Is a Ureteral Access Sheath Necessary for Maintaining Safe Intrarenal Pressures During Retrograde Lithotripsy Using a Flexible 7.5 Fr Scope and a High-Power TFL? In Vivo Experimental Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Experimental Setting
2.2. Preparation of the Porcine Models
2.3. Operating Room Setup and Technique
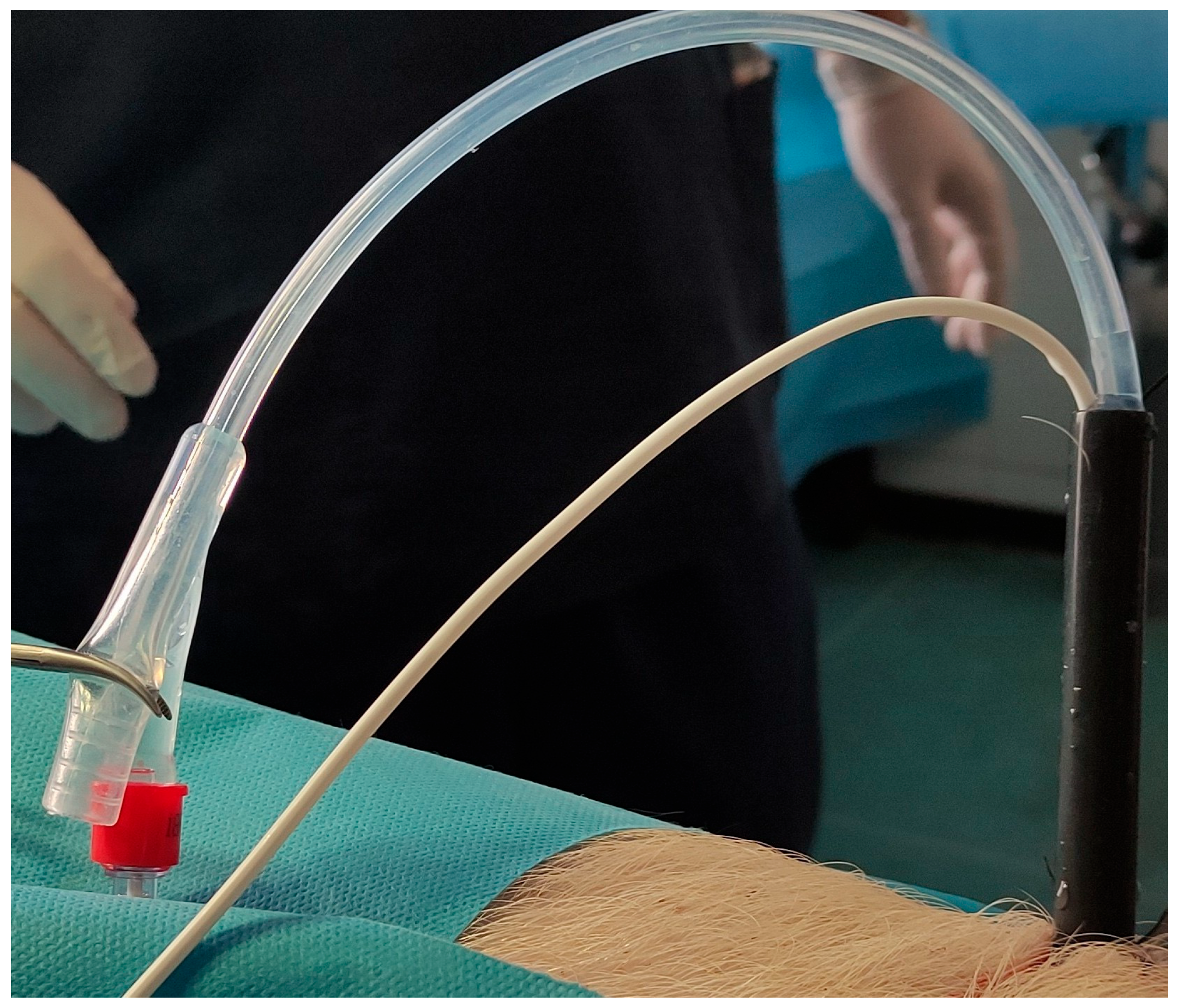
2.4. Measurement of the Intrarenal Pressure
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Türk, C.; Petřík, A.; Sarica, K.; Seitz, C.; Skolarikos, A.; Straub, M.; Knoll, T. EAU Guidelines on Interventional Treatment for Urolithiasis. Eur. Urol. 2016, 69, 475–482. [Google Scholar] [CrossRef]
- Osther, P.J.; Pedersen, K.V.; Lildal, S.K.; Pless, M.S.; Andreassen, K.H.; Osther, S.S.; Jung, H.U. Pathophysiological aspects of ureterorenoscopic management of upper urinary tract calculi. Curr. Opin. Urol. 2016, 26, 63–69. [Google Scholar] [CrossRef]
- Osther, P.J.S. Risks of flexible ureterorenoscopy: Pathophysiology and prevention. Urolithiasis 2018, 46, 59–67. [Google Scholar] [CrossRef]
- Xu, Y.; Min, Z.; Wan, S.P.; Nie, H.; Duan, G. Complications of retrograde intrarenal surgery classified by the modified Clavien grading system. Urolithiasis 2018, 46, 197–202. [Google Scholar] [CrossRef]
- Auge, B.K.; Pietrow, P.K.; Lallas, C.D.; Raj, G.V.; Santa-Cruz, R.W.; Preminger, G.M. Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J. Endourol. 2004, 18, 33–36. [Google Scholar] [CrossRef]
- Traxer, O.; Thomas, A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J. Urol. 2013, 189, 580–584. [Google Scholar] [CrossRef]
- Geavlete, P.; Multescu, R.; Geavlete, B. Pushing the boundaries of ureteroscopy: Current status and future perspectives. Nat. Rev. Urol. 2014, 11, 373–382. [Google Scholar] [CrossRef]
- Fang, L.; Xie, G.; Zheng, Z.; Liu, W.; Zhu, J.; Huang, T.; Lu, Y.; Cheng, Y. The Effect of Ratio of Endoscope-Sheath Diameter on Intrapelvic Pressure During Flexible Ureteroscopic Lasertripsy. J. Endourol. 2019, 33, 132–139. [Google Scholar] [CrossRef]
- Russell, W.J.U.C. On comfort and comfort activities in animals. UFAW Cour. 1959, 16, 14–26. [Google Scholar]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Kallidonis, P.; Vagionis, A.; Vrettos, T.; Adamou, K.; Pagonis, K.; Ntasiotis, P.; Callas, G.A.; Tanaseskou, L.; Al Aown, A.M.; Liatsikos, E. Non papillary mini-percutaneous nephrolithotomy: Early experience. World J. Urol. 2021, 39, 1241–1246. [Google Scholar] [CrossRef]
- Tonyali, S.; von Bargen, M.F.; Ozkan, A.; Gratzke, C.; Miernik, A. The heat is on: The impact of excessive temperature increments on complications of laser treatment for ureteral and renal stones. World J. Urol. 2023, 41, 3853–3865. [Google Scholar] [CrossRef]
- Agrawal, S.; Patil, A.; Sabnis, R.B.; Singh, A.G.; Ganpule, A.P.; Desai, M.R. Initial experience with slimmest single-use flexible ureteroscope Uscope PU3033A (PUSEN™) in retrograde intrarenal surgery and its comparison with Uscope PU3022a: A single-center prospective study. World J. Urol. 2021, 39, 3957–3962. [Google Scholar] [CrossRef]
- Samaras, A.; Tatanis, V.; Peteinaris, A.; Obaidat, M.; Faitatziadis, S.; Vagionis, A.; Spinos, T.; Mylonopoulou, M.; Kallidonis, P.; Liatsikos, E. The Evaluation of Intrarenal Pressure Using a Novel Single-Use Flexible Ureteroscope with Live Intrarenal Pressure Monitoring-An Experimental Study in Porcine Models. Life 2024, 14, 1060. [Google Scholar] [CrossRef]
- Esch, E.; Simmons, W.N.; Sankin, G.; Cocks, H.F.; Preminger, G.M.; Zhong, P. A simple method for fabricating artificial kidney stones of different physical properties. Urol. Res. 2010, 38, 315–319. [Google Scholar] [CrossRef]
- Ballesta Martinez, B.; Magee, D.S.; Tsaturyan, A.; Tatanis, V.; Peteinaris, A.; Tancabel, C.; Chau, M.; Van der Werf, S.; Saluja, M.S.; Aw, I.; et al. Radiological Density, Atomic Numbers, and Stone Fragmentation of Bego Stones Used for Research in Endourology: Comparison to Real Urinary Stones. J. Endourol. 2024, 38, 179–185. [Google Scholar] [CrossRef]
- Traxer, O.; Wendt-Nordahl, G.; Sodha, H.; Rassweiler, J.; Meretyk, S.; Tefekli, A.; Coz, F.; de la Rosette, J.J. Differences in renal stone treatment and outcomes for patients treated either with or without the support of a ureteral access sheath: The Clinical Research Office of the Endourological Society Ureteroscopy Global Study. World J. Urol. 2015, 33, 2137–2144. [Google Scholar] [CrossRef]
- Delvecchio, F.C.; Auge, B.K.; Brizuela, R.M.; Weizer, A.Z.; Silverstein, A.D.; Lallas, C.D.; Pietrow, P.K.; Albala, D.M.; Preminger, G.M. Assessment of stricture formation with the ureteral access sheath. Urology 2003, 61, 518–522. [Google Scholar] [CrossRef]
- Zelenko, N.; Coll, D.; Rosenfeld, A.T.; Smith, R.C. Normal ureter size on unenhanced helical CT. AJR. Am. J. Roentgenol. 2004, 182, 1039–1041. [Google Scholar] [CrossRef]
- Breda, A.; Emiliani, E.; Millán, F.; Scoffone, C.M.; Knoll, T.; Osther, P.J.; Liatsikos, E. The new concept of ureteral access sheath with guidewire disengagement: One wire does it all. World J. Urol. 2016, 34, 603–606. [Google Scholar] [CrossRef]
- Aykanat, C.; Balci, M.; Senel, C.; Ozercan, A.Y.; Coser, S.; Aslan, Y.; Guzel, O.; Asfuroglu, A.; Karabulut, E.; Tuncel, A. The Impact of Ureteral Access Sheath Size on Perioperative Parameters and Postoperative Ureteral Stricture in Retrograde Intrarenal Surgery. J. Endourol. 2022, 36, 1013–1017. [Google Scholar] [CrossRef]
- Erol, E.; Ecer, G.; Kiremit, M.C.; Gokce, M.; Balasar, M.; Sarikaya, A.F.; Babayigit, M.; Karaarslan, U.C.; Aksoy, E.I.; Sarica, K.; et al. Multicentric evaluation of high and low power lasers on RIRS success using propensity score analysis. Urolithiasis 2024, 52, 32. [Google Scholar] [CrossRef]
- Kohler, R. Investigations on backflow in retrograde pyelography; a roentgenological and clinical study. Acta Radiol. 1953, 39, 1–92. [Google Scholar]
- Hong, A.; du Plessis, J.; Browne, C.; Jack, G.; Bolton, D. Mechanism of urosepsis: Relationship between intrarenal pressures and pyelovenous backflow. BJU Int. 2023, 132, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Scotland, K.B.; Lange, D. Prevention and management of urosepsis triggered by ureteroscopy. Res. Rep. Urol. 2018, 10, 43–49. [Google Scholar] [CrossRef]
- Loftus, C.; Byrne, M.; Monga, M. High pressure endoscopic irrigation: Impact on renal histology. Int. Braz J. Urol Off. J. Braz. Soc. Urol. 2021, 47, 350–356. [Google Scholar] [CrossRef]
- Yoshida, T.; Inoue, T.; Abe, T.; Matsuda, T. Evaluation of Intrapelvic Pressure When Using Small-Sized Ureteral Access Sheaths of </=10/12F in an Ex Vivo Porcine Kidney Model. J. Endourol. 2018, 32, 1142–1147. [Google Scholar] [CrossRef]
- Noureldin, Y.A.; Kallidonis, P.; Ntasiotis, P.; Adamou, C.; Zazas, E.; Liatsikos, E.N. The Effect of Irrigation Power and Ureteral Access Sheath Diameter on the Maximal Intra-Pelvic Pressure During Ureteroscopy: In Vivo Experimental Study in a Live Anesthetized Pig. J. Endourol. 2019, 33, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Doizi, S.; Uzan, A.; Keller, E.X.; De Coninck, V.; Kamkoum, H.; Barghouthy, Y.; Ventimiglia, E.; Traxer, O. Comparison of intrapelvic pressures during flexible ureteroscopy, mini-percutaneous nephrolithotomy, standard percutaneous nephrolithotomy, and endoscopic combined intrarenal surgery in a kidney model. World J. Urol. 2021, 39, 2709–2717. [Google Scholar] [CrossRef]
- De Coninck, V.; Keller, E.X.; Rodríguez-Monsalve, M.; Audouin, M.; Doizi, S.; Traxer, O. Systematic review of ureteral access sheaths: Facts and myths. BJU Int. 2018, 122, 959–969. [Google Scholar] [CrossRef]
- Rehman, J.; Monga, M.; Landman, J.; Lee, D.I.; Felfela, T.; Conradie, M.C.; Srinivas, R.; Sundaram, C.P.; Clayman, R.V. Characterization of intrapelvic pressure during ureteropyeloscopy with ureteral access sheaths. Urology 2003, 61, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Huang, T.; Song, B.; Liu, W.; Cheng, Y.; Fang, L. The optimal ratio of endoscope-sheath diameter with negative-pressure ureteral access sheath: An in vitro research. World J. Urol. 2024, 42, 122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xie, T.; Li, F.; Wang, X.; Liu, F.; Jiang, B.; Zou, X.; Zhang, G.; Yuan, Y.; Xiao, R.; et al. Comparison of traditional and novel tip-flexible suctioning ureteral access sheath combined with flexible ureteroscope to treat unilateral renal calculi. World J. Urol. 2023, 41, 3619–3627. [Google Scholar] [CrossRef]
- Peteinaris, A.; Pagonis, K.; Vagionis, A.; Adamou, C.; Tsaturyan, A.; Ballesta Martínez, B.; Karpetas, G.; Farsari, E.; Liatsikos, E.; Kallidonis, P. What is the impact of pulse modulation technology, laser settings and intraoperative irrigation conditions on the irrigation fluid temperature during flexible ureteroscopy? An in vivo experiment using artificial stones. World J. Urol. 2022, 40, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
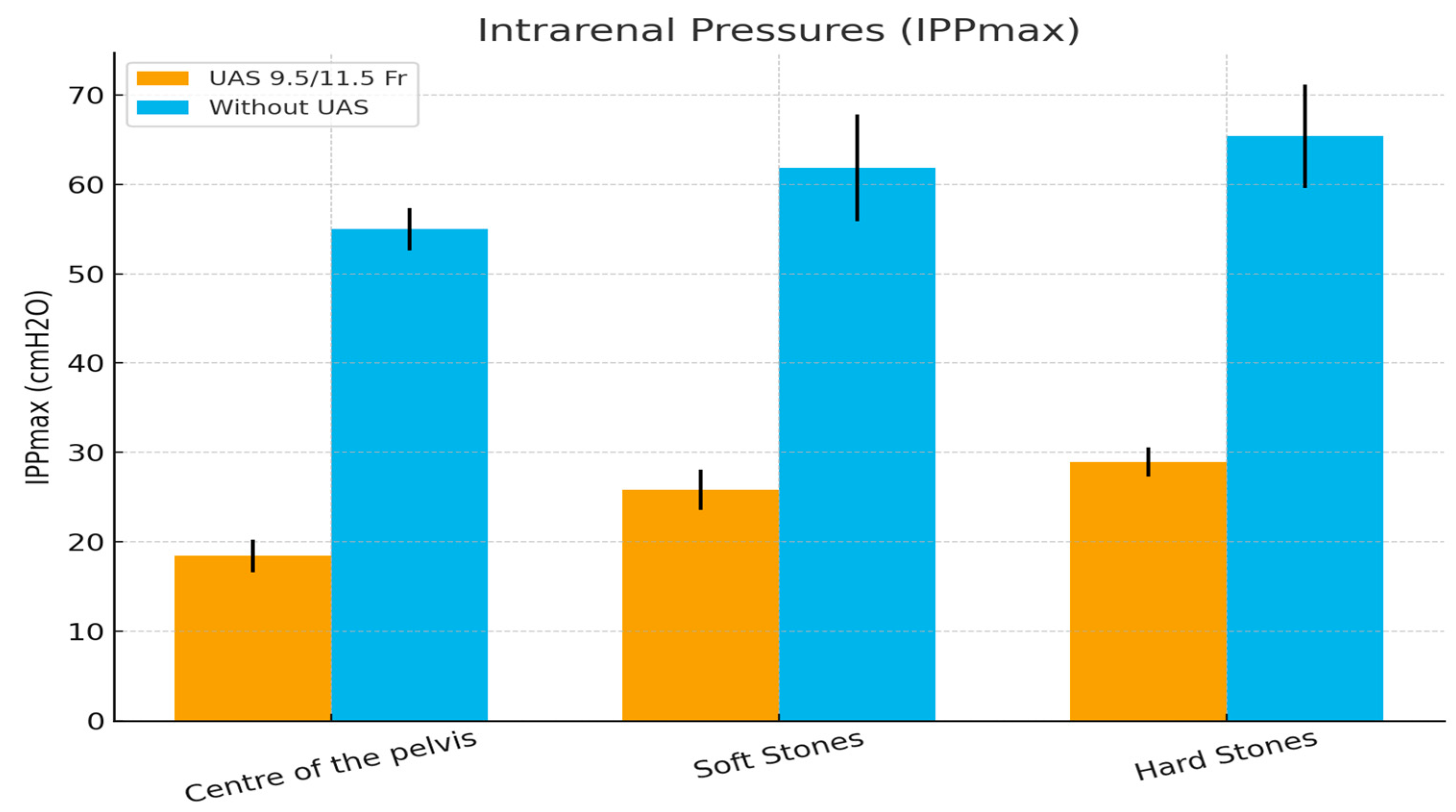
| Intrarenal Pressures IPPmax (cmH2O) | UAS 9.5/11.5 Fr | Without UAS | p-Value |
|---|---|---|---|
| Center of the pelvis | 18.42 ±1.832 | 55 ± 2.374 | p = 0.0005 |
| Soft Stones | 25.83 ± 2.25 | 61.83 ± 5.98 | p = 0.0005 |
| Hard Stones | 28.92 ± 1.62 | 65.42 ± 5.79 | p = 0.0005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vagionis, A.; Tatanis, V.; Peteinaris, A.; Katsakiori, P.; Tsekoura, V.; Pagonis, K.; Vrettos, T.; Liatsikos, E.; Kallidonis, P. Is a Ureteral Access Sheath Necessary for Maintaining Safe Intrarenal Pressures During Retrograde Lithotripsy Using a Flexible 7.5 Fr Scope and a High-Power TFL? In Vivo Experimental Study. Medicina 2025, 61, 1829. https://doi.org/10.3390/medicina61101829
Vagionis A, Tatanis V, Peteinaris A, Katsakiori P, Tsekoura V, Pagonis K, Vrettos T, Liatsikos E, Kallidonis P. Is a Ureteral Access Sheath Necessary for Maintaining Safe Intrarenal Pressures During Retrograde Lithotripsy Using a Flexible 7.5 Fr Scope and a High-Power TFL? In Vivo Experimental Study. Medicina. 2025; 61(10):1829. https://doi.org/10.3390/medicina61101829
Chicago/Turabian StyleVagionis, Athanasios, Vasileios Tatanis, Angelis Peteinaris, Paraskevi Katsakiori, Vasiliki Tsekoura, Konstantinos Pagonis, Theofanis Vrettos, Evangelos Liatsikos, and Panagiotis Kallidonis. 2025. "Is a Ureteral Access Sheath Necessary for Maintaining Safe Intrarenal Pressures During Retrograde Lithotripsy Using a Flexible 7.5 Fr Scope and a High-Power TFL? In Vivo Experimental Study" Medicina 61, no. 10: 1829. https://doi.org/10.3390/medicina61101829
APA StyleVagionis, A., Tatanis, V., Peteinaris, A., Katsakiori, P., Tsekoura, V., Pagonis, K., Vrettos, T., Liatsikos, E., & Kallidonis, P. (2025). Is a Ureteral Access Sheath Necessary for Maintaining Safe Intrarenal Pressures During Retrograde Lithotripsy Using a Flexible 7.5 Fr Scope and a High-Power TFL? In Vivo Experimental Study. Medicina, 61(10), 1829. https://doi.org/10.3390/medicina61101829







