CBCT-Based Online Adaptive, Ultra-Hypofractionated Radiotherapy for Prostate Cancer: First Clinical Experiences
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Preparation and CT Simulation
2.3. Treatment Planning
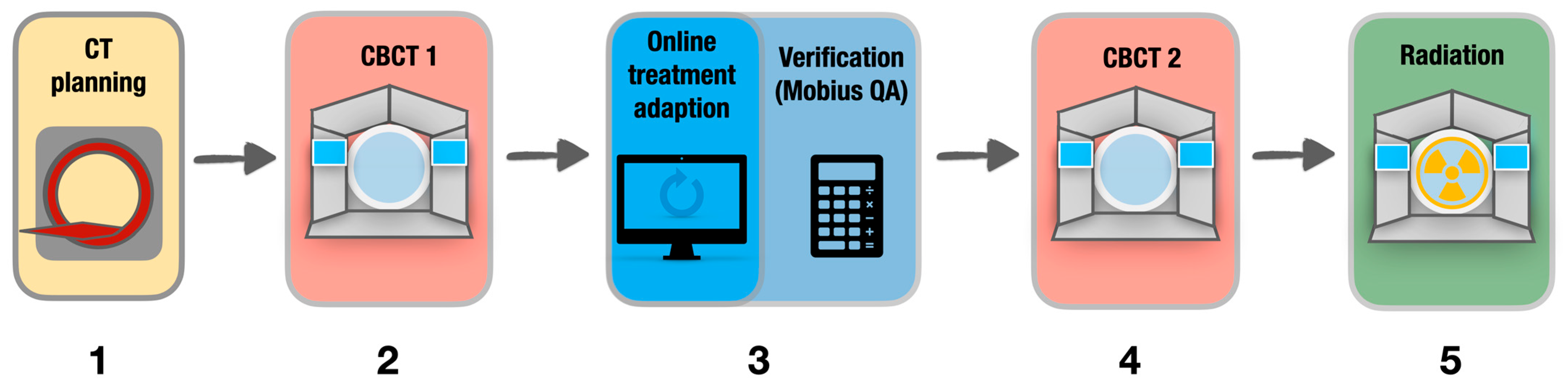
2.4. Online Adaptive Radiotherapy Workflow
2.5. Statistical Analysis and Visualization
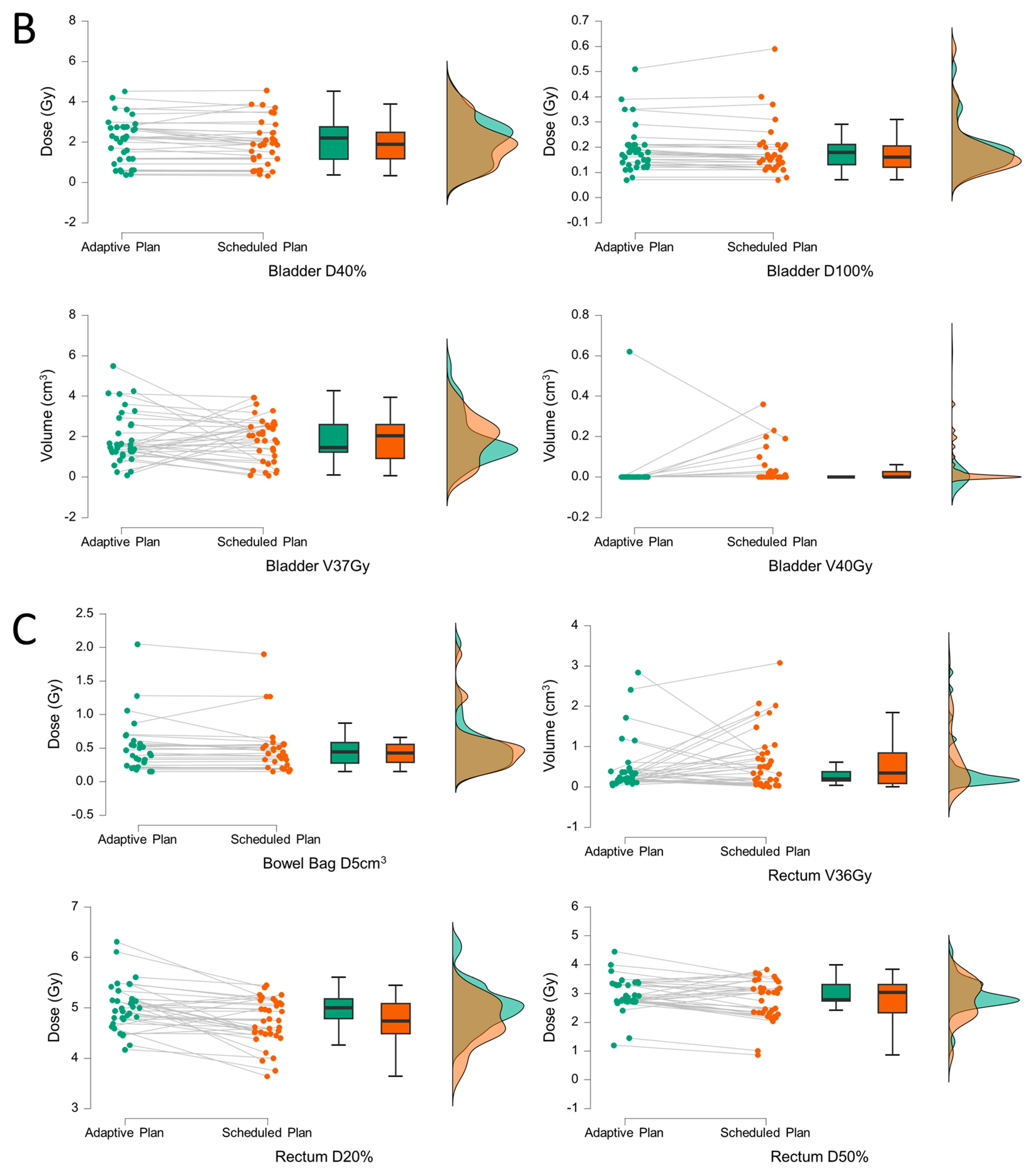
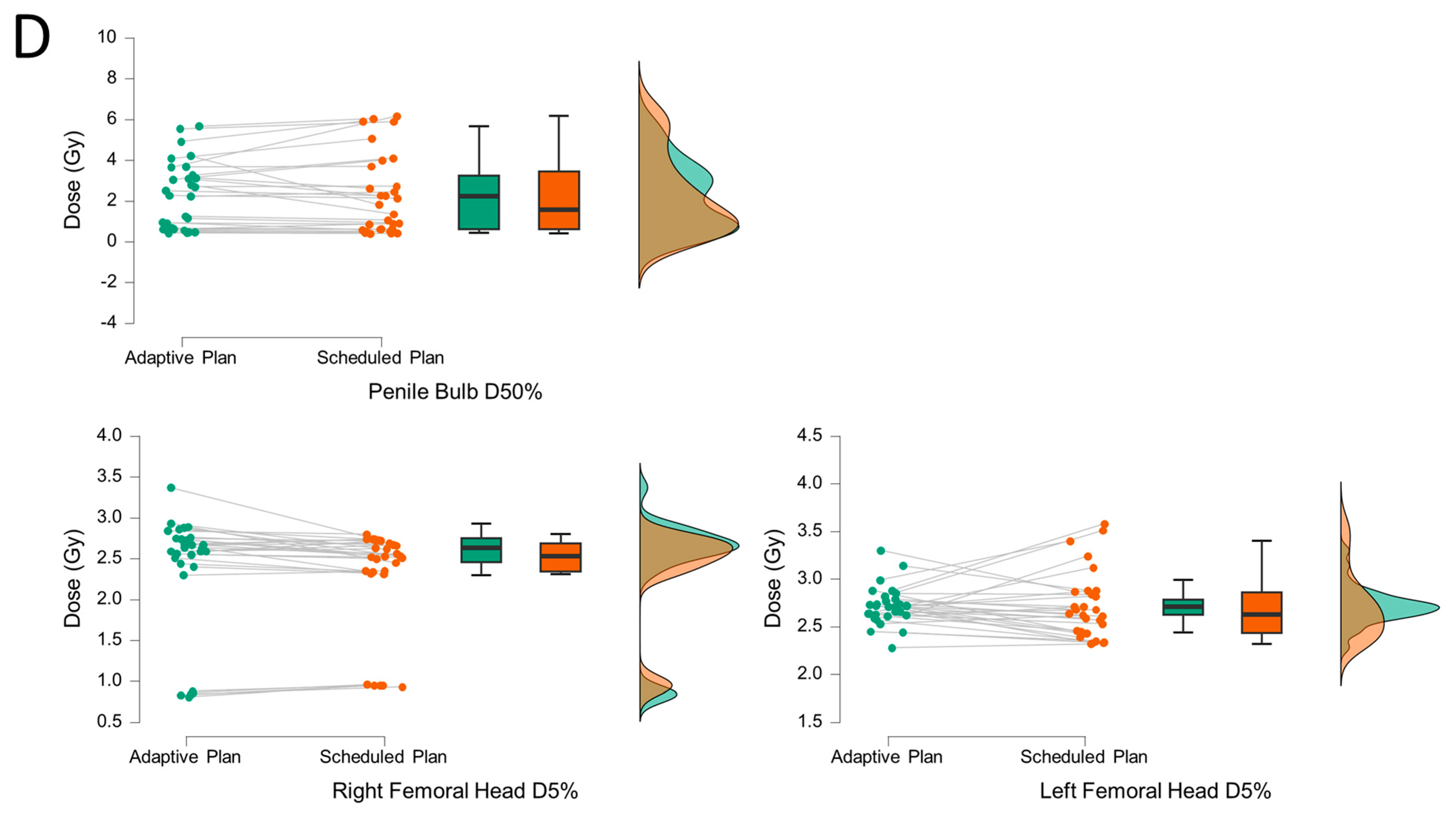
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CBCT | Cone beam computed tomography |
| CT | Computed tomography |
| CTCAE | Common Terminology for Adverse Events |
| CTV | Clinical target volume |
| DVH | Dose–volume histogram |
| GTV | Gross target volume |
| IMRT | Intensity-modulated radiotherapy |
| LINAC | Linear accelerator |
| OAR | Organ at risk |
| oART | Online-adaptive radiotherapy |
| PRO | Patient-reported outcome |
| PTV | Planning target volume |
| SIB | Simultaneous integrated boost |
| uhRT | Ultra-hypofractionated radiotherapy |
| VMAT | Volumetric modulated arc therapy |
Appendix A
Appendix A.1
| Target | Dose Per Fraction | Total Dose |
|---|---|---|
| CTV1 Prostata | 7.25 | 36.25 |
| CTV2 Boost (SIB) | 8.00 | 40.00 |
| Target/Organ at Risk | Dose/Volume Reference | Optimal | Mandatory | Priority |
|---|---|---|---|---|
| PTV1 7.25 Gy | D98% | >97% | ≥95% | 1 |
| D0.1cc | <42.8 Gy | ≤44 Gy | 1 | |
| V95% | ≥99% | ≥95% | 2 | |
| Dmax | ≤42.8 Gy | ≤44 Gy | 2 | |
| CTV1 7.25 Gy | V100% | ≥99% | ≥95% | 1 |
| D98% | >98% | ≥96% | 1 | |
| CTV2 (Boost) = PTV2 8.00 Gy | D0.1% | <105% | ≤107% | 1 |
| D98% | >98% | ≥95% | 1 | |
| D100% | >97% | ≥95% | 1 | |
| V95% | >99% | ≥95% | 2 | |
| D1% | <105% | ≤107% | 2 | |
| Rectum | V36 Gy | <0.8 cc | ≤1 cc | 1 |
| D50% | <15 Gy | ≤18 Gy | 2 | |
| D20% | <25 Gy | ≤29 Gy | 2 | |
| Bladder | V40 Gy | <1 cc | ≤1.5 cc | 1 |
| V37 Gy | <5 cc | ≤10 cc | 1 | |
| D40% | <15 Gy | ≤18 Gy | 2 | |
| D100% | <2.6 Gy | ≤3.6 Gy | 2 | |
| Bowel Bag | V30 Gy | <0.1 cc | ≤1 cc | 1 |
| D5% | <16 Gy | ≤18.10 Gy | 2 | |
| External | Dmax | <42.8 Gy | 2 | |
| Femoral head (L/R) | D5% | <13.5 Gy | ≤14.5 Gy | 2 |
| V30 Gy | < 9 cc | ≤10 cc | 3 | |
| Penile bulb | D50% | <27 Gy | ≤29.5 Gy | 2 |
Appendix A.2
| Target and OAR Volumes | |||||
|---|---|---|---|---|---|
| CTV1, i.e., Prostate + SV [cm3] | PTV1 [cm3] | CTV2 Boost, i.e., Prostate [cm3] | Bladder [cm3] | Rectum [cm3] | |
| Mean | 79.86 | 144.43 | 60.71 | 300.43 | 69.14 |
| Std. Deviation | 17.58 | 25.77 | 13.70 | 103.44 | 23.72 |
| Minimum | 60.00 | 114.00 | 51.00 | 148.00 | 32.00 |
| Maximum | 113.00 | 192.00 | 91.00 | 453.00 | 101.00 |
| Q1 | 68.50 | 129.00 | 54.50 | 230.00 | 57.00 |
| Median | 78.00 | 142.00 | 55.00 | 346.00 | 63.00 |
| Q3 | 85.50 | 152.50 | 59.50 | 348.00 | 87.00 |
Appendix A.3
| Descriptive Statistics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PTV1 D98% | PTV2 Boost D98% | PTV2 Boost D0.1ccm <107% | Bladder V37Gy | Bladder V40Gy | Bladder D100% | Bladder D40% | Rectum V36Gy | Rectum D20% | Rectum D50% | |
| Mean | 93.74 | 96.51 | 102.14 | 2.48 | 0.01 | 0.79 | 9.82 | 0.32 | 24.02 | 14.86 |
| Std. Deviation | 1.80 | 1.29 | 0.60 | 1.40 | 0.03 | 0.23 | 4.20 | 0.20 | 2.40 | 1.53 |
| Minimum | 90.30 | 94.88 | 101.30 | 1.11 | 0.00 | 0.47 | 3.01 | 0.12 | 19.85 | 13.58 |
| Maximum | 95.80 | 98.90 | 102.80 | 5.16 | 0.07 | 1.14 | 15.41 | 0.62 | 26.88 | 17.65 |
| Q1 | 93.25 | 95.95 | 101.70 | 1.72 | 0.00 | 0.64 | 7.44 | 0.15 | 23.32 | 13.71 |
| Median | 93.90 | 96.20 | 102.30 | 1.84 | 0.00 | 0.79 | 10.55 | 0.32 | 23.94 | 14.03 |
| Q3 | 94.85 | 96.85 | 102.60 | 2.89 | 5.00 × 10−3 | 0.94 | 12.46 | 0.45 | 25.41 | 15.67 |
| Bowel Bag V30Gy | Bowel Bag D5ccm | Penile bulb D50% | Femoral Head L D5% | Femoral Head L V30Gy | Femoral Head R D5% | Femoral Head R V30Gy | ||||
| Mean | 0.13 | 2.87 | 9.70 | 13.16 | 0.00 | 11.13 | 0.00 | |||
| Std. Deviation | 0.34 | 1.61 | 7.73 | 0.90 | 0.00 | 3.49 | 0.00 | |||
| Minimum | 0.00 | 1.87 | 2.11 | 12.07 | 0.00 | 4.03 | 0.00 | |||
| Maximum | 0.89 | 6.11 | 20.74 | 14.35 | 0.00 | 13.08 | 0.00 | |||
| Q1 | 0.00 | 2.15 | 3.67 | 12.61 | 0.00 | 12.08 | 0.00 | |||
| Median | 0.00 | 2.25 | 7.54 | 12.93 | 0.00 | 12.46 | 0.00 | |||
| Q3 | 0.00 | 2.51 | 15.29 | 13.87 | 0.00 | 12.69 | 0.00 | |||
Appendix A.4
| Target and Organ at Risk (OAR) Volumes (V) | |||||
|---|---|---|---|---|---|
| V CTV1 [cm3] | V PTV 1 [cm3] | V CTV 2 Boost [cm3] | V Bladder [cm3] | V Rectum [cm3] | |
| Mean | 88.14 | 157.57 | 60.84 | 259.42 | 64.44 |
| Std. Deviation | 22.79 | 32.96 | 15.19 | 115.76 | 27.11 |
| Minimum | 63.70 | 119.00 | 38.20 | 69.90 | 29.00 |
| Maximum | 164.00 | 266.00 | 107.00 | 589.00 | 149.00 |
| Q1 | 74.90 | 139.00 | 53.75 | 146.00 | 46.65 |
| Median | 81.80 | 149.00 | 57.50 | 267.00 | 56.30 |
| Q3 | 92.40 | 163.50 | 61.45 | 331.00 | 72.65 |
Appendix A.5
| Relative Volume Change (%) | |||||
|---|---|---|---|---|---|
| Bladder Volume | Rectum Volume | CTV1 Volume | PTV1 Volume | CTV2 (Boost) Volume | |
| Mean | −8.20 | −3.37 | 10.56 | 9.23 | 0.05 |
| Std. Deviation | 39.24 | 32.54 | 14.98 | 12.58 | 11.05 |
| Minimum | −84.57 | −53.84 | −7.56 | −6.34 | −25.10 |
| Maximum | 75.73 | 84.75 | 45.13 | 38.54 | 17.58 |
| 25th percentile | −30.76 | −24.22 | −0.30 | 0.35 | −5.45 |
| Median | −8.67 | −9.38 | 4.42 | 4.40 | 0.55 |
| 75th percentile | 16.64 | 14.85 | 19.70 | 15.65 | 6.88 |
Appendix A.6
| Descriptive Statistics | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bladder D40% | Bladder D100% | Bladder V40Gy | Bladder V37Gy | Bowel Bag V30Gy | Bowel Bag D5ccm | |||||||||||
| Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | |||||
| Std. Deviation | 1.12 | 1.12 | 0.09 | 0.10 | 0.10 | 0.08 | 1.27 | 1.11 | 0.07 | 0.01 | 0.40 | 0.39 | ||||
| Minimum | 0.37 | 0.33 | 0.07 | 0.07 | 0.00 | 0.00 | 0.09 | 0.06 | 0.00 | 0.00 | 0.15 | 0.15 | ||||
| Maximum | 4.52 | 4.56 | 0.51 | 0.59 | 0.62 | 0.36 | 5.50 | 3.93 | 0.39 | 0.06 | 2.05 | 1.90 | ||||
| Q1 | 1.15 | 1.16 | 0.13 | 0.12 | 0.00 | 0.00 | 1.23 | 0.92 | 0.00 | 0.00 | 0.28 | 0.29 | ||||
| Median | 2.20 | 1.90 | 0.18 | 0.16 | 0.00 | 0.00 | 1.46 | 2.05 | 0.00 | 0.00 | 0.44 | 0.43 | ||||
| Q3 | 2.75 | 2.48 | 0.21 | 0.21 | 0.00 | 0.03 | 2.58 | 2.58 | 0.00 | 0.00 | 0.58 | 0.55 | ||||
| Rectum D50% | Rectum D20% | Rectum V36Gy | Penile bulb D50% | Femoral head L D5% | Femoral Head L V30Gy | Femoral head R D5% | Femoral Head R V30Gy | |||||||||
| Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | Adaptive Plan | Scheduled Plan | |
| Mean | 2.94 | 2.79 | 5.03 | 4.71 | 0.46 | 0.64 | 2.22 | 2.24 | 2.72 | 2.71 | 0.00 | 0.00 | 2.38 | 2.30 | 0.00 | 0.00 |
| Std. Deviation | 0.59 | 0.71 | 0.46 | 0.46 | 0.65 | 0.74 | 1.65 | 1.96 | 0.20 | 0.35 | 0.00 | 0.00 | 0.73 | 0.63 | 0.00 | 0.00 |
| Minimum | 1.20 | 0.86 | 4.17 | 3.64 | 0.04 | 0.00 | 0.43 | 0.40 | 2.28 | 2.32 | 0.00 | 0.00 | 0.81 | 0.93 | 0.00 | 0.00 |
| Maximum | 4.45 | 3.83 | 6.31 | 5.45 | 2.84 | 3.08 | 5.67 | 6.17 | 3.30 | 3.58 | 0.00 | 0.00 | 3.37 | 2.80 | 0.00 | 0.00 |
| Q1 | 2.74 | 2.33 | 4.79 | 4.49 | 0.15 | 0.08 | 0.62 | 0.61 | 2.62 | 2.44 | 0.00 | 0.00 | 2.46 | 2.34 | 0.00 | 0.00 |
| Median | 2.78 | 3.04 | 5.00 | 4.74 | 0.20 | 0.35 | 2.25 | 1.58 | 2.71 | 2.63 | 0.00 | 0.00 | 2.63 | 2.53 | 0.00 | 0.00 |
| Q3 | 3.30 | 3.31 | 5.17 | 5.08 | 0.38 | 0.83 | 3.24 | 3.46 | 2.79 | 2.86 | 0.00 | 0.00 | 2.75 | 2.69 | 0.00 | 0.00 |
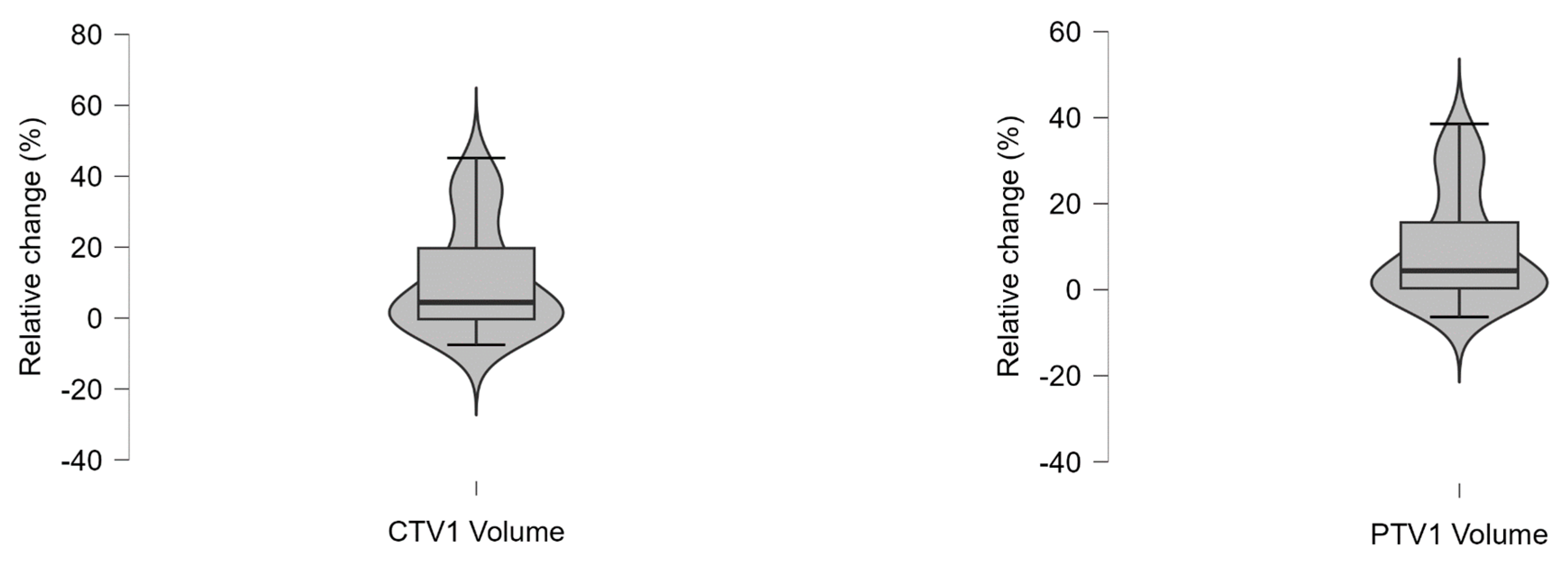
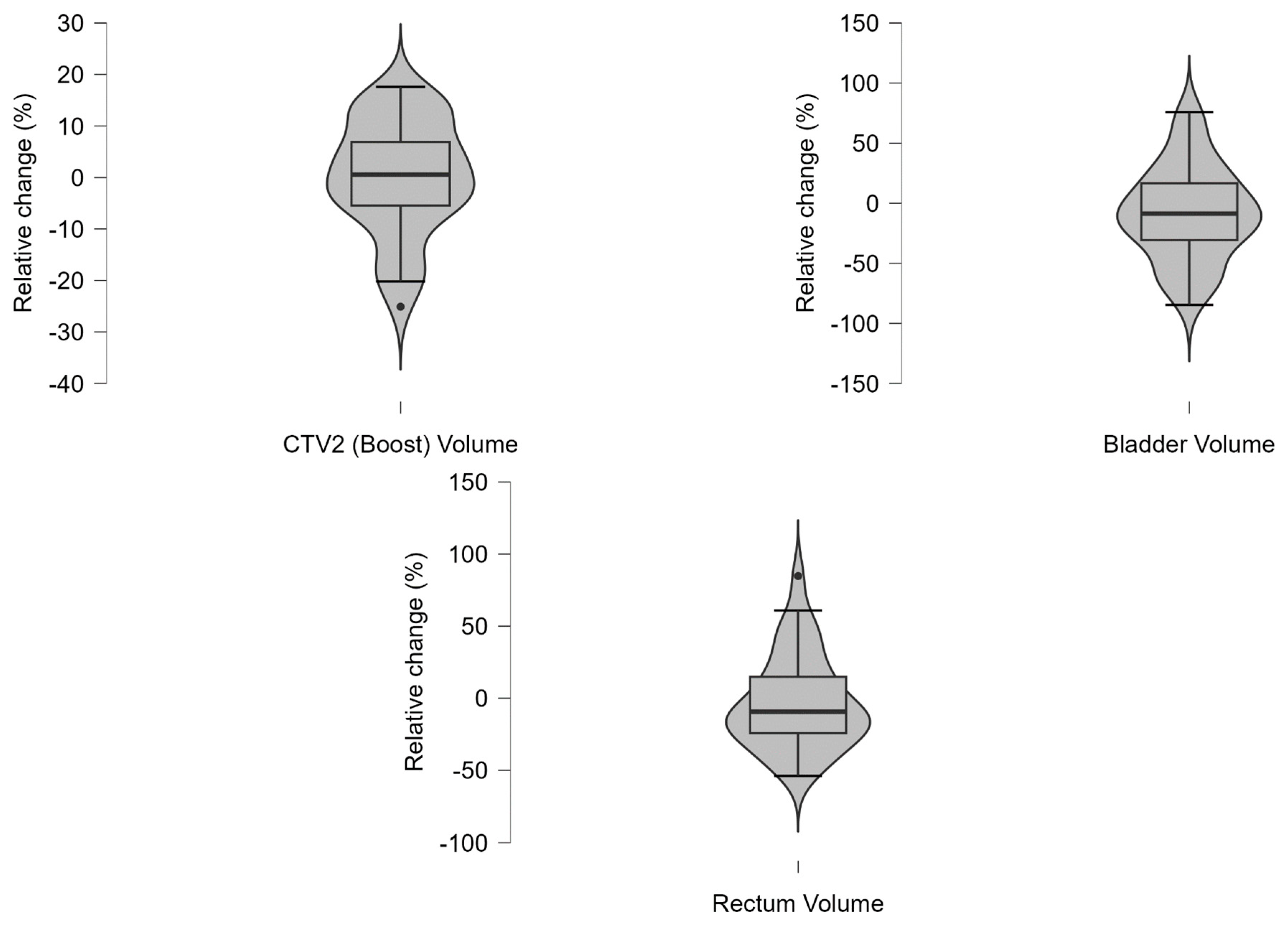
Appendix A.7
| Spearman’s Correlations | ||||
|---|---|---|---|---|
| Variable | longitudinal couch shift (z, absolute values [cm]) | lateral couch shift (x, absolute values [cm]) | vertical couch shift (y, absolute values [cm]) | |
| Interval between CBCT1–CBCT2 [s] | Spearman’s ρ | −0.022 | 0.011 | −0.082 |
| p-value | 0.902 | 0.950 | 0.642 | |
| Lower 95% CI | −0.352 | −0.323 | −0.404 | |
| Upper 95% CI | 0.314 | 0.343 | 0.259 | |
| Contouring/Adaptation time [s] | Spearman’s ρ | −0.025 | 0.002 | −0.181 |
| p-value | 0.886 | 0.991 | 0.297 | |
| Lower 95% CI | −0.355 | −0.332 | −0.485 | |
| Upper 95% CI | 0.311 | 0.335 | 0.162 | |
| QA time [s] | Spearman’s ρ | −0.075 | −0.077 | 0.129 |
| p-value | 0.670 | 0.662 | 0.460 | |
| Lower 95% CI | −0.398 | −0.400 | −0.213 | |
| Upper 95% CI | 0.265 | 0.263 | 0.443 | |
References
- Lapierre, A.; Hennequin, C.; Beneux, A.; Belhomme, S.; Benziane Ouaritini, N.; Biston, M.C.; Crehange, G.; de Crevoisier, R.; Dumas, J.L.; Fawzi, M.; et al. Highly hypofractionated schedules for localized prostate cancer: Recommendations of the GETUG radiation oncology group. Crit. Rev. Oncol. Hematol. 2022, 173, 103661. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Bitting, R.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; Desai, N.; Dorff, T.; et al. Prostate Cancer, Version 3.2024: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2024, 22, 140–150. [Google Scholar] [CrossRef]
- Wurschi, G.W.; Graf, M.; Weimann, S.; Mäurer, M.; Straube, C.; Medenwald, D.; Domschikowski, J.; Münter, M.; Pietschmann, K. Travel costs and ecologic imprint associated with different fractionation schedules in prostate cancer radiotherapy. Das Gesundheitswesen 2025. [Google Scholar] [CrossRef]
- He, J.; Wang, Q.; Hu, Q.; Li, C. Cost-effectiveness analysis of ultra-hypofractionated radiotherapy and conventionally fractionated radiotherapy for intermediate- to high-risk localized prostate cancer. Front. Oncol. 2022, 12, 841356. [Google Scholar] [CrossRef]
- Sun, S.; Jonsson, H.; Salén, K.G.; Andén, M.; Beckman, L.; Fransson, P. Is ultra-hypo-fractionated radiotherapy more cost-effective relative to conventional fractionation in treatment of prostate cancer? A cost-utility analysis alongside a randomized HYPO-RT-PC trial. Eur. J. Health Econ. 2023, 24, 237–246. [Google Scholar] [CrossRef]
- Byun, D.J.; Gorovets, D.J.; Jacobs, L.M.; Happersett, L.; Zhang, P.; Pei, X.; Burleson, S.; Zhang, Z.; Hunt, M.; McBride, S.; et al. Strict bladder filling and rectal emptying during prostate SBRT: Does it make a dosimetric or clinical difference? Radiat. Oncol. 2020, 15, 239. [Google Scholar] [CrossRef]
- Lavrova, E.; Garrett, M.D.; Wang, Y.F.; Chin, C.; Elliston, C.; Savacool, M.; Price, M.; Kachnic, L.A.; Horowitz, D.P. Adaptive Radiation Therapy: A Review of CT-based Techniques. Radiol. Imaging Cancer 2023, 5, e230011. [Google Scholar] [CrossRef]
- Murr, M.; Wegener, D.; Böke, S.; Gani, C.; Mönnich, D.; Niyazi, M.; Schneider, M.; Zips, D.; Müller, A.C.; Thorwarth, D. Comparison of online adaptive and non-adaptive magnetic resonance image-guided radiation therapy in prostate cancer using dose accumulation. Phys. Imaging Radiat. Oncol. 2024, 32, 100662. [Google Scholar] [CrossRef]
- Bertelsen, A.S.; Schytte, T.; Møller, P.K.; Mahmood, F.; Riis, H.L.; Gottlieb, K.L.; Agergaard, S.N.; Dysager, L.; Hansen, O.; Gornitzka, J.; et al. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol. 2019, 58, 1352–1357. [Google Scholar] [CrossRef]
- Winkel, D.; Bol, G.H.; Kroon, P.S.; van Asselen, B.; Hackett, S.S.; Werensteijn-Honingh, A.M.; Intven, M.P.; Eppinga, W.S.; Tijssen, R.H.; Kerkmeijer, L.G. Adaptive radiotherapy: The Elekta Unity MR-linac concept. Clin. Transl. Radiat. Oncol. 2019, 18, 54–59. [Google Scholar] [CrossRef]
- Alongi, F.; Rigo, M.; Figlia, V.; Cuccia, F.; Giaj-Levra, N.; Nicosia, L.; Ricchetti, F.; Sicignano, G.; De Simone, A.; Naccarato, S. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: Feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat. Oncol. 2020, 15, 69. [Google Scholar] [CrossRef]
- Tetar, S.U.; Bruynzeel, A.M.; Lagerwaard, F.J.; Slotman, B.J.; Bohoudi, O.; Palacios, M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys. Imaging Radiat. Oncol. 2019, 9, 69–76. [Google Scholar] [CrossRef]
- Zwart, L.G.M.; Ong, F.; Ten Asbroek, L.A.; van Dieren, E.B.; Koch, S.A.; Bhawanie, A.; de Wit, E.; Dasselaar, J.J. Cone-beam computed tomography-guided online adaptive radiotherapy is feasible for prostate cancer patients. Phys. Imaging Radiat. Oncol. 2022, 22, 98–103. [Google Scholar] [CrossRef]
- Moazzezi, M.; Rose, B.; Kisling, K.; Moore, K.L.; Ray, X. Prospects for daily online adaptive radiotherapy via ethos for prostate cancer patients without nodal involvement using unedited CBCT auto-segmentation. J. Appl. Clin. Med. Phys. 2021, 22, 82–93. [Google Scholar] [CrossRef]
- Lawes, R.; Barnes, H.; Herbert, T.; Mitchell, A.; Nill, S.; Oelfke, U.; Pathmanathan, A.; Smith, G.A.; Sritharan, K.; Tree, A.; et al. MRI-guided adaptive radiotherapy for prostate cancer: When do we need to account for intra-fraction motion? Clin. Transl. Radiat. Oncol. 2022, 37, 85–88. [Google Scholar] [CrossRef]
- Calvo-Ortega, J.F.; Laosa-Bello, C.; Moragues-Femenía, S.; Torices-Caballero, J.; Pozo-Massó, M.; Jones, A.; Hermida-López, M. Online Adaptive Five-Fraction Ablative Radiotherapy for Prostate Cancer Using a Conventional Linear Accelerator. Pract. Radiat. Oncol. 2025, 15, e371–e381. [Google Scholar] [CrossRef]
- Calvo-Ortega, J.F.; Moragues-Femenía, S.; Laosa-Bello, C.; Torices-Caballero, J.; Hermida-López, M.; Casals-Farran, J. Clinical Experience in Prostate Ultrahypofractionated Radiation Therapy With an Online Adaptive Method. Pract. Radiat. Oncol. 2022, 12, e144–e152. [Google Scholar] [CrossRef]
- Waters, M.; Price, A.; Laugeman, E.; Henke, L.; Hugo, G.; Stowe, H.; Andruska, N.; Brenneman, R.; Hao, Y.; Green, O.; et al. CT-based online adaptive radiotherapy improves target coverage and organ at risk (OAR) avoidance in stereotactic body radiation therapy (SBRT) for prostate cancer. Clin. Transl. Radiat. Oncol. 2024, 44, 100693. [Google Scholar] [CrossRef]
- Yoganathan, S.A.; Riyas, M.; Sukumaran, R.; Hammoud, R.; Al-Hammadi, N. Ultra-Hypofractionated Prostate Radiotherapy With Online Adaptive Technique: A Case Report. Cureus 2024, 16, e64101. [Google Scholar] [CrossRef]
- Kunnen, B.; van de Schoot, A.J.; Fremeijer, K.P.; Nicolai-Koornneef, E.M.; Offereins-van Harten, K.; Sluijter, J.H.; Sijtsema, N.D.; Oomen-de Hoop, E.; el Yaakoubi, A.; Froklage, F.E.; et al. The added value of a new high-performance ring-gantry CBCT imaging system for prostate cancer patients. Radiother. Oncol. 2024, 200, 110458. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- van As, N.; Griffin, C.; Tree, A.; Patel, J.; Ostler, P.; van der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; et al. Phase 3 Trial of Stereotactic Body Radiotherapy in Localized Prostate Cancer. N. Engl. J. Med. 2024, 391, 1413–1425. [Google Scholar] [CrossRef]
- Brand, D.H.; Tree, A.C.; Ostler, P.; van der Voet, H.; Loblaw, A.; Chu, W.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): Acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019, 20, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- JASP Team. JASP; Version 0.19.3; JASP Team: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Byrne, M.; Archibald-Heeren, B.; Hu, Y.; Teh, A.; Beserminji, R.; Cai, E.; Liu, G.; Yates, A.; Rijken, J.; Collett, N.; et al. Varian ethos online adaptive radiotherapy for prostate cancer: Early results of contouring accuracy, treatment plan quality, and treatment time. J. Appl. Clin. Med. Phys. 2022, 23, e13479. [Google Scholar] [CrossRef]
- Valencia Lozano, I.; Buss, E.; Spina, C.S.; Horowitz, D.P.; Kachnic, L.A.; Price, M.; Wang, Y.F.; Munbodh, R. Quantification and dosimetric impact of intra-fractional bladder changes during CBCT-guided online adaptive radiotherapy for pelvic cancer treatments. J. Appl. Clin. Med. Phys. 2025, 26, e70074. [Google Scholar] [CrossRef]
- Khouya, A.; Pöttgen, C.; Hoffmann, C.; Ringbaek, T.P.; Lübcke, W.; Indenkämpen, F.; Guberina, M.; Guberina, N.; Gauler, T.; Stuschke, M.; et al. Adaptation Time as a Determinant of the Dosimetric Effectiveness of Online Adaptive Radiotherapy for Bladder Cancer. Cancers 2023, 15, 5629. [Google Scholar] [CrossRef]
- Bruynzeel, A.M.E.; Tetar, S.U.; Oei, S.S.; Senan, S.; Haasbeek, C.J.A.; Spoelstra, F.O.B.; Piet, A.H.M.; Meijnen, P.; Bakker van der Jagt, M.A.B.; Fraikin, T.; et al. A Prospective Single-Arm Phase 2 Study of Stereotactic Magnetic Resonance Guided Adaptive Radiation Therapy for Prostate Cancer: Early Toxicity Results. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1086–1094. [Google Scholar] [CrossRef]
- Fink, C.A.; Ristau, J.; Buchele, C.; Klüter, S.; Liermann, J.; Hoegen-Saßmannshausen, P.; Sandrini, E.; Lentz-Hommertgen, A.; Baumann, L.; Andratschke, N.; et al. Stereotactic ultrahypofractionated MR-guided radiotherapy for localized prostate cancer—Acute toxicity and patient-reported outcomes in the prospective, multicenter SMILE phase II trial. Clin. Transl. Radiat. Oncol. 2024, 46, 100771. [Google Scholar] [CrossRef]

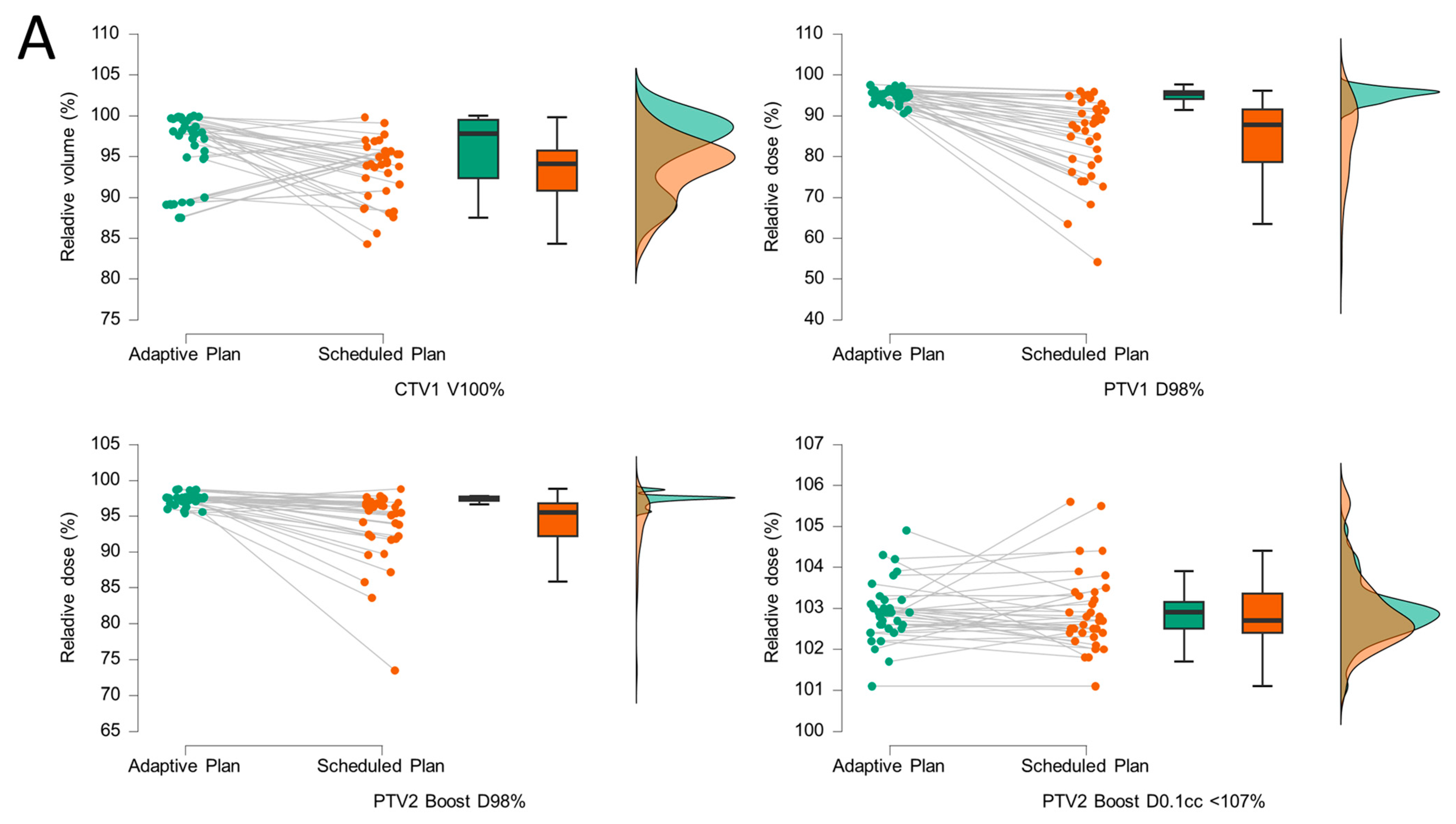
| ID | Age at Start | Gleason Score | Gleason Sum Score | ISUP Grading | Initial PSA (ng/mL) | TNM T Stage | RiskClass (NCCN) | RT Delivery |
|---|---|---|---|---|---|---|---|---|
| 1 | 67 | 3 + 4 | 7a | 2 | 9.92 | 2c | intermediate unfavorable | q2d |
| 2 | 71 | 3 + 4 | 7a | 2 | 6.33 | 2b | intermediate favorable | q1d |
| 3 | 72 | 4 + 3 | 7b | 3 | 4.76 | 2a | intermediate unfavorable | q1d |
| 4 | 62 | 3 + 3 | 6 | 1 | 3.7 | 2a | low | q2d |
| 5 | 73 | 3 + 3 | 6 | 1 | 7.18 | 2a | low | q1d |
| 6 | 67 | 4 + 3 | 7b | 3 | 7.8 | 2b | intermediate unfavorable | q1d |
| 7 | 64 | 4 + 3 | 7b | 3 | 15.2 | 1c | intermediate unfavorable | q1d |
| (A) | Measure 1 | Measure 2 | W | z | p |
| CTV1 V100% (adaptive) | CTV1 V100% (scheduled) | 448.50 | 2.19 | 0.01 | |
| PTV1 D98% (adaptive) | PTV1 D98% (scheduled) | 629.00 | 5.14 | <0.001 | |
| PTV2 Boost D98% (adaptive) | PTV2 Boost D98% (scheduled) | 564.50 | 4.56 | <0.001 | |
| PTV2 Boost D0.1ccm <107% (adaptive) | PTV2 Boost D0.1ccm <107% (scheduled) | 281.50 | 0.33 | 0.38 | |
| (B) | Measure 1 | Measure 2 | W | z | p |
| Bladder D40% (adaptive) | Bladder D40% (scheduled) | 399.50 | 2.13 | 0.98 | |
| Bladder D100% (adaptive) | Bladder D100% (scheduled) | 153.50 | 2.35 | 0.99 | |
| Bladder V40Gy (adaptive) | Bladder V40Gy (scheduled) | 12.00 | −2.12 | 0.02 | |
| Bladder V37Gy (adaptive) | Bladder V37Gy (scheduled) | 321.00 | 0.40 | 0.66 | |
| Bowel Bag V30Gy (adaptive) | Bowel Bag V30Gy (scheduled) | 3.00 | 1.34 | 0.96 | |
| Bowel Bag D5ccm (adaptive) | Bowel Bag D5ccm (scheduled) | 146.00 | 1.53 | 0.94 | |
| Rectum D50% (adaptive) | Rectum D50% (scheduled) | 424.00 | 1.79 | 0.96 | |
| Rectum D20% (adaptive) | Rectum D20% (scheduled) | 533.50 | 3.58 | 1.00 | |
| Rectum V36Gy (adaptive) | Rectum V36Gy (scheduled) | 253.50 | −1.01 | 0.16 | |
| Penile bulb D50% (adaptive) | Penile bulb D50% (scheduled) | 229.50 | 0.26 | 0.61 | |
| Longitudinal (z) in cm | Lateral (x) in cm | Vertical (y) in cm | |
|---|---|---|---|
| Mean | −0.01 | −0.04 | −0.01 |
| Std. Dev. | 0.16 | 0.10 | 0.21 |
| Minimum | −0.68 | −0.26 | −0.72 |
| Maximum | 0.11 | 0.18 | 0.28 |
| 2.5th percentile | −0.48 | −0.21 | −0.58 |
| 25th percentile | 0.00 | −0.12 | 0.00 |
| Median | 0.03 | −0.01 | 0.07 |
| 75th percentile | 0.06 | 0.01 | 0.09 |
| 97.5th percentile | 0.11 | 0.16 | 0.19 |
| Contouring/Adaptation [min] | QA Time [min] | Beam-on Time [min] | Interval Between CBCT1–CBCT2 [min] | Total Treatment Time [min] | |
|---|---|---|---|---|---|
| Mean | 12:11 | 05:27 | 05:22 | 17:38 | 30:17 |
| Std. Dev. | 05:14 | 01:23 | 00:44 | 05:02 | 05:49 |
| Minimum | 04:09 | 03:00 | 03:58 | 11:00 | 22:37 |
| Maximum | 24:51 | 08:42 | 06:39 | 28:35 | 43:07 |
| 25th percentile | 07:59 | 04:35 | 04:43 | 16:00 | 25:07 |
| Median | 10:37 | 05:00 | 05:29 | 13:30 | 30:31 |
| 75th percentile | 16:20 | 06:04 | 05:56 | 21:49 | 33:00 |
| CTCAE Event | Diarrhea | Proctitis | Rectal Bleeding | Cystitis | Urinary Retention | Hematuria | Erectile Dysfunction | Fatigue |
|---|---|---|---|---|---|---|---|---|
| Grade 0 | 6 (87.5%) | 3 (42.9%) | 6 (85.7%) | 3 (42.9%) | 6 (85.7%) | 7 (100.0%) | 5 (71.4%) | 7 (100.0%) |
| Grade 1 | 1 (14.3%) | 1 (14.3%) | - | 3 (42.9%) | 1 (14.3%) | - | 1 (14.3%) | - |
| Grade 2 | - | 3 (42.9%) | 1 (14.3%) | 1 (14.3%) | - | - | 1 (14.3%) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurschi, G.; Voigt, A.; Murr, N.; Riede, C.; Schwedas, M.; Römer, M.; Drozdz, S.; Pietschmann, K. CBCT-Based Online Adaptive, Ultra-Hypofractionated Radiotherapy for Prostate Cancer: First Clinical Experiences. Medicina 2025, 61, 1839. https://doi.org/10.3390/medicina61101839
Wurschi G, Voigt A, Murr N, Riede C, Schwedas M, Römer M, Drozdz S, Pietschmann K. CBCT-Based Online Adaptive, Ultra-Hypofractionated Radiotherapy for Prostate Cancer: First Clinical Experiences. Medicina. 2025; 61(10):1839. https://doi.org/10.3390/medicina61101839
Chicago/Turabian StyleWurschi, Georg, Alexander Voigt, Noreen Murr, Cora Riede, Michael Schwedas, Maximilian Römer, Sonia Drozdz, and Klaus Pietschmann. 2025. "CBCT-Based Online Adaptive, Ultra-Hypofractionated Radiotherapy for Prostate Cancer: First Clinical Experiences" Medicina 61, no. 10: 1839. https://doi.org/10.3390/medicina61101839
APA StyleWurschi, G., Voigt, A., Murr, N., Riede, C., Schwedas, M., Römer, M., Drozdz, S., & Pietschmann, K. (2025). CBCT-Based Online Adaptive, Ultra-Hypofractionated Radiotherapy for Prostate Cancer: First Clinical Experiences. Medicina, 61(10), 1839. https://doi.org/10.3390/medicina61101839






