Sleep Paralysis Among Higher Education Students: A Possible Role of Antidepressant and Recreational Stimulant Use
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Questionnaire
2.3. Statistical Analysis
2.4. Ethics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefani, A.; Tang, Q. Recurrent Isolated Sleep Paralysis. Sleep. Med. Clin. 2024, 19, 101–109. [Google Scholar] [CrossRef]
- American Academy of Sleep Medicine Recurrent Isolated Sleep Paralysis. In International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014; pp. 254–256.
- Denis, D.; French, C.C.; Gregory, A.M. A Systematic Review of Variables Associated with Sleep Paralysis. Sleep. Med. Rev. 2018, 38, 141–157. [Google Scholar] [CrossRef]
- Sharpless, B.A.; Barber, J.P. Lifetime Prevalence Rates of Sleep Paralysis: A Systematic Review. Sleep. Med. Rev. 2011, 15, 311–315. [Google Scholar] [CrossRef]
- Campillo-Ferrer, T.; Alcaraz-Sánchez, A.; Demšar, E.; Wu, H.P.; Dresler, M.; Windt, J.; Blanke, O. Out-of-Body Experiences in Relation to Lucid Dreaming and Sleep Paralysis: A Theoretical Review and Conceptual Model. Neurosci. Biobehav. Rev. 2024, 163, 105770. [Google Scholar] [CrossRef]
- Auerbach, R.P.; Mortier, P.; Bruffaerts, R.; Alonso, J.; Benjet, C.; Cuijpers, P.; Demyttenaere, K.; Ebert, D.D.; Green, J.G.; Hasking, P.; et al. The WHO World Mental Health Surveys International College Student Project: Prevalence and Distribution of Mental Disorders on Behalf of the WHO WMH-ICS Collaborators HHS Public Access. J. Abnorm. Psychol. 2018, 127, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Gardani, M.; Bradford, D.R.R.; Russell, K.; Allan, S.; Beattie, L.; Ellis, J.G.; Akram, U. A Systematic Review and Meta-Analysis of Poor Sleep, Insomnia Symptoms and Stress in Undergraduate Students. Sleep. Med. Rev. 2022, 61, 101565. [Google Scholar] [CrossRef] [PubMed]
- Wróbel-Knybel, P.; Flis, M.; Rog, J.; Jalal, B.; Karakuła-Juchnowicz, H. Risk Factors of Sleep Paralysis in a Population of Polish Students. BMC Psychiatry 2022, 22, 383. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Cellini, N. Lifetime Prevalence and Characteristics of Sleep Paralysis in Italian University Students Population. Sleep. Med. 2024, 122, 106–112. [Google Scholar] [CrossRef]
- Duarte, J.M.; Lisi, G.R.; Carroll, B.T.; Garro, M.F.; Appiani, F.J. The Prevalence of Sleep Paralysis in Medical Students in Buenos Aires, Argentina. J. Neurosci. Rural. Pract. 2023, 14, 272–275. [Google Scholar] [CrossRef]
- Benham, G. Sleep Paralysis in College Students. J. Am. Coll. Health 2022, 70, 1286–1291. [Google Scholar] [CrossRef]
- Wróbel-Knybel, P.; Karakuła-Juchnowicz, H.; Flis, M.; Rog, J.; Hinton, D.E.; Boguta, P.; Jalal, B. Prevalence and Clinical Picture of Sleep Paralysis in a Polish Student Sample. Int. J. Environ. Res. Public Health 2020, 17, 3529. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, B.A.; Kliková, M. Clinical Features of Isolated Sleep Paralysis. Sleep. Med. 2019, 58, 102–106. [Google Scholar] [CrossRef]
- Johnson, C. Sleep Paralysis: A Brief Overview of the Intersections of Neurophysiology and Culture. Am. J. Psychiatry Resid. J. 2023, 18, 2–5. [Google Scholar] [CrossRef]
- Rauf, B.; Sharpless, B.A.; Denis, D.; Perach, R.; Madrid-Valero, J.J.; French, C.C.; Gregory, A.M. Isolated Sleep Paralysis: Clinical Features, Perception of Aetiology, Prevention and Disruption Strategies in a Large International Sample. Sleep. Med. 2023, 104, 105–112. [Google Scholar] [CrossRef]
- Denis, D. Relationships between Sleep Paralysis and Sleep Quality: Current Insights. Nat. Sci. Sleep. 2018, 10, 355–367. [Google Scholar] [CrossRef]
- Lišková, M.; Janečková, D.; Klůzová Kráčmarová, L.; Mladá, K.; Bušková, J. The Occurrence and Predictive Factors of Sleep Paralysis in University Students. Neuropsychiatr. Dis. Treat. 2016, 12, 2957–2962. [Google Scholar] [CrossRef]
- Munezawa, T.; Kaneita, Y.; Osaki, Y.; Kanda, H.; Ohtsu, T.; Suzuki, H.; Minowa, M.; Suzuki, K.; Higuchi, S.; Mori, J.; et al. Nightmare and Sleep Paralysis among Japanese Adolescents: A Nationwide Representative Survey. Sleep. Med. 2011, 12, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wu, T.; Pi, G. Sleep Paralysis in Chinese Adolescents: A Representative Survey. Sleep. Biol. Rhythms 2014, 12, 46–52. [Google Scholar] [CrossRef]
- Manni, R.; Toscano, G.; Terzaghi, M. Therapeutic Symptomatic Strategies in the Parasomnias. Curr. Treat. Options Neurol. 2018, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Palagini, L.; Baglioni, C.; Ciapparelli, A.; Gemignani, A.; Riemann, D. REM Sleep Dysregulation in Depression: State of the Art. Sleep. Med. Rev. 2013, 17, 377–390. [Google Scholar] [CrossRef]
- Kierlin, L.; Littner, M.R. Parasomnias and Antidepressant Therapy: A Review of the Literature. Front. Psychiatry 2011, 2, 71. [Google Scholar] [CrossRef]
- Sohi, M.; Jain, L.; Ang-Rabanes, M.; Mogallapu, R. Sertraline-Induced Sleep Paralysis: A Case Report. Cureus 2023, 15, e49014. [Google Scholar] [CrossRef]
- Thorpy, M.J.; Bogan, R.K. Update on the Pharmacologic Management of Narcolepsy: Mechanisms of Action and Clinical Implications. Sleep. Med. 2020, 68, 97–109. [Google Scholar] [CrossRef]
- Bassetti, C.L.A.; Adamantidis, A.; Burdakov, D.; Han, F.; Gay, S.; Kallweit, U.; Khatami, R.; Koning, F.; Kornum, B.R.; Lammers, G.J.; et al. Narcolepsy—Clinical Spectrum, Aetiopathophysiology, Diagnosis and Treatment. Nat. Rev. Neurol. 2019, 15, 519–539. [Google Scholar] [CrossRef]
- Nyberg, F. Structural Plasticity of the Brain to Psychostimulant Use. Neuropharmacology 2014, 87, 115–124. [Google Scholar] [CrossRef]
- Brooks, P.L.; Peever, J.H. Identification of the Transmitter and Receptor Mechanisms Responsible for REM Sleep Paralysis. J. Neurosci. 2012, 32, 9785–9795. [Google Scholar] [CrossRef]
- Jalal, B. The Neuropharmacology of Sleep Paralysis Hallucinations: Serotonin 2A Activation and a Novel Therapeutic Drug. Psychopharmacology 2018, 235, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V. The Pharmacology of Amphetamine and Methylphenidate: Relevance to the Neurobiology of Attention-Deficit/Hyperactivity Disorder and Other Psychiatric Comorbidities. Neurosci. Biobehav. Rev. 2018, 87, 255–270. [Google Scholar] [CrossRef] [PubMed]
- van der Ham, M.; Bijlenga, D.; Böhmer, M.; Beekman, A.T.F.; Kooij, S. Sleep Problems in Adults With ADHD: Prevalences and Their Relationship With Psychiatric Comorbidity. J. Atten. Disord. 2024, 28, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Čekanauskaitė, A.; Peičius, E.; Urbonas, G.; Lukaševičienė, V. NEBIOMEDICININIŲ MOKSLINIŲ TYRIMŲ, KURIŲ OBJEKTAS YRA ŽMOGAUS SVEIKATA, ETINIAI PRINCIPAI. Public Health 2021, 2, 72–76. [Google Scholar]
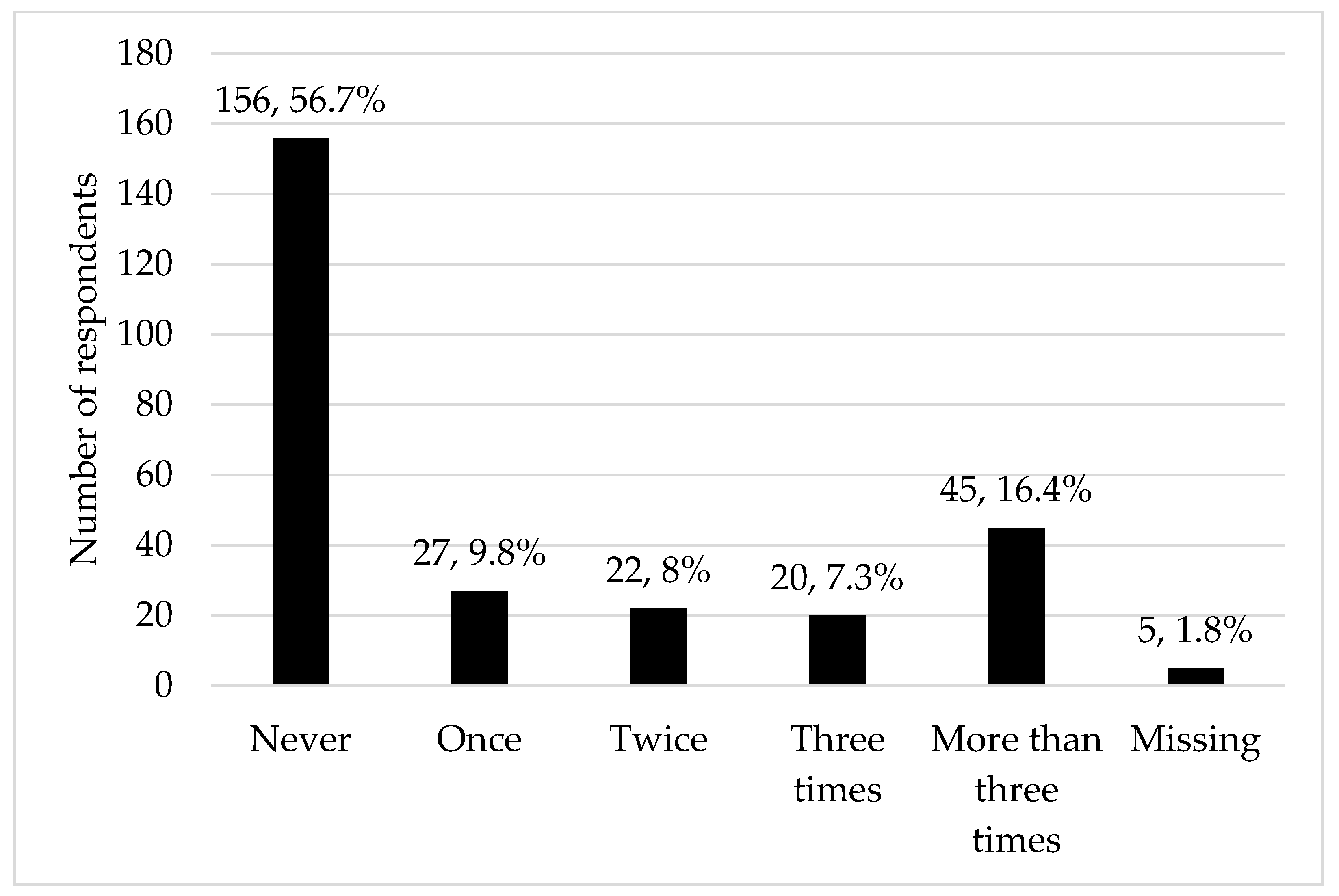
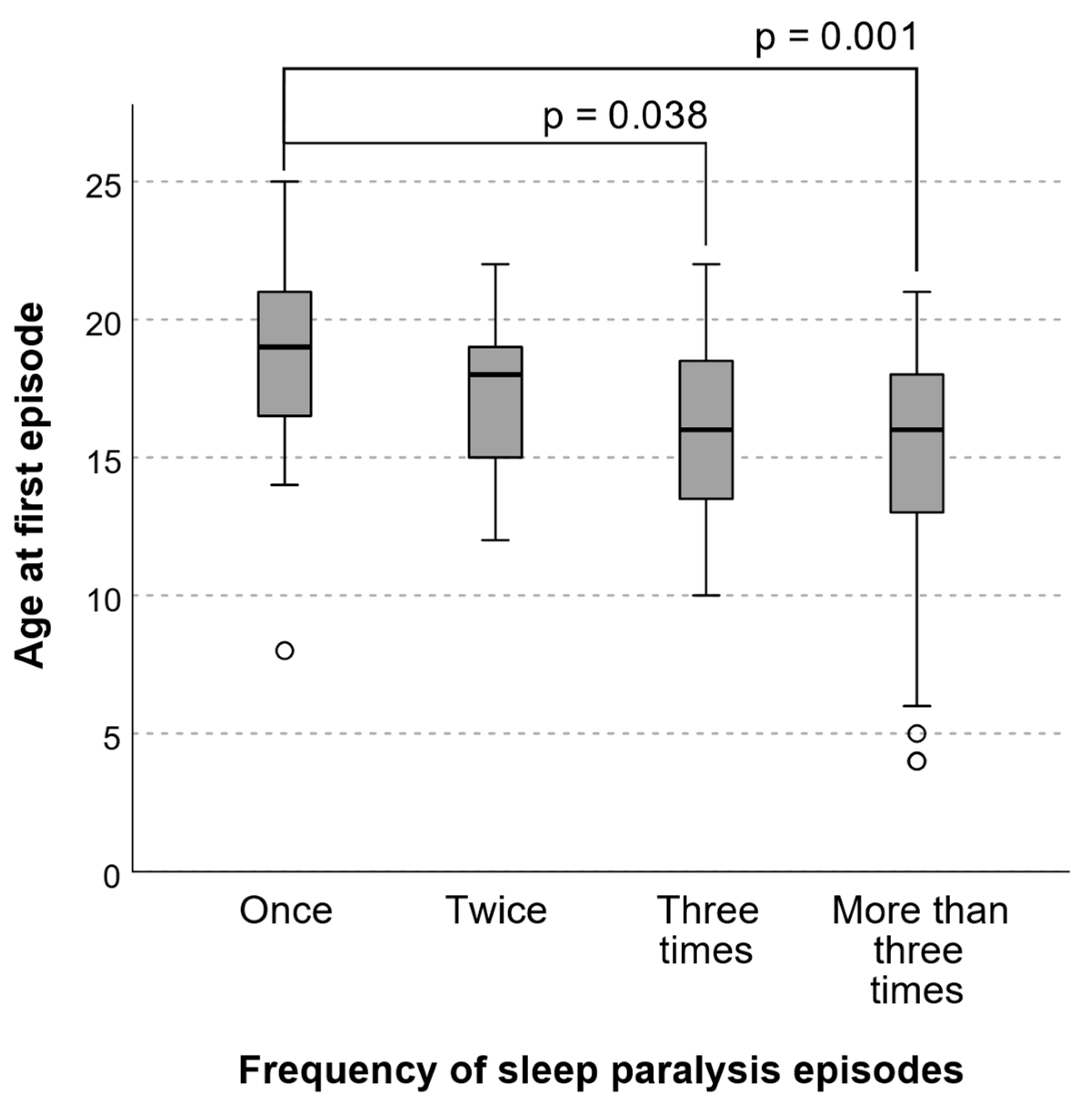
| Characteristic | n, % or Mean, SD |
|---|---|
| Age, years | 22.9 ± 4.7 |
| Sex | |
| Male | 29, 10.5% |
| Female | 240, 87.3% |
| Other | 6, 2.2% |
| Household situation | |
| Lives alone | 56, 20.4% |
| Lives with a partner | 79, 28.7% |
| Lives with parents | 80, 29.1% |
| Lives with family | 8, 2.9% |
| Lives with friends | 38, 13.8% |
| Other | 14, 5.1% |
| Study type | |
| Undergraduate | 170, 61.8% |
| Masters | 58, 21.1% |
| PhD | 1, 0.4% |
| Continuous studies (medicine, law) | 46, 16.7% |
| Study year | |
| 1 | 50, 18.2% |
| 2 | 110, 40% |
| 3 | 54, 19.6% |
| 4 | 51, 18.5% |
| 5 | 9, 3.3% |
| 6 | 1, 0.4% |
| Study program | |
| Life sciences | 8, 2.9% |
| Technology sciences | 6, 2.2% |
| Medicine and health sciences | 85, 30.9% |
| Social sciences | 150, 54.5% |
| Humanitarian sciences | 26, 9.5% |
| Employment during studies | |
| Yes | 151, 54.9% |
| No | 124, 45.1% |
| Place of residence | |
| Village | 11, 4.0% |
| Town | 10, 3.6% |
| City | 60, 21.8% |
| Large city | 194, 70.5% |
| Characteristic | n, % or Mean, SD |
|---|---|
| Current smoker status (cigarettes, electronic cigarettes, heated tobacco, pipe, hookah) | |
| Yes | 110, 40.0% |
| No | 165, 60.0% |
| Wakes up to smoke at night | |
| Yes | 12, 10.9% |
| No | 98, 89.1% |
| Current use of any amount of alcohol | |
| Yes | 182, 66.2% |
| No | 93, 33.8% |
| Mean weekly standard units of alcohol consumed | 2.2 ± 2.3 |
| Any psychoactive substance use | |
| Never | 175, 63.6% |
| Yes, during lifetime | 63, 22.9% |
| Yes, in the past year | 27, 9.8% |
| Yes, in the past month | 10, 3.6% |
| Substances used (use in lifetime, reported by respondents in free text): | |
| Cannabis and synthetic cannabinoids | 78, 78.0% |
| Hallucinogens (e.g., LSD, DMT, psychedelic mushrooms) | 17, 17.0% |
| Stimulants (e.g., cocaine, amphetamine, methamphetamine) | 19, 19.0% |
| Current use of sleep medication | |
| No | 246, 89.5% |
| Yes | 29, 10.5% |
| Sleep medication use frequency | |
| No medication | 246, 89.5% |
| Less than several times per month | 10, 3.6% |
| Several times per month | 9, 3.3% |
| Several times per week | 3, 1.1% |
| Every day | 7, 2.5% |
| Current use of sedatives/anxiolytics | |
| No | 232, 84.4% |
| Yes | 43, 15.6% |
| Sedative/anxiolytic use frequency | |
| No medication | 232, 84.4% |
| Less than several times per month | 17, 6.2% |
| Several times per month | 12, 4.4% |
| Several times per week | 6, 2.2% |
| Every day | 6, 2.2% |
| Missing | 2, 0.7% |
| Current use of antidepressants | |
| No | 256, 93.1% |
| Yes | 19, 6.9% |
| Antidepressant use frequency | |
| No medication | 256, 93.1% |
| Less than several times per month | 0, 0 |
| Several times per month | 0, 0 |
| Several times per week | 0, 0 |
| Every day | 19, 6.9% |
| Characteristic | n, % or Mean, SD |
|---|---|
| Any current comorbid disorder | |
| No | 179, 65.1% |
| Yes | 61, 22.2% |
| Unknown/cannot say | 35, 12.7% |
| Any current sleep disorder | |
| No | 254, 92.4% |
| Yes | 8, 2.9% (5 insomnia, 1 periodic limb movement disorder, 2 unspecified) |
| Unknown/cannot say | 13, 4.7% |
| Usual nightmare frequency | |
| Never | 19, 6.9% |
| Less than twice a year | 47, 17.1% |
| About twice a year | 94, 34.2% |
| Around twice per month | 88, 32.0% |
| Around twice per week | 24, 8.7% |
| Every night | 3, 1.1% |
| Usual time to sleep onset after going to bed | |
| <5 min | 9, 3.3% |
| 5 to 10 min | 50, 18.2% |
| 10 to 20 min | 80, 29.1% |
| 20 to 30 min | 53, 19.3% |
| 30 to 60 min | 62, 22.5% |
| >60 min | 16, 5.8% |
| Missing | 5, 1.8% |
| Self-rated health | 7.6 ± 1.4 |
| Self-rated quality of sleep | 7.2 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumbis, G.; Puteikis, K.; Mameniškienė, R. Sleep Paralysis Among Higher Education Students: A Possible Role of Antidepressant and Recreational Stimulant Use. Medicina 2025, 61, 1844. https://doi.org/10.3390/medicina61101844
Gumbis G, Puteikis K, Mameniškienė R. Sleep Paralysis Among Higher Education Students: A Possible Role of Antidepressant and Recreational Stimulant Use. Medicina. 2025; 61(10):1844. https://doi.org/10.3390/medicina61101844
Chicago/Turabian StyleGumbis, Gediminas, Kristijonas Puteikis, and Rūta Mameniškienė. 2025. "Sleep Paralysis Among Higher Education Students: A Possible Role of Antidepressant and Recreational Stimulant Use" Medicina 61, no. 10: 1844. https://doi.org/10.3390/medicina61101844
APA StyleGumbis, G., Puteikis, K., & Mameniškienė, R. (2025). Sleep Paralysis Among Higher Education Students: A Possible Role of Antidepressant and Recreational Stimulant Use. Medicina, 61(10), 1844. https://doi.org/10.3390/medicina61101844






