Blood Flow Restriction Training in Knee Arthroplasty: A Systematic Review of Current Evidence on Postoperative Muscle Strength and Function
Abstract
1. Introduction
2. Methods
2.1. Protocol Registration and Reporting Guidelines
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Study Selection Process
2.5. Data Extraction Protocol
2.6. Risk of Bias Assessment
2.7. Data Synthesis and Analysis
3. Results
3.1. Study Selection and Characteristics
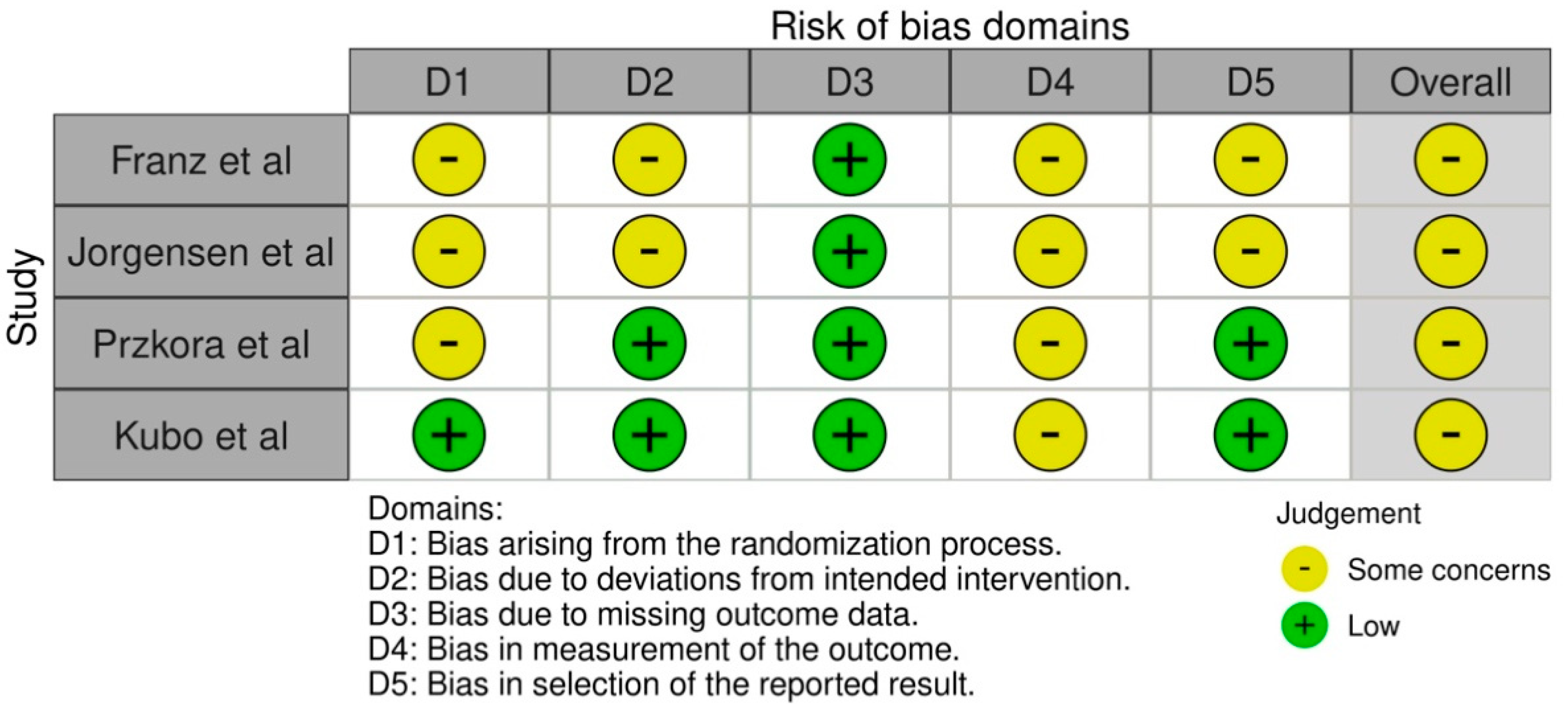
3.2. Risk of Bias Assessment
3.3. Intervention Characteristics and Protocols
3.4. Muscle Strength Outcomes
3.4.1. Significant Improvements in Muscle Strength
3.4.2. Non-Significant Strength Outcomes
3.5. Functional Performance Outcomes
3.5.1. Significant Functional Improvements
3.5.2. Mixed Functional Outcomes
3.6. Safety Profile and Adverse Events
4. Discussion
4.1. Individual Result Interpretation
4.1.1. Muscle Strength Improvements
4.1.2. Functional Performance Outcomes
4.1.3. Safety Profile and Tolerability
4.2. Non-Significant Results
4.3. Practical Implications and Applications
4.3.1. Clinical Practice Implications
4.3.2. Health System Implications
4.3.3. Patient Care Implications
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erivan, R.; Tardieu, A.; Villatte, G.; Ollivier, M.; Jacquet, C.; Descamps, S.; Boisgard, S. Knee surgery trends and projections in France from 2008 to 2070. Orthop. Traumatol. Surg. Res. OTSR 2020, 106, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Le Stum, M.; Le Goff-Pronost, M.; Stindel, E.; Dardenne, G. Incidence rate of total knee arthroplasties in eleven European countries: Do they reach a plateau? PLoS ONE 2025, 20, e0312701. [Google Scholar] [CrossRef] [PubMed]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040–2060. JB JS Open Access 2023, 8, e22.00112. [Google Scholar] [CrossRef]
- Klug, A.; Gramlich, Y.; Rudert, M.; Drees, P.; Hoffmann, R.; Weißenberger, M.; Kutzner, K.P. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2021, 29, 3287–3298. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development (OECD). Hip and Knee Replacement: Health at a Glance 2023. 2023. Available online: https://www.oecd.org/en/publications/health-at-a-glance-2023_7a7afb35-en/full-report/hip-and-knee-replacement_687dec46.html (accessed on 27 September 2025).
- Al-Dadah, O.; Hing, C. Advancing indications for total knee arthroplasty. Knee 2024, 50, A1–A2. [Google Scholar] [CrossRef]
- Canovas, F.; Dagneaux, L. Quality of life after total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2018, 104, S41–S46. [Google Scholar] [CrossRef]
- Gunaratne, R.; Pratt, D.N.; Banda, J.; Fick, D.P.; Khan, R.J.K.; Robertson, B.W. Patient Dissatisfaction Following Total Knee Arthroplasty: A Systematic Review of the Literature. J. Arthroplast. 2017, 32, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Shoman, H.; Olavarria, F.; Matsumoto, T.; Kuroda, R.; Khanduja, V. Why are patients dissatisfied following a total knee replacement? A systematic review. Int. Orthop. 2020, 44, 1971–2007. [Google Scholar] [CrossRef]
- DeFrance, M.J.; Scuderi, G.R. Are 20% of Patients Actually Dissatisfied Following Total Knee Arthroplasty? A Systematic Review of the Literature. J. Arthroplast. 2023, 38, 594–599. [Google Scholar] [CrossRef]
- Le Stum, M.; Gicquel, T.; Dardenne, G.; Le Goff-Pronost, M.; Stindel, E.; Clavé, A. Prothèses Totale de Genou en France: Une croissance portée par les Hommes entre 2009 et 2019. Projections à 2050. Rev. Chir. Orthopédique Traumatol. 2023, 109, 733–739. [Google Scholar] [CrossRef]
- Bily, W.; Sarabon, N.; Löfler, S.; Franz, C.; Wakolbinger, R.; Kern, H. Relationship Between Strength Parameters and Functional Performance Tests in Patients With Severe Knee Osteoarthritis. PMR 2019, 11, 834–842. [Google Scholar] [CrossRef]
- Meier, W.; Mizner, R.; Marcus, R.; Dibble, L.; Peters, C.; Lastayo, P.C. Total Knee Arthroplasty: Muscle Impairments, Functional Limitations, and Recommended Rehabilitation Approaches. J. Orthop. Sports Phys. Ther. 2008, 38, 246–256. [Google Scholar] [CrossRef]
- Silva, M.; Shepherd, E.F.; Jackson, W.O.; Pratt, J.A.; McClung, C.D.; Schmalzried, T.P. Knee strength after total knee arthroplasty. J. Arthroplast. 2003, 18, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Poenaru, D.; Sandulescu, M.I.; Potcovaru, C.G.; Cinteza, D. High-Intensity Laser Therapy in Pain Management of Knee Osteoarthritis. Biomedicines 2024, 12, 1679. [Google Scholar] [CrossRef]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K.P. Risk factors for onset of osteoarthritis of the knee in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yan, Y.; Zhou, J.; Zhou, Q.; Wei, H. Evidence on risk factors for knee osteoarthritis in middle-older aged: A systematic review and meta analysis. J. Orthop. Surg. 2023, 18, 634. [Google Scholar] [CrossRef] [PubMed]
- Skou, S.T.; Roos, E.M. Good Life with osteoArthritis in Denmark (GLA:DTM): Evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet. Disord. 2017, 18, 72. [Google Scholar] [CrossRef]
- Vechin, F.C.; Libardi, C.A.; Conceição, M.S.; Damas, F.R.; Lixandrão, M.E.; Berton, R.P.B.; Tricoli, V.A.A.; Roschel, H.A.; Cavaglieri, C.R.; Chacon-Mikahil, M.P.T.; et al. Comparisons between low-intensity resistance training with blood flow restriction and high-intensity resistance training on quadriceps muscle mass and strength in elderly. J. Strength Cond. Res. 2015, 29, 1071–1076. [Google Scholar] [CrossRef]
- Wang, D.; Wu, T.; Li, Y.; Jia, L.; Ren, J.; Yang, L. A systematic review and meta-analysis of the effect of preoperative exercise intervention on rehabilitation after total knee arthroplasty. Ann. Palliat. Med. 2021, 10, 109860996. [Google Scholar] [CrossRef]
- Hughes, L.; Paton, B.; Rosenblatt, B.; Gissane, C.; Patterson, S.D. Blood flow restriction training in clinical musculoskeletal rehabilitation: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1003–1011. [Google Scholar] [CrossRef]
- Lixandrão, M.E.; Ugrinowitsch, C.; Berton, R.; Vechin, F.C.; Conceição, M.S.; Damas, F.; Libardi, C.A.; Roschel, H. Magnitude of Muscle Strength and Mass Adaptations Between High-Load Resistance Training Versus Low-Load Resistance Training Associated with Blood-Flow Restriction: A Systematic Review and Meta-Analysis. Sports Med. 2018, 48, 361–378. [Google Scholar] [CrossRef]
- Slysz, J.; Stultz, J.; Burr, J.F. The efficacy of blood flow restricted exercise: A systematic review & meta-analysis. J. Sci. Med. Sport 2016, 19, 669–675. [Google Scholar] [CrossRef]
- Patterson, S.D.; Hughes, L.; Warmington, S.; Burr, J.; Scott, B.R.; Owens, J.; Abe, T.; Nielsen, J.L.; Libardi, C.A.; Laurentino, G.; et al. Blood Flow Restriction Exercise: Considerations of Methodology, Application, and Safety. Front. Physiol. 2019, 10, 533. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef]
- Thomas, J.; Kneale, D.; McKenzie, J.E.; Brennan, S.E.; Bhaumik, S. Determining the scope of the review and the questions it will address. In Cochrane Handbook for Systematic Reviews of Interventions [Internet], 1st ed.; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 13–31. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024); Cochrane: Chichester, UK, 2024. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Franz, A.; Ji, S.; Bittersohl, B.; Zilkens, C.; Behringer, M. Impact of a Six-Week Prehabilitation With Blood-Flow Restriction Training on Pre- and Postoperative Skeletal Muscle Mass and Strength in Patients Receiving Primary Total Knee Arthroplasty. Front. Physiol. 2022, 13, 881484. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, S.L.; Aagaard, P.; Bohn, M.B.; Hansen, P.; Hansen, P.M.; Holm, C.; Mortensen, L.; Garval, M.; Tønning, L.U.; Mechlenburg, I. The Effect of Blood Flow Restriction Exercise Prior to Total Knee Arthroplasty on Postoperative Physical Function, Lower Limb Strength and Patient-Reported Outcomes: A Randomized Controlled Trial. Scand. J. Med. Sci. Sports 2024, 34, e14750. [Google Scholar] [CrossRef]
- Przkora, R.; Sibille, K.; Victor, S.; Meroney, M.; Leeuwenburgh, C.; Gardner, A.; Vasilopoulos, T.; Parvataneni, H.K. Blood flow restriction exercise to attenuate postoperative loss of function after total knee replacement: A randomized pilot study. Eur. J. Transl. Myol. 2021, 31, 9932. [Google Scholar] [CrossRef] [PubMed]
- Kubo, Y.; Fujita, D.; Sugiyama, S.; Takachu, R.; Sugiura, T.; Sawada, M.; Yamashita, K.; Kobori, K.; Kobori, M. Safety and Effects of a Four-Week Preoperative Low-Load Resistance Training With Blood Flow Restriction on Pre- and Postoperative Quadriceps Strength in Patients Undergoing Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial. Cureus 2024, 16, e64466. [Google Scholar] [CrossRef] [PubMed]
- Bykov, E.V.; Sverchkov, V.V. Metabolic effects of training with blood flow restriction. Sci. Educ. Basics Phys. Cult. Sports 2024, 2, 16–21. [Google Scholar] [CrossRef]
- García-Rodríguez, P.; Pecci, J.; Vázquez-González, S.; Pareja-Galeano, H. Acute and Chronic Effects of Blood Flow Restriction Training in Physically Active Patients With Anterior Cruciate Ligament Reconstruction: A Systematic Review. Sports Health 2024, 16, 820–828. [Google Scholar] [CrossRef]
- Barber-Westin, S.; Noyes, F.R. Blood Flow-Restricted Training for Lower Extremity Muscle Weakness due to Knee Pathology: A Systematic Review. Sports Health 2019, 11, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Pitsillides, A.; Stasinopoulos, D.; Mamais, I. Blood flow restriction training in patients with knee osteoarthritis: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2021, 27, 477–486. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.P.; Santo, R.C.d.E.; Ramis, T.R.; Portes, J.K.S.; Chakr, R.M.d.S.; Xavier, R.M. The effects of resistance training with blood flow restriction on muscle strength, muscle hypertrophy and functionality in patients with osteoarthritis and rheumatoid arthritis: A systematic review with meta-analysis. PLoS ONE 2021, 16, e0259574. [Google Scholar] [CrossRef] [PubMed]
- Fraca-Fernández, E.; Ceballos-Laita, L.; Hernández-Lázaro, H.; Jiménez-del-Barrio, S.; Mingo-Gómez, M.T.; Medrano-de-la-Fuente, R.; Hernando-Garijo, I. Effects of Blood Flow Restriction Training in Patients before and after Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 1231. [Google Scholar] [CrossRef]
- Johns, W.; Wiafe, B.M.; Hammoud, S. Blood Flow Restriction Following ACL Reconstruction. Video J. Sports Med. 2023, 3, 26350254221148215. [Google Scholar] [CrossRef]
- Karanasios, S.; Korakakis, V.; Moutzouri, M.; Xergia, S.A.; Tsepis, E.; Gioftsos, G. Low-Load Resistance Training With Blood Flow Restriction Is Effective for Managing Lateral Elbow Tendinopathy: A Randomized, Sham-Controlled Trial. J. Orthop. Sports Phys. Ther. 2022, 52, 803–825. [Google Scholar] [CrossRef]
- Bowman, E.N.; Elshaar, R.; Milligan, H.; Jue, G.; Mohr, K.; Brown, P.; Watanabe, D.M.; Limpisvasti, O. Upper-extremity blood flow restriction: The proximal, distal, and contralateral effects-a randomized controlled trial. J. Shoulder Elb. Surg. 2020, 29, 1267–1274. [Google Scholar] [CrossRef]
- Yamanashi, Y.; Allahabadi, S.; Ma, C.B.; Arriaga, I. Blood Flow Restriction Training for Meniscus Repair Surgery. Video J. Sports Med. 2024, 4, 26350254231202532. [Google Scholar] [CrossRef]

| Pubmed | Query | Results |
|---|---|---|
| #4 | #1 AND #2 AND #3 | 12 |
| #1 | (“Blood Flow Restriction Training”[MeSH] OR “Blood Flow Restriction Exercise” OR “BFR Therapy” OR “BFR Therapies” OR “KAATSU Training” OR “Occlusion Training” OR “Ischemic Training” OR “Ischemic Exercise” OR “Vascular Occlusion Training” OR “Tourniquet Training”) | 1491 |
| #2 | (“Arthroplasty, Replacement, Knee”[MeSH] OR “Arthroplasty, Knee Replacement” OR “Knee Replacement Arthroplasty” OR “Knee Arthroplasty” OR “Knee Replacement” OR “Total Knee Arthroplasty” OR “Total Knee Replacement” OR “TKA” OR “TKR” OR “Partial Knee Arthroplasty” OR “Unicompartmental Knee Arthroplasty” OR “Unicondylar Knee Arthroplasty” OR “Unicompartmental Knee Replacement” OR “Partial Knee Replacement”) | 51,916 |
| #3 | (“Muscle Strength” [MeSH] OR “Quadriceps Strength” OR “Lower Limb Strength” OR “Muscle Power” OR “Postoperative Muscle Weakness” OR “Muscle Endurance” OR “Recovery of Function” [MeSH] OR “Functional Outcome” OR “Functional Performance” OR “Activities of Daily Living” [MeSH] OR “ADL” OR “Gait” [MeSH] OR “Mobility Limitation” [MeSH] OR “Mobility” OR “Physical Function” OR “Functional Independence” OR “Walking Ability” OR “Sit-to-Stand Performance” OR “Timed Up and Go” OR “TUG” OR “Balance”) | 877,859 |
| Embase | Query | |
| #4 | #1 AND #2 AND #3 | 13 |
| #1 | (‘blood flow restriction training’/exp OR ‘blood flow restriction exercise’ OR ‘BFR therapy’ OR ‘BFR therapies’ OR ‘KAATSU training’ OR ‘occlusion training’ OR ‘ischemic training’ OR ‘ischemic exercise’ OR ‘vascular occlusion training’ OR ‘tourniquet training’) | 1046 |
| #2 | (‘knee arthroplasty’/exp OR ‘arthroplasty, knee replacement’ OR ‘knee replacement arthroplasty’ OR ‘knee arthroplasty’ OR ‘knee replacement’ OR ‘total knee arthroplasty’ OR ‘total knee replacement’ OR ‘TKA’ OR ‘TKR’ OR ‘partial knee arthroplasty’ OR ‘unicompartmental knee arthroplasty’ OR ‘unicondylar knee arthroplasty’ OR ‘unicompartmental knee replacement’ OR ‘partial knee replacement’) | 70,865 |
| #3 | (‘muscle strength’/exp OR ‘quadriceps strength’ OR ‘lower limb strength’ OR ‘muscle power’ OR ‘postoperative muscle weakness’ OR ‘muscle endurance’ OR ‘functional outcome’/exp OR ‘recovery of function’/exp OR ‘activities of daily living’/exp OR ‘mobility’/exp OR ‘gait’/exp OR ‘functional performance’ OR ‘physical function’ OR ‘functional independence’ OR ‘walking ability’ OR ‘sit-to-stand performance’ OR ‘timed up and go’ OR ‘TUG’ OR ‘balance’) | 854,915 |
| Cochrane Library | Query | |
| (“Blood Flow Restriction Training” OR “Blood Flow Restriction Exercise” OR “BFR Therapy” OR “BFR Therapies” OR “KAATSU Training” OR “Occlusion Training” OR “Ischemic Training” OR “Ischemic Exercise” OR “Vascular Occlusion Training” OR “Tourniquet Training”) AND (“Total Knee Arthroplasty” OR “Total Knee Replacement” OR “TKA” OR “TKR” OR “Knee Replacement” OR “Knee Arthroplasty” OR “Partial Knee Replacement” OR “Unicompartmental Knee Arthroplasty” OR “Unicondylar Knee Arthroplasty”) AND (“Muscle Strength” OR “Quadriceps Strength” OR “Lower Limb Strength” OR “Muscle Power” OR “Postoperative Muscle Weakness” OR “Muscle Endurance” OR “Functional Outcome” OR “Recovery of Function” OR “Functional Performance” OR “Activities of Daily Living” OR “ADL” OR “Gait” OR “Mobility” OR “Physical Function” OR “Functional Independence” OR “Walking Ability” OR “Sit-to-Stand Performance” OR “Timed Up and Go” OR “TUG” OR “Balance”) IN Title Abstract Keyword | 12 |
| Source | Study Design | Setting | Sample Characteristics | Description of the Intervention | BFR Protocol | Function and/or Muscle Strength Measurement Tools | Results |
|---|---|---|---|---|---|---|---|
| [30] | Single-blinded RCT with parallel group | Location of the study: Germany Enrollment period: NR | n = 30 total, n = 10 control group, n = 10 active control (AC) group, n = 10 BFR group mean age: 63.5 ± 8.1 years sex distribution: 18 males and 12 females Inclusion criteria: patients with end-stage knee OA undergoing unilateral TKA | 6 weeks before a TKA The control group had a standard clinical treatment (surgery and 3 weeks inpatient post op rehabilitation) The active control group had a prehabilitation program with sham-BFR (tourniquet in alternation between both legs with a standard pressure of 20 mmHg while training with a cycling ergometer) The BFR group had the same rehabilitation protocol as the active control group, but loaded with 40% of the individual LOP Assessment timepoints: Baseline 3w prehab Preop 3m-post op 6m-post op | 2 sessions of 50 min a week Pressure was 40% of the individual LOP, alternating between both legs during cycling ergometer training For each session, BFR was applied 3 times, for 1 min in the first week and 6 min in the sixth week, in each leg -Cuffs were applied to the thigh | Muscle strength: 6 RM test of leg extension and leg curl Function: Active knee joint mobility (ROM) 30 STS 6 MWT | Muscle strength: At Pre-op assessment, the BFR group showed significantly higher values in all strength measures than the other groups Significant differences in leg strength between the BFR group and other groups during the overall postoperative period concerning the operated and the non-operated leg Function: Only the BFR group showed a significant improvement of 6 MWT with a large effect size in all timepoints except for 3m post op Both AC and BFR groups showed improvements of the 30 STS, but BFR improvements were faster (occurring at 3w prehab), without a drop at 3m postop, unlike the AC group. No significant changes in the ROM Adverse event: None |
| [31] | Multicenter, randomized, assessor-blinded, controlled trial | Location of the study: Denmark Enrollment period: from September 2019 to October 2022 | n = 86 (total), n = 42 (control group) and n = 44 (BFR group) mean age: 66.6 ± 6.62 years sex distribution: 37 males and 49 females Inclusion criteria: patients aged ≥ 50 years scheduled for primary TKA for advanced knee OA | 8 weeks before a primary TKA The control group: 2 to 3 weeks of usual preoperative care, including information and physical activity, and usual postop care The BFR group: same as the control group, but including pre-op BFR training Assessment time points: baseline (12 weeks preop) Pre-op 3m-post op 12m-post op | 3 sessions a week 10 min warm-up on an ergometer bike THEN 4 sessions of Leg press exercise and four sessions of Leg extension exercise The first session: 30 reps The second and third: 15 reps The fourth until volitional contraction failure 5 min rest between the two exercises Pressure was 60% of the LOP The load was 30% 1 RM, but increased if the fourth session surpassed 15 reps The contralateral leg was not trained Cuffs were applied to the thigh | Muscle strength: -5 RM and estimated 1 RM of leg press -Max isometric knee extensors and flexors strength Function: TUG 30 STS 40m fast-paced walk test knee ROM | Muscle strength: 1 RM leg press: BFR significantly better in pre-op 3m-post but not in 12m-post With large effect sizes at all time points 1 RM knee extension: BFR significantly better in all time points than the control group, with a large effect size in all time points Max iso knee ext: BFR only sig better at 3m-post op, moderate to large effect size in all time points, favoring BFR Max iso knee flex: No difference between groups, but a moderate to large effect size favoring BFR at all time points Function: -None between group difference at any time point -Effect size 30 STS favoring BFR pre-op, moderate effect size TUG at 3m-post op -At 12 m Post, large Effect sizes favoring CON emerged for 30 STS, TUG, and 40mFWT -Adverse event: None |
| [32] | Non-blinded Randomized controlled trial | Location of the study: USA Enrollment period: NR | n = 10 (total), n = 4 (control) and n = 6 (BFR group) mean age: 67.2 ± 7.1 years sex distribution: 7 females and three males Inclusion criteria: -age: 60 to 75 years -scheduled for unilateral TKA for knee OA | 4 weeks before surgery The control group: no exercise The BFR group: 2 to 3 sessions a week with a minimum of 8 sessions and a maximum of 12 (one patient did only six sessions) Assessment time points: Baseline: 4 to 5 weeks pre-op 2w-post op | 2 to 3 sessions a week -Brief warm-up THEN Leg press, leg extension, leg curl, and calf extension at an intensity of 30% of 1 RM Exercises were performed to volitional fatigue (no standardized reps) -Cuff pressures for each An automated cuff inflator determined the individual depending on systolic blood pressure and thigh circumference -Cuffs remained inflated during exercise, but they were deflated for a 3 min rest between exercises. -Cuffs were applied to the thigh | Muscle strength: Quadriceps Peak torque strength Function: SPPB 6 MWT | Muscle strength: Peak torque: Similar decrease in both groups Function: SPPB: after 2 weeks- post op, both groups decreased values, but a greater decrease in the control group 6 MWT: similar decrease Adverse event: NR |
| [33] | Single-blinded randomized controlled trial | Location of study: Japan Enrollment period: from September 2019 to December 2021 | n = 22 (total), n = 11 (BFR group) and n = 11 (LST group) mean age: 73 years sex distribution: 4 males and 18 females Inclusion criteria: Age between 60 and 79 years, with an advanced knee OA condition scheduled for unilateral TKA | 4weeks before surgery Both groups had a 10 min warm-up, and each session lasted 50 min, including resistance and aerobic exercises The LST group: 6 ± 3 training sessions before surgery, and performed the same exercises as the BFR group while using two cuffs inflated at only 20 mmHg on the thigh and on the calf The BFR group: 7 ± 2 training sessions before surgery Surgery: tricompartmental uncemented TKA using a low-contact-stress implant with Tourniquet use at 300 mmHg After surgery, both groups received the same inpatient and outpatient rehabilitation treatment Assessment time points: Baseline: 6w preop Multiple preop and postop assessments until 3 months | 2 to 3 sessions a week -Squats, forward lunges using body weight for resistance, and seated bilateral knee extensions at 30% of the maximum isometric voluntary contraction. The exercise consisted of three sets of 10 reps, with each rep including 3 s each of eccentric, isometric, and concentric phases There was a 30 s rest between sets and a 60 s rest between different exercises -Cuffs were inflated at 100–120 mmHg on the thigh and calf | Muscle strength: isometric quadriceps strength Function: 30 STS TUG SCT | Muscle strength: Quadriceps strength: no significant differences in the rate of increase in the quadriceps strength before and after the intervention, or in the rate of reduction in quadriceps strength before and after surgery between the groups Function: no difference between the two groups concerning the 30 STS, TUG, and SCT after the intervention, nor after the surgery Adverse event: None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiss, B.; Layouni, S.; Ghali, H.; Ceylan, H.İ.; Nticha, I.; Jemni, S.; Muntean, R.I.; Bragazzi, N.L.; Dergaa, I. Blood Flow Restriction Training in Knee Arthroplasty: A Systematic Review of Current Evidence on Postoperative Muscle Strength and Function. Medicina 2025, 61, 1879. https://doi.org/10.3390/medicina61101879
Tiss B, Layouni S, Ghali H, Ceylan Hİ, Nticha I, Jemni S, Muntean RI, Bragazzi NL, Dergaa I. Blood Flow Restriction Training in Knee Arthroplasty: A Systematic Review of Current Evidence on Postoperative Muscle Strength and Function. Medicina. 2025; 61(10):1879. https://doi.org/10.3390/medicina61101879
Chicago/Turabian StyleTiss, Bassem, Saoussen Layouni, Hela Ghali, Halil İbrahim Ceylan, Iheb Nticha, Sonia Jemni, Raul Ioan Muntean, Nicola Luigi Bragazzi, and Ismail Dergaa. 2025. "Blood Flow Restriction Training in Knee Arthroplasty: A Systematic Review of Current Evidence on Postoperative Muscle Strength and Function" Medicina 61, no. 10: 1879. https://doi.org/10.3390/medicina61101879
APA StyleTiss, B., Layouni, S., Ghali, H., Ceylan, H. İ., Nticha, I., Jemni, S., Muntean, R. I., Bragazzi, N. L., & Dergaa, I. (2025). Blood Flow Restriction Training in Knee Arthroplasty: A Systematic Review of Current Evidence on Postoperative Muscle Strength and Function. Medicina, 61(10), 1879. https://doi.org/10.3390/medicina61101879









