; Sánchez, P.J.; Van Meurs, K.P.; Wyckoff, M.; et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993-2012. JAMA 2015, 314, 1039–1051. [Google Scholar] [CrossRef]
- Koc, E.; Unal, S. Viability of extremely premature neonates: Clinical approaches and outcomes. J. Perinat. Med. 2024, 53, 706–715. [Google Scholar] [CrossRef]
- Werner, E.F.; Savitz, D.A.; Janevic, T.M.; Ehsanipoor, R.M.; Thung, S.F.; Funai, E.F.; Lipkind, H.S. Mode of Delivery and Neonatal Outcomes in Preterm, Small-for-Gestational-Age Newborns. Obstet. Gynecol. 2012, 120, 560–564. [Google Scholar] [CrossRef]
- Riskin, A.; Riskin-Mashiah, S.; Lusky, A.; Reichman, B. In collaboration with the Israel Neonatal Network The relationship between delivery mode and mortality in very low birthweight singleton vertex—Presenting infants. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Unger, V.; Gasparics, Á.; Nagy, Z.; Hernádfői, M.; Nagy, R.; Walter, A.; Farkas, N.; Szabó, M.; Hegyi, P.; Garami, M.; et al. Cesarean delivery is associated with lower neonatal mortality among breech pregnancies: A systematic review and meta-analysis of preterm deliveries ≤32 weeks of gestation. Am. J. Obstet. Gynecol. 2024, 231, 589–598.e21. [Google Scholar] [CrossRef]
- Weyrich, J.; Setter, A.; Müller, A.; Schmidt, G.; Brambs, C.E.; Ortiz, J.U.; Lobmaier, S.M. Longitudinal progression of fetal short-term variation and average acceleration and deceleration capacity after antenatal maternal betamethasone application. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 212, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Son, J.; Park, H.-K. Surgical necrotizing enterocolitis risk factors in extremely preterm infants: A Korean nationwide cohort study. Pediatr. Res. 2024, 97, 1575–1581. [Google Scholar] [CrossRef]
- Venkatesh, K.K.; Nadel, H.; Blewett, D.; Freeman, M.P.; Kaimal, A.J.; Riley, L.E. Implementation of universal screening for depression during pregnancy: Feasibility and impact on obstetric care. Am. J. Obstet. Gynecol. 2016, 215, 517.e1–517.e8. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernández, N.; Solís, G.; Hernandez-Barranco, A.; Margolles, A.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef]
- Reznik, S.E.; Akinyemi, A.J.; Harary, D.; Latuga, M.S.; Fuloria, M.; Charron, M.J. The effect of cesarean delivery on the neonatal gut microbiome in an under-resourced population in the Bronx, NY, USA. BMC Pediatr. 2024, 24, 1–12. [Google Scholar] [CrossRef]
- Green, V.L. Foreword. Clin. Obstet. Gynecol. 2016, 59, 320. [Google Scholar] [CrossRef]
- Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF). S3-Leitlinie, Frühgeborene an der Grenze der Lebensfähigkeit (Registernummer 024/019). DGGG; 2023. Available online: https://register.awmf.org/de/leitlinien/detail/024-019 (accessed on 28 August 2025).
- Higgins, B.V.; Baer, R.J.; Steurer, M.A.; Karvonen, K.L.; Oltman, S.P.; Jelliffe-Pawlowski, L.L.; Rogers, E.E. Resuscitation, survival and morbidity of extremely preterm infants in California 2011–2019. J. Perinatol. 2023, 44, 209–216. [Google Scholar] [CrossRef]
- Jayaprakash, T.P.; Wagner, E.C.; van Schalkwyk, J.; Albert, A.Y.K.; Hill, J.E.; Money, D.M. PPROM Study Group High Diversity and Variability in the Vaginal Microbiome in Women following Preterm Premature Rupture of Membranes (PPROM): A Prospective Cohort Study. PLoS ONE 2016, 11, e0166794. [Google Scholar] [CrossRef]
- dos Anjos Borges, L.G.; Pastuschek, J.; Heimann, Y.; Dawczynski, K.; PEONS Study Group; Bergner, M.; Haase, R.; Stubert, J.; Olbertz, D.; Plumeier, I.; et al. Vaginal and neonatal microbiota in pregnant women with preterm premature rupture of membranes and consecutive early onset neonatal sepsis. BMC Med. 2023, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, J.M.; Serrano, M.G.; Brooks, J.P.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.G.; Marchesi, J.R.; Lee, Y.S.; Smith, A.; Lehne, B.; Kindinger, L.M.; Terzidou, V.; Holmes, E.; Nicholson, J.K.; Bennett, P.R.; et al. Vaginal dysbiosis increases risk of preterm fetal membrane rupture, neonatal sepsis and is exacerbated by erythromycin. BMC Med. 2018, 16, 1–15. [Google Scholar] [CrossRef]
- Saucedo, A.M.; Calvert, C.; Chiem, A.; Groves, A.; Ghartey, K.; Cahill, A.G.; Harper, L.M. Periviable Premature Rupture of Membranes—Maternal and Neonatal Risks: A Systematic Review and Meta-analysis. Am. J. Perinatol. 2024, 41, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Papile, L.-A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Leistner, R.; Piening, B.; Gastmeier, P.; Geffers, C.; Schwab, F. Nosocomial infections in very low birthweight infants in Germany: Current data from the National Surveillance System NEO-KISS. Klin. Padiatr. 2013, 225, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Travers, C.P.; Carlo, W.A.; McDonald, S.A.; Das, A.; Bell, E.F.; Ambalavanan, N.; Jobe, A.H.; Goldberg, R.N.; D’ANgio, C.T.; Stoll, B.J.; et al. Mortality and pulmonary outcomes of extremely preterm infants exposed to antenatal corticosteroids. Am. J. Obstet. Gynecol. 2018, 218, 130.e1–130.e13. [Google Scholar] [CrossRef] [PubMed]
- Rysavy, M.A.; Li, L.; Bell, E.F.; Das, A.; Hintz, S.R.; Stoll, B.J.; Vohr, B.R.; Carlo, W.A.; Shankaran, S.; Walsh, M.C.; et al. Between-Hospital Variation in Treatment and Outcomes in Extremely Preterm Infants. N. Engl. J. Med. 2015, 372, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, H.S.; Jung, E.; Kim, E.S.; Shim, G.H.; Lee, H.J.; Lee, J.A.; Choi, C.W.; Kim, E.-K.; Kim, B.I.; et al. Risk Factors for Periventricular-Intraventricular Hemorrhage in Premature Infants. J. Korean Med. Sci. 2010, 25, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Santhakumaran, S.; Statnikov, Y.; Gray, D.; Battersby, C.; Ashby, D.; Modi, N. Medicines for Neonates Investigator Group Survival of very preterm infants admitted to neonatal care in England 2008–2014: Time trends and regional variation. Arch. Dis. Child.-Fetal Neonatal Ed. 2017, 103, F208–F215. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2020, 2021, CD004454. [Google Scholar] [CrossRef]
| Variable | Vaginal Birth n = 46 | Cesarean Section n = 46 | p-Value |
|---|---|---|---|
| Maternal age (years) | 30 (15–41) P25–P75: 25–33 | 31 (15–41) P25–P75: 26–35 | 0.409 |
| Primiparae | 7 (15.2%) | 7 (15.2%) | - |
| Multiparae | 39 (84.8%) | 39 (84.8%) | 0.500 |
| Multiparae: previous birth Term birth | 7 (15.2%) | 12 (24.0%) | 0.396 |
| Multiparae: previous birth Preterm birth | 32 (84.8%) | 34 (76.0%) | 0.021 |
| PPROM | 17.4% (8) | 21.7% (10) | 0.396 |
| No PPROM | 82.6% (38) | 78.3% (36) | |
| Vaginal microbial colonization | 35 (76.0%) | 24 (52.2%) | 0.021 |
| No Vaginal microbial colonization | 11 (24.0%) | 22 (47.8) |
| Parameter | Vaginal Delivery (n = 46) | Cesarean Section (n = 46) | p-Value |
|---|---|---|---|
| Gestational age at birth (days) | 165 (154–182; 161–165–171) | 166 (150–170; 164–166–168) | 0.971 |
| Birth weight (g) | 595 (415–830; 555–595–663.5) | 620 (335–1001; 588.8–620–706.3) | 0.622 |
| Antenatal corticosteroids (ANCS) | 0.021 | ||
| None | 9 (19.5%) | 4 (8.7%) | |
| One dose | 19 (41.3%) | 10 (21.7%) | |
| Two doses | 18 (39.2%) | 32 (69.6%) | |
| Sex (male/female) | 26 (56.5%)/20 (43.5%) | 25 (54.3%)/21 (45.7%) | 0.405 |
| Variable | Vaginal Birth (n = 46) | Cesarean Section (n = 46) | p-Value |
|---|---|---|---|
| APGAR 1 min Median (Range) P25–P50–P75 | 3 (0–8) 2–3–5 | 3.5 (1–8) 2–3.5–5 | 0.286 |
| APGAR 5 min Median (Range) P25–P50–P75 | 6 (0–9) 4–6–7 | 6 (2–9) 5–6–8 | 0.264 |
| APGAR 10 min Median (Range) P25–P50–P75 | 7.5 (0–10) 6–7.5–8 | 7(4–10) 7–7–9 | 0.281 |
| Umbilical arterial cord pH | 7.35 (6.95–7.50) 7.285–7.30–7.39 | 7.32 (7.12–7.44) 7.283–7.32–7.38 | 0.229 |
| Survival until NICU Discharge | Yes 39 (84.8%) No 7 (15.2%) | Yes 43 (93.5%) No 3 (6.5%) | 0.140 |
| NEC | Yes 3 (6.5%) No 43 (93.5%) | Yes 4 (8.7%) No 42 (91.3%) | 0.513 |
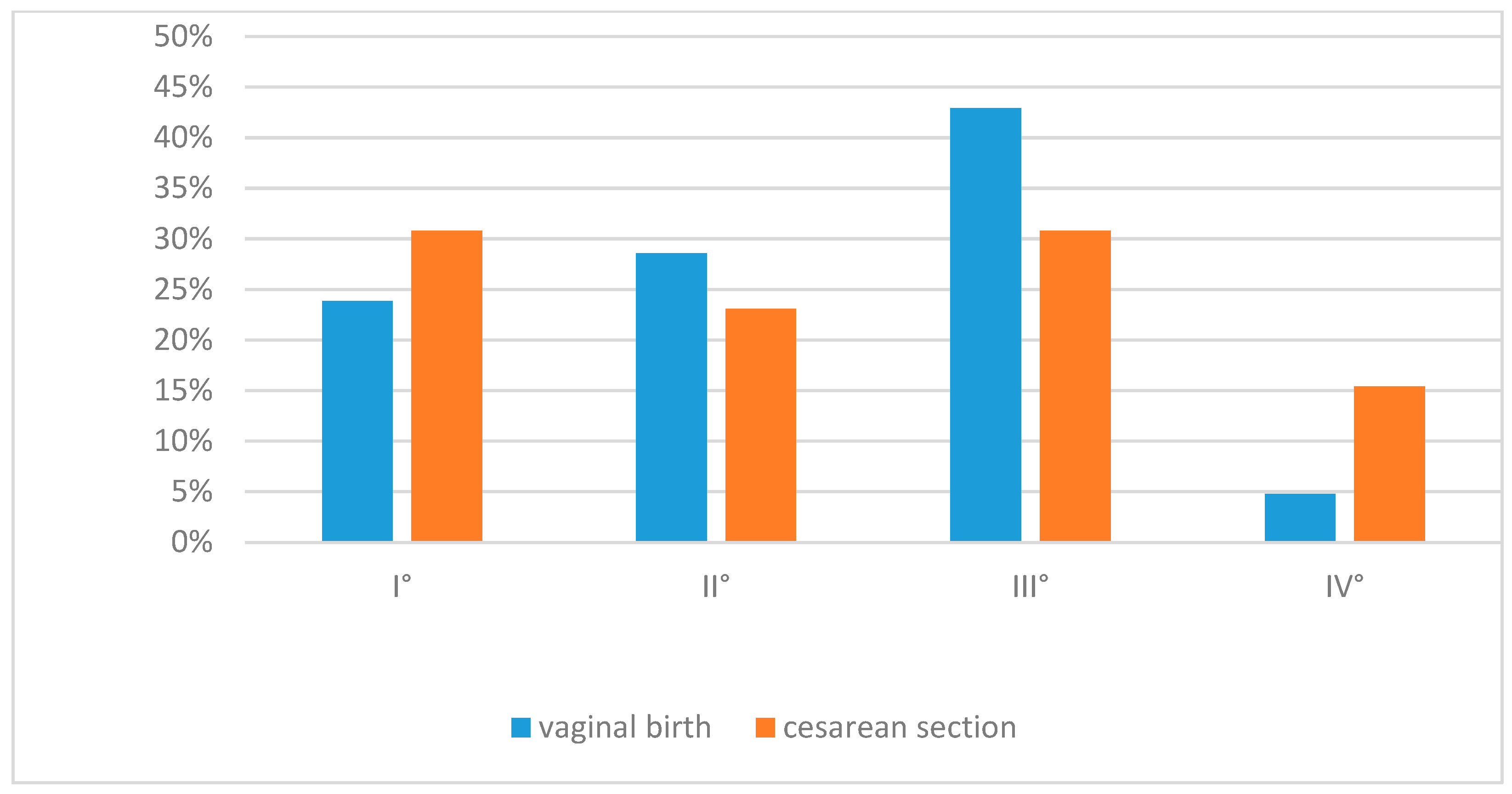
| IVH any | Yes 22 (47.8%) No 24 (52.2%) | Yes 14 (30.4%) No 32 (69.6%) | 0.080 |
| Variable | Regression Coefficient (B) | Standard Error | p = Value |
|---|---|---|---|
| Mode of birth | –0.657 | 0.558 | p = 0.239 |
| Arterial pH | –0.236 | 0.275 | p = 0.391 |
| APGAR 1 min | –0.255 | 0.192 | p = 0.185 |
| APGAR 5 min | –0.100 | 0.256 | p = 0.697 |
| APGAR 10 min | 0.047 | 0.252 | p = 0.854 |
| Pathological vaginal colonization | 0.135 | 0.605 | p = 0.824 |
| PPROM | 1.648 | 0.938 | p = 0.079 |
| Birthweight | –0.002 | 0.002 | p = 0.486 |
| Gestational age | 0.021 | 0.057 | p = 0.708 |
| Male sex | –1.318 | 0.643 | p = 0.041 |
| ANCS (incomplete) | –0.440 | 0.431 | p = 0.308 |
| Constant term | 0.062 | 9.030 | p = 0.995 |
| Parameter | Vaginal Delivery (n = 46) | Cesarean Section (n = 46) | p-Value | Interpretation |
|---|---|---|---|---|
| Maternal age (years) | 30 (15–41) | 31 (15–41) | 0.409 | Comparable maternal age |
| Multiparity with prior preterm birth, n (%) | 32 (84.8) | 34 (76.0) | 0.021 | Higher rate after vaginal delivery |
| Vaginal microbial colonization, n (%) | 35 (76.1) | 24 (52.2) | 0.021 | Significantly more frequent in vaginal group |
| Gestational age at birth (days) | 165 (154–182) | 166 (150–170) | 0.971 | No difference |
| Birth weight (g) | 595 (415–830) | 620 (335–1001) | 0.622 | No difference |
| Antenatal corticosteroids (two doses), n (%) | 18 (39.2) | 32 (69.6) | 0.021 | More frequent in the cesarean group |
| Survival to NICU discharge, n (%) | 39 (84.8) | 43 (93.5) | 0.140 | Higher survival trend in the cesarean group, not significant |
| NEC, n (%) | 3 (6.5) | 4 (8.7) | 0.513 | Comparable incidence |
| IVH, n (%) | 22 (47.8) | 14 (30.4) | 0.080 | Higher after vaginal delivery, not significant |
| Parameter | Vaginal Birth | Cesarean Section | Power |
|---|---|---|---|
| Survival until discharge | 84.8% | 93.5% | 26.6% |
| IVH | 47.8% | 30.4% | 40.0% |
| NEC | 6.5% | 8.7% | 5.9% |
| Parameter | Spearman’s Rho | p-Wert | Interpretation |
|---|---|---|---|
| Neonatal infection | −0.344 | 0.001 | highly significant correlation |
| Survival until discharge | 0.013 | 0.902 | no association |
| NEC | −0.213 | 0.052 | non-significant trend/trend without statistical significance on-significant trend/trend without statistical significance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markfeld-Erol, F.; Kuntz, M.; Laufs, V.; Tippmann, S.; Juhasz-Böss, I.; Hasenburg, A.; Steetskamp, J. Impact of Delivery Mode on Neonatal Outcomes in Extremely Preterm Infants Born at 22 + 0 to 25 + 6 Weeks of Gestation. Medicina 2025, 61, 1880. https://doi.org/10.3390/medicina61101880
Markfeld-Erol F, Kuntz M, Laufs V, Tippmann S, Juhasz-Böss I, Hasenburg A, Steetskamp J. Impact of Delivery Mode on Neonatal Outcomes in Extremely Preterm Infants Born at 22 + 0 to 25 + 6 Weeks of Gestation. Medicina. 2025; 61(10):1880. https://doi.org/10.3390/medicina61101880
Chicago/Turabian StyleMarkfeld-Erol, Filiz, Martin Kuntz, Valeria Laufs, Susanne Tippmann, Ingolf Juhasz-Böss, Annette Hasenburg, and Joscha Steetskamp. 2025. "Impact of Delivery Mode on Neonatal Outcomes in Extremely Preterm Infants Born at 22 + 0 to 25 + 6 Weeks of Gestation" Medicina 61, no. 10: 1880. https://doi.org/10.3390/medicina61101880
APA StyleMarkfeld-Erol, F., Kuntz, M., Laufs, V., Tippmann, S., Juhasz-Böss, I., Hasenburg, A., & Steetskamp, J. (2025). Impact of Delivery Mode on Neonatal Outcomes in Extremely Preterm Infants Born at 22 + 0 to 25 + 6 Weeks of Gestation. Medicina, 61(10), 1880. https://doi.org/10.3390/medicina61101880






