Comparison of Ultrapreservation and Retzius-Sparing Techniques in Robotic Radical Prostatectomy: Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Sample Size and Power Calculation
2.3. Surgical Techniques
2.4. Ultrapreservation Technique
2.5. Retzius-Sparing Technique
2.6. Outcome Definitions
2.7. Evaluation Parameters
2.8. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Perioperative Outcomes
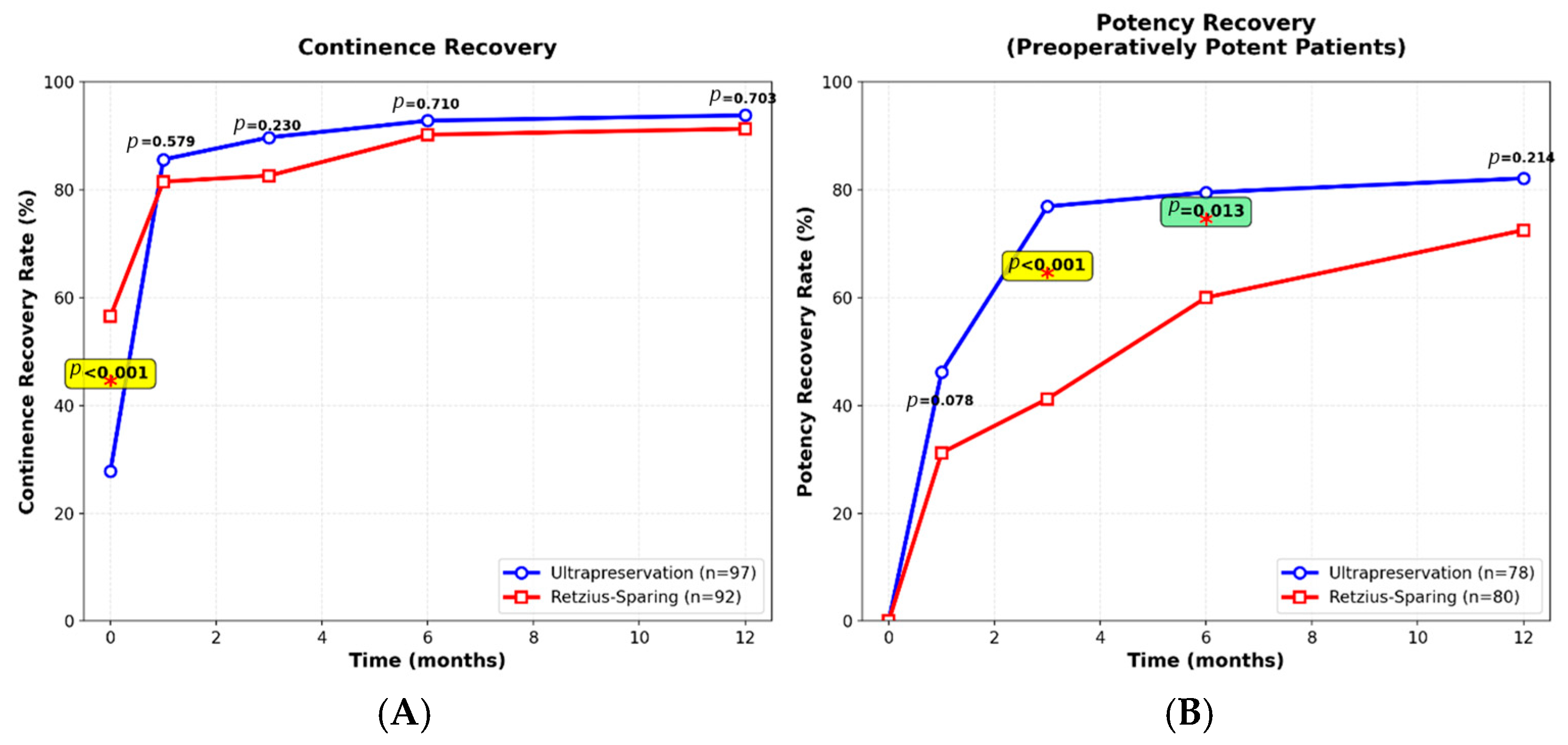
3.3. Functional Outcomes
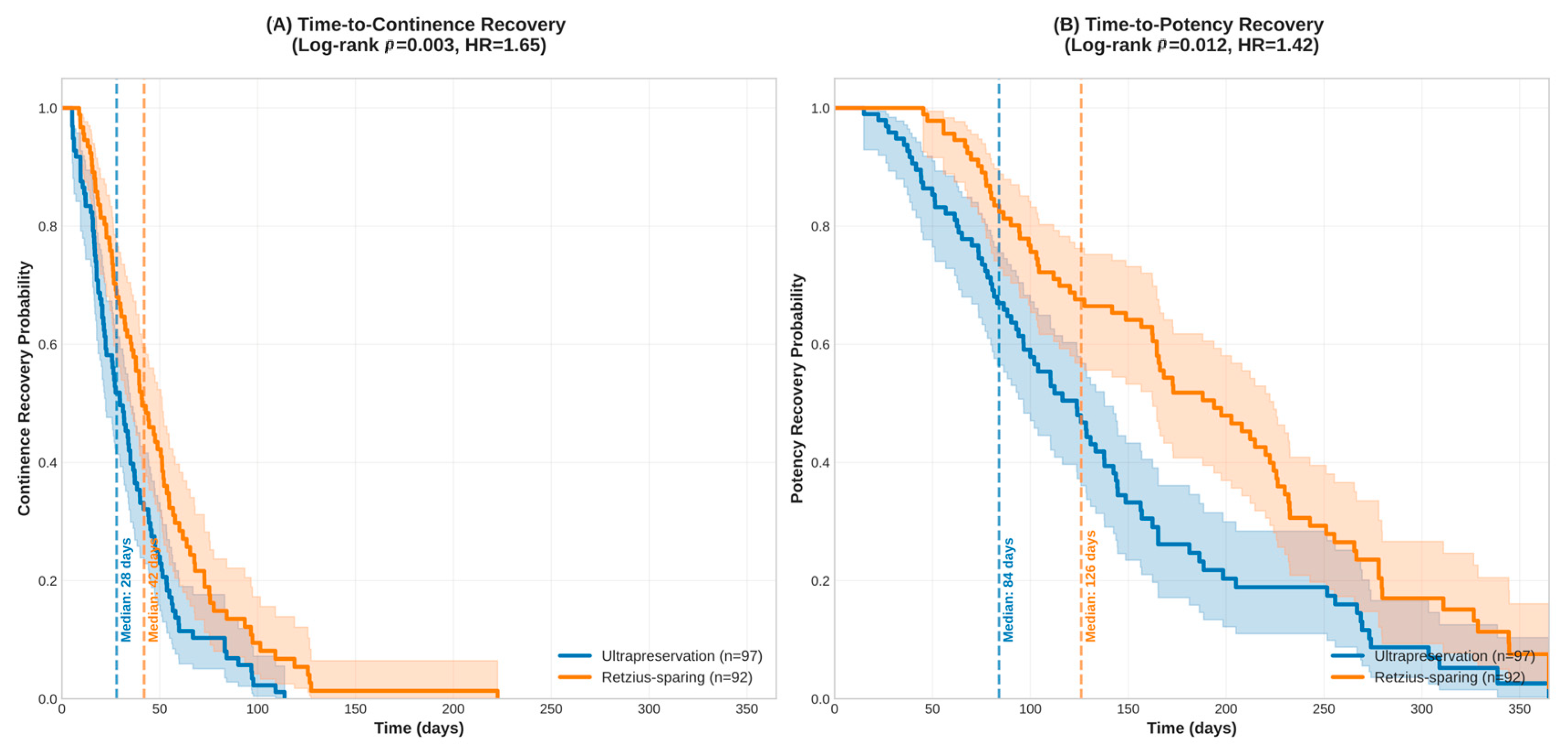
3.4. Time-to-Recovery Analysis
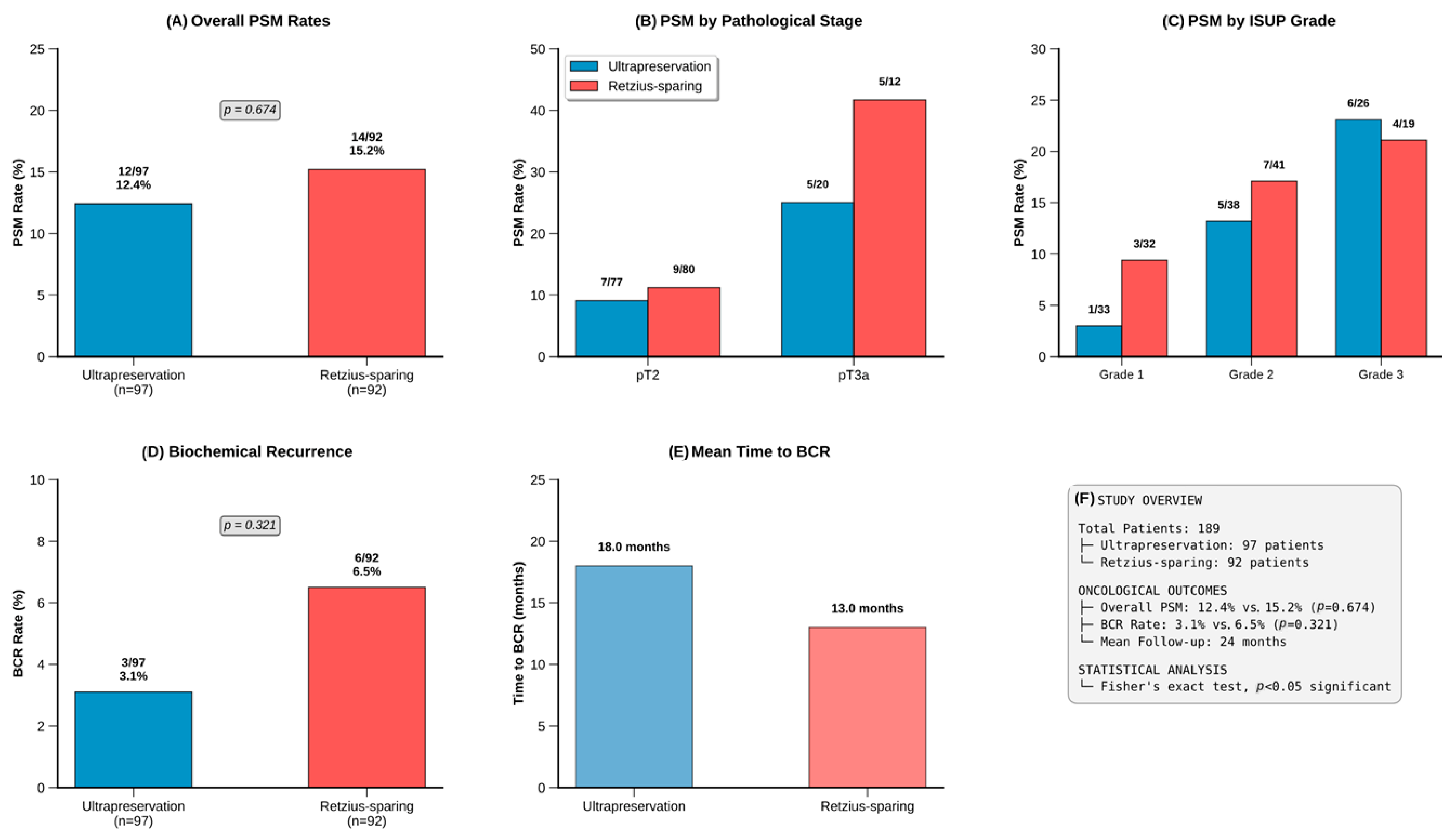
3.5. Oncological Outcomes
3.6. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CI | Confidence Interval |
| DVC | Dorsal Venous Complex |
| HR | Hazard Ratio |
| ICIQ-UI | International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form |
| IIEF-5 | International Index of Erectile Function-5 |
| IPSS | International Prostate Symptom Score |
| ISUP | International Society of Urological Pathology |
| NVBs | Neurovascular Bundles |
| PSA | Prostate-Specific Antigen |
| PSM | Positive Surgical Margin |
| RARP | Robotic-Assisted Radical Prostatectomy |
| SD | Standard Deviation |
| SHIM | Sexual Health Inventory for Men |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Culp, M.B.; Soerjomataram, I.; Efstathiou, J.A.; Bray, F.; Jemal, A. Recent global patterns in prostate cancer incidence and mortality rates. Eur. Urol. 2020, 77, 38–52. [Google Scholar] [CrossRef]
- Ganapathi, H.P.; Ogaya-Pinies, G.; Rogers, T.; Patel, V.R. Robotic Assisted Radical Prostatectomy. In Operative Atlas of Laparoscopic and Robotic Reconstructive Urology, 2nd ed.; Patel, V.R., Ramalingam, M., Eds.; Springer International Publishing: Cham, Germany, 2017; pp. 581–591. [Google Scholar] [CrossRef]
- Ferretti, S.; Dell’Oglio, P.; Ciavarella, D.; Galfano, A.; Schips, L.; Marchioni, M. Retzius-sparing robotic-assisted prostatectomy: Technical challenges for surgeons and key prospective refinements. Res. Rep. Urol. 2023, 15, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Montorsi, F.; Karakiewicz, P.I.; Sun, M. Robot-Assisted Radical Prostatectomy in Prostate Cancer. Future Oncol. 2015, 11, 2767–2773. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, S.; Wiklund, N.P. Robot-Assisted Radical Prostatectomy. In Management of Prostate Cancer: A Multidisciplinary Approach; Bolla, M., van Poppel, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 105–111. [Google Scholar] [CrossRef]
- Cortés, Á.G.; Vives, J.C.; Castañé, C.G.; San Román, S.C.; López, P.D.; Marckert, F.A.; Suárez, M.H.; Narro, I.M.; Campillo, J.M.V.; Grima, F.G.; et al. Comparison of surgical approaches to radical prostatectomy in our series beyond oncological and functional outcomes. Actas Urológicas Españolas (Engl. Ed.) 2022, 46, 275–284. [Google Scholar] [CrossRef]
- Donovan, J.L.; Hamdy, F.C.; Lane, J.A.; Mason, M.; Metcalfe, C.; Walsh, E.; Blazeby, J.M.; Peters, T.J.; Holding, P.; Bonnington, S.; et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. New Engl. J. Med. 2016, 375, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Yan, J.; Cui, Y.; Zhang, D.; Ma, Y. Comparison of efficacy of Retzius-sparing radical prostatectomy versus standard radical prostatectomy in the treatment of prostate cancer: A systematic review and meta-analysis. Front. Oncol. 2025, 15, 1547687. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arroyo, C.; Martini, A.; Wang, J.; Tewari, A.K. Anatomical, surgical and technical factors influencing continence after radical prostatectomy. Ther. Adv. Urol. 2019, 11, 1756287218813787. [Google Scholar] [CrossRef]
- Checcucci, E.; San Luigi Study Group; Pecoraro, A.; De Cillis, S.; Manfredi, M.; Amparore, D.; Aimar, R.; Piramide, F.; Granato, S.; Volpi, G.; et al. The importance of anatomical reconstruction for continence recovery after robot assisted radical prostatectomy: A systematic review and pooled analysis from referral centers. Minerva Urol. Nephrol. 2020, 73, 165–177. [Google Scholar] [CrossRef]
- Kucuk, E.V.; Sobay, R.; Tahra, A. Ultrapreservation in robotic assisted radical prostatectomy provides early continence recovery. J. Soc. Laparosc. Robot. Surg. 2023, 27, e2022.00077. [Google Scholar] [CrossRef]
- Galfano, A.; Ascione, A.; Grimaldi, S.; Petralia, G.; Strada, E.; Bocciardi, A.M. A new anatomic approach for robot-assisted laparoscopic prostatectomy: A feasibility study for completely intrafascial surgery. Eur. Urol. 2010, 58, 457–461. [Google Scholar] [CrossRef]
- Galfano, A.; Di Trapani, D.; Sozzi, F.; Strada, E.; Petralia, G.; Bramerio, M.; Ascione, A.; Gambacorta, M.; Bocciardi, A.M. Beyond the learning curve of the Retzius-sparing approach for robot-assisted laparoscopic radical prostatectomy: Oncologic and functional results of the first 200 patients with ≥1 year of follow-up. Eur. Urol. 2013, 64, 974–980. [Google Scholar] [CrossRef]
- Egan, J.; Marhamati, S.; Carvalho, F.L.; Davis, M.; O’Neill, J.; Lee, H.; Lynch, J.H.; Hankins, R.A.; Hu, J.C.; Kowalczyk, K.J. Retzius-sparing robot-assisted radical prostatectomy leads to durable improvement in urinary function and quality of life versus standard robot-assisted radical prostatectomy without compromise on oncologic efficacy: Single-surgeon series and step-by-step guide. Eur. Urol. 2021, 79, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Asimakopoulos, A.D.; Topazio, L.; De Angelis, M.; Agrò, E.F.; Pastore, A.L.; Fuschi, A.; Annino, F. Retzius-sparing versus standard robot-assisted radical prostatectomy: A prospective randomized comparison on immediate continence rates. Surg. Endosc. 2019, 33, 2187–2196. [Google Scholar] [CrossRef]
- Dalela, D.; Jeong, W.; Prasad, M.-A.; Sood, A.; Abdollah, F.; Diaz, M.; Karabon, P.; Sammon, J.; Jamil, M.; Baize, B.; et al. A pragmatic randomized controlled trial examining the impact of the Retzius-sparing approach on early urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2017, 72, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Barakat, B.; Othman, H.; Gauger, U.; Wolff, I.; Hadaschik, B.; Rehme, C. Retzius sparing radical prostatectomy versus robot-assisted radical prostatectomy: Which technique is more beneficial for prostate cancer patients (MASTER study)? A systematic review and meta-analysis. Eur. Urol. Focus 2022, 8, 1060–1071. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205. [Google Scholar] [CrossRef]
- García Cortés, Á.; Colombás Vives, J.; Gutiérrez Castañé, C.; Chiva San Román, S.; Doménech López, P.; Ancizu Marckert, F.J.; Suárez, M.H.; Narro, I.M.; Campillo, J.M.V.; Grima, F.G.; et al. What is the impact of post-radical prostatectomy urinary incontinence on everyday quality of life? Linking Pad usage and International Consultation on Incontinence Questionnaire Short-Form (ICIQ-SF) for a COMBined definition (PICOMB definition). Neurourol. Urodyn. 2021, 40, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.L.; Nehra, A.; Breau, R.H.; Culkin, D.J.; Faraday, M.M.; Hakim, L.S.; Heidelbaugh, J.; Khera, M.; McVary, K.T.; Miner, M.M.; et al. Erectile Dysfunction: AUA Guideline. J. Urol. 2018, 200, 633–641. [Google Scholar] [CrossRef]
- Eastham, J.A.; Boorjian, S.A.; Kirkby, E. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline. J. Urol. 2022, 208, 505–507. [Google Scholar] [CrossRef]
- Eden, C.G. Retzius-sparing robotic radical prostatectomy. Asian J. Androl. 2020, 22, 149–151. [Google Scholar] [CrossRef]
- Borregales, L.D.; Berg, W.T.; Tal, O.; Wambi, C.; Kaufman, S.; Gaya, J.M.; Urzúa, C.; Badani, K.K. ‘Trifecta’ after radical prostatectomy: Is there a standard definition? BJU Int. 2013, 112, 60–67. [Google Scholar] [CrossRef]
- Liao, P.-C.; Hung, S.-C.; Hu, J.-C.; Chiu, K.-Y. Retzius-sparing Robotic-assisted Radical Prostatectomy Facilitates Early Continence Regardless of Neurovascular Bundle Sparing. Anticancer. Res. 2020, 40, 4075–4080. [Google Scholar] [CrossRef]
- Albisinni, S.; Dasnoy, C.; Diamand, R.; Mjaess, G.; Aoun, F.; Esperto, F.; Porpiglia, F.; Fiori, C.; Roumeguere, T.; DE Nunzio, C. Anterior vs. Retzius-sparing robotic assisted radical prostatectomy: Can the approach really make a difference? Minerva Urol. Nephrol. 2022, 74, 137–145. [Google Scholar] [CrossRef]
- Checcucci, E.; Veccia, A.; Fiori, C.; Amparore, D.; Manfredi, M.; Di Dio, M.; Morra, I.; Galfano, A.; Autorino, R.; Bocciardi, A.M.; et al. Retzius—sparing robot—assisted radical prostatectomy vs the standard approach: A systematic review and analysis of comparative outcomes. BJU Int. 2020, 125, 8–16. [Google Scholar] [CrossRef]
- Anıl, H.; Karamık, K.; Yıldız, A.; Savaş, M. Does transition from standard to Retzius-sparing technique in robot-assisted radical prostatectomy affect the functional and oncological outcomes? Arch. Ital. Di Urol. E Androl. 2021, 93, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.W.; Herlemann, A.; Cowan, J.E.; Broering, J.M.; Ten Ham, R.M.; Wilson, L.S.; Carroll, P.R.; Cooperberg, M.R. The relative impact of urinary and sexual function vs bother on health utility for men with prostate cancer. JNCI Cancer Spectr. 2020, 4, pkaa044. [Google Scholar] [CrossRef] [PubMed]
- Helgason, A.R.; Adolfsson, J.; Dickman, P.; Fredrikson, M.; Arver, S.; Steineck, G. Waning sexual function-the most important disease-specific distress for patients with prostate cancer. Br. J. Cancer 1996, 73, 1417–1421. [Google Scholar] [CrossRef]
- Novara, G.; Ficarra, V.; Mocellin, S.; Ahlering, T.E.; Carroll, P.R.; Graefen, M.; Guazzoni, G.; Menon, M.; Patel, V.R.; Shariat, S.F.; et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 382–404. [Google Scholar] [CrossRef]
- Tourinho-Barbosa, R.; Srougi, V.; Nunes-Silva, I.; Baghdadi, M.; Rembeyo, G.; Eiffel, S.S.; Barret, E.; Rozet, F.; Galiano, M.; Cathelineau, X.; et al. Biochemical recurrence after radical prostatectomy: What does it mean? Int. Braz. J. Urol. 2018, 44, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, A.; Satkunasivam, R.; Gill, I.S.; Lieskovsky, G.; Daneshmand, S.; Pinski, J.K.; Stern, M.C. Predictors of time to biochemical recurrence in a radical prostatectomy cohort within the PSA-era. Can. Urol. Assoc. J. 2016, 10, E17–E22. [Google Scholar] [CrossRef] [PubMed]

| Variable | Ultrapreservation (n = 97) | Retzius-Sparing (n = 92) | p-Value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 61.5 (3.1) | 60.9 (3.2) | 0.2 | |
| BMI (kg/m2), mean (SD) | 26.3 (2.1) | 26.0 (2.0) | 0.353 | |
| Charlson Comorbidity Index | 2.0 ± 0.7 | 2.4 ± 0.6 | <0.001 | |
| Pre-operative Potency, n (%) | 78 (80.4) | 80 (87.0) | 0.309 | |
| PSA (ng/mL), mean (SD) | 6.8 (1.4) | 6.6 (1.6) | 0.438 | |
| Prostate Volume (mL), mean (SD) | 45.8 (7.0) | 44.9 (8.1) | 0.436 | |
| D’Amico Risk Group, n (%) | 0.581 | |||
| Low Risk | 33 (34.0) | 32 (34.8) | ||
| Intermediate Risk | 38 (39.2) | 41 (44.6) | ||
| High Risk | 26 (26.8) | 19 (20.7) | ||
| ISUP Grade Group, n (%) | 1 | 33 (34.0) | 32 (34.8) | 0.581 |
| 2 | 38 (39.2) | 41 (44.6) | ||
| 3 | 26 (26.8) | 19 (20.7) | ||
| Pathologic Stage, n (%) | pT2 | 77 (79.4) | 80 (87.0) | 0.233 |
| pT3a | 20 (20.6) | 12 (13.0) | ||
| Nerve Sparing, n (%) | None | 5 (5.2) | 12 (13.0) | 0.077 |
| Unilateral | 11 (11.3) | 15 (16.3) | ||
| Bilateral | 81 (83.5) | 65 (70.7) | ||
| Follow-up, months, mean (range) | 22.0 (14–32) | 22.9 (14–36) | 0.196 | |
| Variable | Ultrapreservation (n = 97) | Retzius-Sparing (n = 92) | p-Value |
|---|---|---|---|
| Operative time (min), mean (SD) | 174.8 (19.5) | 188.7 (14.9) | <0.001 |
| Console time (min), mean (SD) | 112.4 (14.3) | 132.0 (18.5) | <0.001 |
| Blood loss (mL), mean (SD) | 119.0 (30.7) | 133.3 (33.4) | 0.002 |
| Hospital stay (days), mean (SD) | 2.3 (0.5) | 2.5 (0.6) | 0.004 |
| Variable | Ultrapreservation (n = 97) | Retzius-Sparing (n = 92) | p-Value |
|---|---|---|---|
| Overall PSM, n (%) | 12 (12.4) | 14 (15.2) | 0.674 |
| PSM by Pathologic Stage | |||
| pT2 (n = 157) | 7/77 (9.1) | 9/80 (11.2) | 0.674 |
| pT3a (n = 32) | 5/20 (25.0) | 5/12 (41.7) | 0.440 |
| PSM by ISUP Grade | |||
| Grade 1 (n = 65) | 1/33 (3.0) | 3/32 (9.4) | 0.349 |
| Grade 2 (n = 79) | 5/38 (13.2) | 7/41 (17.1) | 0.771 |
| Grade 3 (n = 45) | 6/26 (23.1) | 4/19 (21.1) | 1.000 |
| PSM by D’Amico Risk Group | |||
| Low Risk (n = 65) | 1/33 (3.0) | 3/32 (9.4) | 0.349 |
| Intermediate Risk (n = 79) | 5/38 (13.2) | 7/41 (17.1) | 0.771 |
| High Risk (n = 45) | 6/26 (23.1) | 4/19 (21.1) | 1.000 |
| Clavien-Dindo | Ultrapreservation | Retzius-Sparing |
|---|---|---|
| Grade 0 (No Complication) | 89 (91.8%) | 82 (89.13%) |
| Grade 1 (Minor) | 6 (6.2%) | 7 (7.6%) |
| Grade 2 | 2 (2.1%) | 3 (3.26%) |
| Grade ≥ 3 (Major) | 0 (0.0%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beyatlı, M.; Güngör, H.S.; Haberal, H.B.; İnkaya, A.; Sobay, R.; Tahra, A.; Küçük, E.V. Comparison of Ultrapreservation and Retzius-Sparing Techniques in Robotic Radical Prostatectomy: Single-Center Experience. Medicina 2025, 61, 1851. https://doi.org/10.3390/medicina61101851
Beyatlı M, Güngör HS, Haberal HB, İnkaya A, Sobay R, Tahra A, Küçük EV. Comparison of Ultrapreservation and Retzius-Sparing Techniques in Robotic Radical Prostatectomy: Single-Center Experience. Medicina. 2025; 61(10):1851. https://doi.org/10.3390/medicina61101851
Chicago/Turabian StyleBeyatlı, Murat, Hasan Samet Güngör, Hakan Bahadir Haberal, Abdurrahman İnkaya, Resul Sobay, Ahmet Tahra, and Eyüp Veli Küçük. 2025. "Comparison of Ultrapreservation and Retzius-Sparing Techniques in Robotic Radical Prostatectomy: Single-Center Experience" Medicina 61, no. 10: 1851. https://doi.org/10.3390/medicina61101851
APA StyleBeyatlı, M., Güngör, H. S., Haberal, H. B., İnkaya, A., Sobay, R., Tahra, A., & Küçük, E. V. (2025). Comparison of Ultrapreservation and Retzius-Sparing Techniques in Robotic Radical Prostatectomy: Single-Center Experience. Medicina, 61(10), 1851. https://doi.org/10.3390/medicina61101851






