Prognostic Impact of Concomitant Beta-Blocker Use on Survival in EGFR-Mutant Metastatic Non-Small Cell Lung Cancer Patients Treated with Erlotinib
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TKI | Tyrosine kinase inhibitor |
| mNSCLC | metastatic non-small cell lung cancer |
| PFS | Progression-free survival |
| OS | Overall Survival |
References
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef] [PubMed]
- Midha, A.; Dearden, S.; McCormack, R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am. J. Cancer Res. 2015, 5, 2892. [Google Scholar] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lee, C.-H.; Ko, J.-C.; Chang, L.-Y.; Lee, M.-C.; Zhang, J.-F.; Wang, J.-Y.; Shih, J.-Y.; Yu, C.-J. Effect of β-blocker in treatment-naïve patients with advanced lung adenocarcinoma receiving first-generation EGFR-TKIs. Front. Oncol. 2020, 10, 583529. [Google Scholar] [CrossRef]
- Chaudhary, K.R.; Yan, S.X.; Heilbroner, S.P.; Sonett, J.R.; Stoopler, M.B.; Shu, C.; Halmos, B.; Wang, T.J.C.; Hei, T.K.; Cheng, S.K. Effects of β-adrenergic antagonists on chemoradiation therapy for locally advanced non-small cell lung cancer. J. Clin. Med. 2019, 8, 575. [Google Scholar] [CrossRef]
- Diaconu, C.C.; Marcu, D.R.; Bratu, O.G.; Stanescu, A.M.A.; Gheorghe, G.; Hlescu, A.A.; Mischianu, D.L.; Manea, M. Beta-blockers in cardiovascular therapy: A review. J. Mind Med. Sci. 2019, 6, 216–223. [Google Scholar] [CrossRef]
- Yap, A.; Lopez-Olivo, M.; Dubowitz, J.; Pratt, G.; Hiller, J.; Gottumukkala, V.; Sloan, E.; Riedel, B.; Schier, R. Effect of beta-blockers on cancer recurrence and survival: A meta-analysis of epidemiological and perioperative studies. Br. J. Anaesth. 2018, 121, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, M.; Eryılmaz, M.K.; Koçak, M.Z.; Er, M.M.; Hendem, E.; Demirkıran, A.; Araz, M.; Artaç, M. The effect of concomitant beta-blocker use on survival in patients with metastatic renal cell carcinoma treated with a vascular endothelial growth factor receptor inhibitors in the first line. Eur. J. Clin. Pharmacol. 2024, 80, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Kocak, M.Z.; Er, M.; Ugrakli, M.; Hendem, E.; Araz, M.; Eryilmaz, M.K.; Artac, M. Could the concomitant use of beta blockers with bevacizumab improve survival in metastatic colon cancer? Eur. J. Clin. Pharmacol. 2023, 79, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Aydiner, A.; Ciftci, R.; Karabulut, S.; Kilic, L. Does beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pac. J. Cancer Prev. 2013, 14, 6109–6114. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, I.; Matsukawa, A.; Parizi, M.K.; Miszczyk, M.; Fazekas, T.; Schulz, R.J.; Mancon, S.; Litterio, G.; Laukhtina, E.; Kawada, T.; et al. The impact of concomitant medications on the overall survival of patients treated with systemic therapy for advanced or metastatic renal cell carcinoma: A systematic review and meta-analysis. Clin. Genitourin. Cancer 2024, 22, 102237. [Google Scholar] [CrossRef] [PubMed]
- Fiala, O.; Hošek, P.; Tkadlecová, M.; Melichar, B.; Zemánková, A.; Kopecký, J.; Vočka, M.; Matějů, M.; Lohynská, R.; Šiková, D.; et al. Impact of concomitant use of proton pump inhibitors or cardiovascular medication on survival outcomes of patients with metastatic renal cell carcinoma treated with nivolumab. Clin. Exp. Metastasis 2025, 42, 43. [Google Scholar] [CrossRef] [PubMed]
- Melhem-Bertrandt, A.; Mac Gregor, M.C.; Lei, X.; Brown, E.N.; Lee, R.T.; Meric-Bernstam, F.; Sood, A.K.; Conzen, S.D.; Hortobagyi, G.N.; Gonzalez-Angulo, A.-M. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2011, 29, 2645–2652. [Google Scholar] [PubMed]
- De Giorgi, V.; Grazzini, M.; Gandini, S.; Benemei, S.; Lotti, T.; Marchionni, N.; Geppetti, P. Treatment with β-blockers and reduced disease progression in patients with thick melanoma. Arch. Intern. Med. 2011, 171, 779–781. [Google Scholar] [CrossRef] [PubMed]
- Grytli, H.H.; Fagerland, M.W.; Fosså, S.D.; Taskén, K.A. Association between use of β-blockers and prostate cancer–specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur. Urol. 2014, 65, 635–641. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 103) | No Beta-Blocker (n = 68) | Beta-Blocker (n = 35) | p-Value |
|---|---|---|---|---|
| Age Group | 0.328 | |||
| <65 | 54 (52.4%) | 38 (55.9%) | 16 (45.7%) | |
| ≥65 | 49 (47.6%) | 30 (44.1%) | 19 (54.3%) | |
| Sex | 0.984 | |||
| Female | 59 (57.3%) | 39 (57.4%) | 20 (57.1%) | |
| Male | 44 (42.7%) | 29 (42.6%) | 15 (42.9%) | |
| Comorbid Conditions | 0.005 | |||
| Absent | 58 (56.3%) | 45 (66.2%) | 13 (37.1%) | |
| Present | 45 (43.7%) | 23 (33.8%) | 22 (62.9%) | |
| Smoking History | 0.924 | |||
| Never-smoker | 70 (68.0%) | 46 (67.6%) | 24 (68.6%) | |
| Smoker | 33 (32.0%) | 22 (32.4%) | 11 (31.4%) | |
| ECOG at Diagnosis | 0.548 | |||
| ECOG 0 | 40 (38.8%) | 25 (36.8%) | 15 (42.9%) | |
| ECOG ≥ 1 | 63 (61.2%) | 43 (63.2%) | 20 (57.1%) | |
| Metastasis at Diagnosis | <0.001 | |||
| Non-metastatic | 18 (17.5%) | 5 (7.4%) | 13 (37.1%) | |
| Metastatic | 85 (82.5%) | 63 (92.6%) | 22 (62.9%) | |
| Primary Tumor Surgery | 0.004 | |||
| No | 89 (86.4%) | 64 (94.1%) | 25 (71.4%) | |
| Yes | 14 (13.6%) | 4 (5.9%) | 10 (28.6%) | |
| Bone Metastasis | 0.060 | |||
| Absent | 40 (38.8%) | 22 (32.4%) | 18 (51.4%) | |
| Present | 63 (61.2%) | 46 (67.6%) | 17 (48.6%) | |
| Liver Metastasis | 0.007 | |||
| Absent | 86 (83.5%) | 52 (76.5%) | 34 (97.1%) | |
| Present | 17 (16.5%) | 16 (23.5%) | 1 (2.9%) | |
| Adrenal Metastasis | 0.209 | |||
| Absent | 81 (78.6%) | 51 (75.0%) | 30 (85.7%) | |
| Present | 22 (21.4%) | 17 (25.0%) | 5 (14.3%) | |
| Brain Metastasis | 0.663 | |||
| Absent | 86 (83.5%) | 56 (82.4%) | 30 (85.7%) | |
| Present | 17 (16.5%) | 12 (17.6%) | 5 (14.3%) | |
| 1st-Line Treatment | 0.780 | |||
| Chemotherapy | 52 (50.5%) | 35 (51.5%) | 17 (48.6%) | |
| Erlotinib | 51 (49.5%) | 33 (48.5%) | 18 (51.4%) | |
| Response to TKI | 0.072 | |||
| Non-responsive | 45 (43.7%) | 34 (50.0%) | 11 (31.4%) | |
| Responsive | 58 (56.3%) | 34 (50.0%) | 24 (68.6%) | |
| Post-TKI Treatment | 0.493 | |||
| No | 86 (83.5%) | 58 (85.3%) | 28 (80.0%) | |
| Yes | 17 (16.5%) | 10 (14.7%) | 7 (20.0%) |
| Variable | Median PFS (Months) | 95% Confidence Interval | p-Value | Variable | Median OS (Months) | 95% Confidence Interval | p-Value |
|---|---|---|---|---|---|---|---|
| Beta-Blocker Use | 0.003 | Beta-Blocker Use | 0.010 | ||||
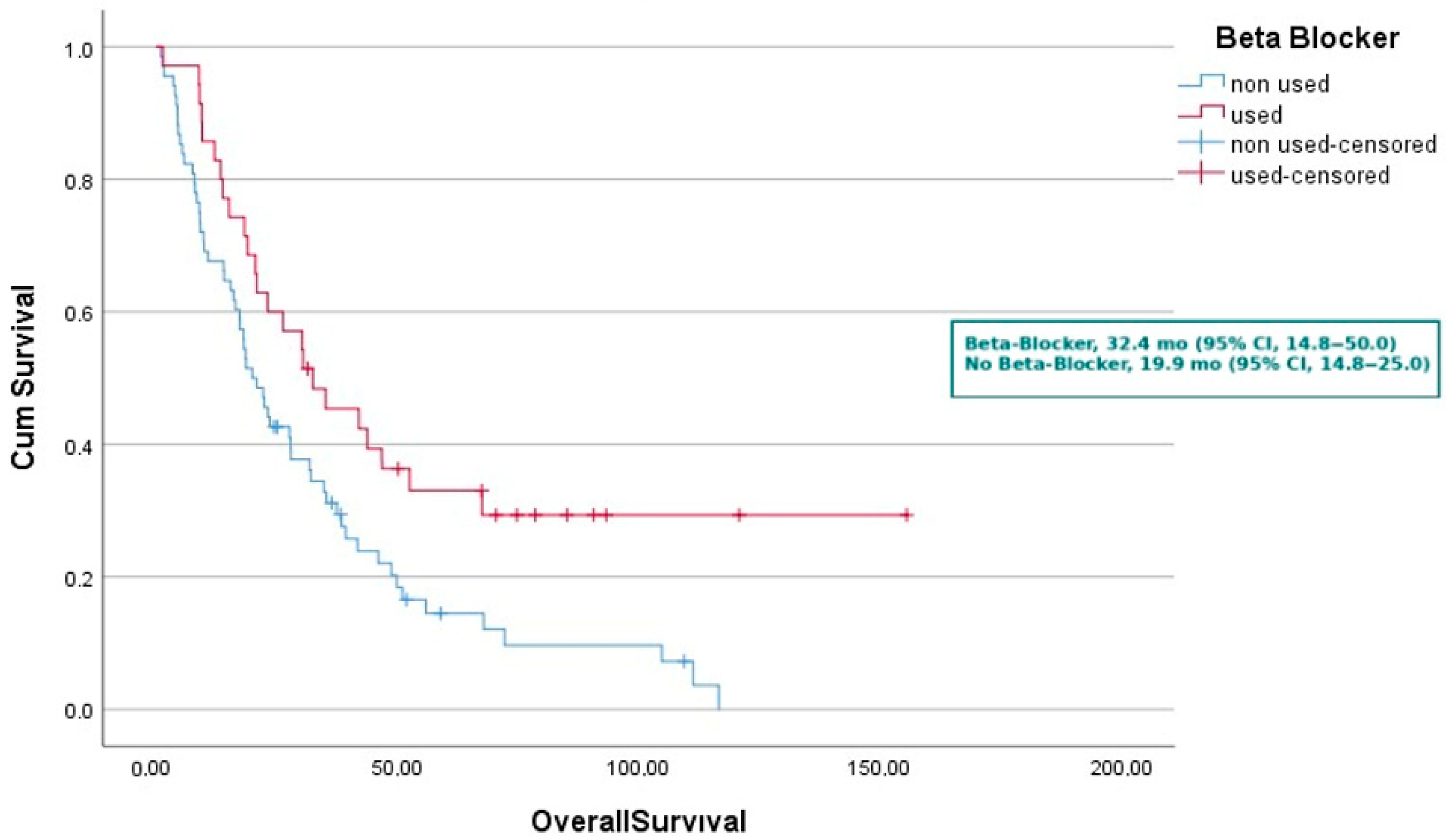
| Not used | 9.7 | 6.7–12.7 | Not used | 19.9 | 14.8–25.0 | ||
| Used | 21.4 | 13.1–29.7 | Used | 32.4 | 14.8–50.0 | ||
| Age Group | 0.150 | Age Group | 0.723 | ||||
| <65 years | 9.4 | 5.5–13.3 | <65 years | 23.5 | 12.2–34.8 | ||
| ≥65 years | 17 | 11.6–22.3 | ≥65 years | 23.0 | 14.4–31.6 | ||
| Sex | 0.531 | Sex | 0.209 | ||||
| Female | 15.4 | 10.2–20.6 | Female | 34.7 | 20.5–48.9 | ||
| Male | 11.3 | 4.7–18 | Male | 18.9 | 13.3–24.5 | ||
| Comorbid Conditions | 0.367 | Comorbid Conditions | 0.464 | ||||
| Absent | 12.4 | 6.8–18 | Absent | 30.2 | 16.9–43.5 | ||
| Present | 12.8 | 36–22 | Present | 20.7 | 16.0–25.4 | ||
| Smoking History | 0.150 | Smoking History | 0.027 | ||||
| Never-smoker | 15.4 | 10.7–20.1 | Never-smoker | 30.2 | 14.3–46.1 | ||
| Smoker | 9.2 | 1.7–16.6 | Smoker | 18.2 | 8.6–27.8 | ||
| ECOG at Diagnosis | 0.106 | ECOG at Diagnosis | 0.022 | ||||
| ECOG 0 | 17.2 | 6.6–27.8 | ECOG 0 | 39.2 | 23.6–54.9 | ||
| ECOG ≥ 1 | 12.4 | 9–15.7 | ECOG ≥ 1 | 20.5 | 15.1–25.9 | ||
| Metastasis at Diagnosis | 0.034 | Metastasis at Diagnosis | 0.035 | ||||
| Non-metastatic | 22.5 | 0–71.9 | Non-metastatic | 30.2 | 0–75 | ||
| Metastatic | 11.4 | 8.6–14.1 | Metastatic | 23.0 | 14.1–31.9 | ||
| Primary Tumor Surgery | 0.070 | Primary Tumor Surgery | 0.033 | ||||
| No | 12.2 | 7.8–16.7 | No | 22.3 | 14.8–29.8 | ||
| Yes | 22.5 | 0–68.9 | Yes | 31.8 | 7.1–56.4 | ||
| Bone Metastasis | <0.001 | Bone Metastasis | <0.001 | ||||
| Absent | 24.4 | 15.4–33.4 | Absent | 52.5 | 17.5–87.4 | ||
| Present | 9.8 | 7.4–12.2 | Present | 18.2 | 14.2–22.2 | ||
| Liver Metastasis | 0.136 | Liver Metastasis | 0.016 | ||||
| Absent | 14.3 | 9.7–18.8 | Absent | 27.8 | 18.6–37.0 | ||
| Present | 6.9 | 0–14.1 | Present | 8.4 | 0–16.8 | ||
| Adrenal Metastasis | 0.774 | Adrenal Metastasis | 0.789 | ||||
| Absent | 12.4 | 7.1–17.6 | Absent | 23.0 | 13.5–32.6 | ||
| Present | 12.5 | 0–26.2 | Present | 23.1 | 8.3–37.9 | ||
| Brain Metastasis | 0.398 | Brain Metastasis | 0.012 | ||||
| Absent | 12.5 | 8–17 | Absent | 26.3 | 19.0–33.5 | ||
| Present | 10.1 | 0–24.2 | Present | 9.7 | 0–23.7 | ||
| 1st-Line Treatment | 0.590 | 1st-Line Treatment | 0.264 | ||||
| Chemotherapy | 11.3 | 6.6–16.1 | Chemotherapy | 20.5 | 14.6–26.4 | ||
| Erlotinib | 16.3 | 9.8–22.8 | Erlotinib | 27.9 | 15.2–40.6 | ||
| Response to TKI | <0.001 | Response to TKI | <0.001 | ||||
| Non-responsive | 4.2 | 3.6–4.8 | Non-responsive | 13.2 | 4.6–21.8 | ||
| Responsive | 22.5 | 16.1–28.9 | Responsive | 41.6 | 32.0–51.2 | ||
| Post-TKI Treatment | 0.031 | Post-TKI Treatment | 0.031 | ||||
| Not received | 11.4 | 6.3–16.4 | Not received | 20.7 | 15.7–25.8 | ||
| Received | 24.4 | 7.3–41.4 | Received | 48.7 | 18.9–78.4 | ||
| Median Progression Free Survival | Median Overall Survival | ||||||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Smoking History | 0.722 (0.463–1.127) | 0.152 | ||
| ECOG > 0 at Diagnosis | 0.701 (0.455–1.081) | 0.108 | ||
| Primary Tumor Surgery | 1.759 (0.947–3.268) | 0.074 | ||
| Beta-Blocker Use | 0.508 (0.321–0.803) | 0.004 | 0.508 (0.321–0.803) | 0.004 |
| Metastasis Status | 0.545 (0.309–0.962) | 0.036 | ||
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Smoking History | 1.685 (1.057–2.686) | 0.028 | ||
| ECOG > 0 at Diagnosis | 1.681 (1.072–2.637) | 0.024 | 1.644 (1.048–2.579) | 0.030 |
| Primary Tumor Surgery | 0.459 (0.220–0.956) | 0.037 | ||
| Beta-Blocker Use | 0.537 (0.333–0.866) | 0.011 | 0.548 (0.340–0.883) | 0.014 |
| Metastasis at diagnosis | 1.966 (1.038–3.722) | 0.038 | ||
| ECOG > 0 at Diagnosis | 1.644 (1.048–2.579) | 0.030 | ||
| Beta-Blocker Use | 0.548 (0.340–0.883) | 0.014 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıldız, O.; Aykut, T.; Kaya, B.E.; Genç, Ö.; Gürbüz, A.F.; Saçkan, F.; Eryılmaz, M.K.; Koçak, M.Z.; Araz, M.; Artaç, M. Prognostic Impact of Concomitant Beta-Blocker Use on Survival in EGFR-Mutant Metastatic Non-Small Cell Lung Cancer Patients Treated with Erlotinib. Medicina 2025, 61, 1843. https://doi.org/10.3390/medicina61101843
Yıldız O, Aykut T, Kaya BE, Genç Ö, Gürbüz AF, Saçkan F, Eryılmaz MK, Koçak MZ, Araz M, Artaç M. Prognostic Impact of Concomitant Beta-Blocker Use on Survival in EGFR-Mutant Metastatic Non-Small Cell Lung Cancer Patients Treated with Erlotinib. Medicina. 2025; 61(10):1843. https://doi.org/10.3390/medicina61101843
Chicago/Turabian StyleYıldız, Oğuzhan, Talat Aykut, Bahattin Engin Kaya, Ömer Genç, Ali Fuat Gürbüz, Fatih Saçkan, Melek Karakurt Eryılmaz, Mehmet Zahid Koçak, Murat Araz, and Mehmet Artaç. 2025. "Prognostic Impact of Concomitant Beta-Blocker Use on Survival in EGFR-Mutant Metastatic Non-Small Cell Lung Cancer Patients Treated with Erlotinib" Medicina 61, no. 10: 1843. https://doi.org/10.3390/medicina61101843
APA StyleYıldız, O., Aykut, T., Kaya, B. E., Genç, Ö., Gürbüz, A. F., Saçkan, F., Eryılmaz, M. K., Koçak, M. Z., Araz, M., & Artaç, M. (2025). Prognostic Impact of Concomitant Beta-Blocker Use on Survival in EGFR-Mutant Metastatic Non-Small Cell Lung Cancer Patients Treated with Erlotinib. Medicina, 61(10), 1843. https://doi.org/10.3390/medicina61101843






