The Age Factor in Ixekizumab Survival: Older Patients Show Higher Long-Term Treatment Survival
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics
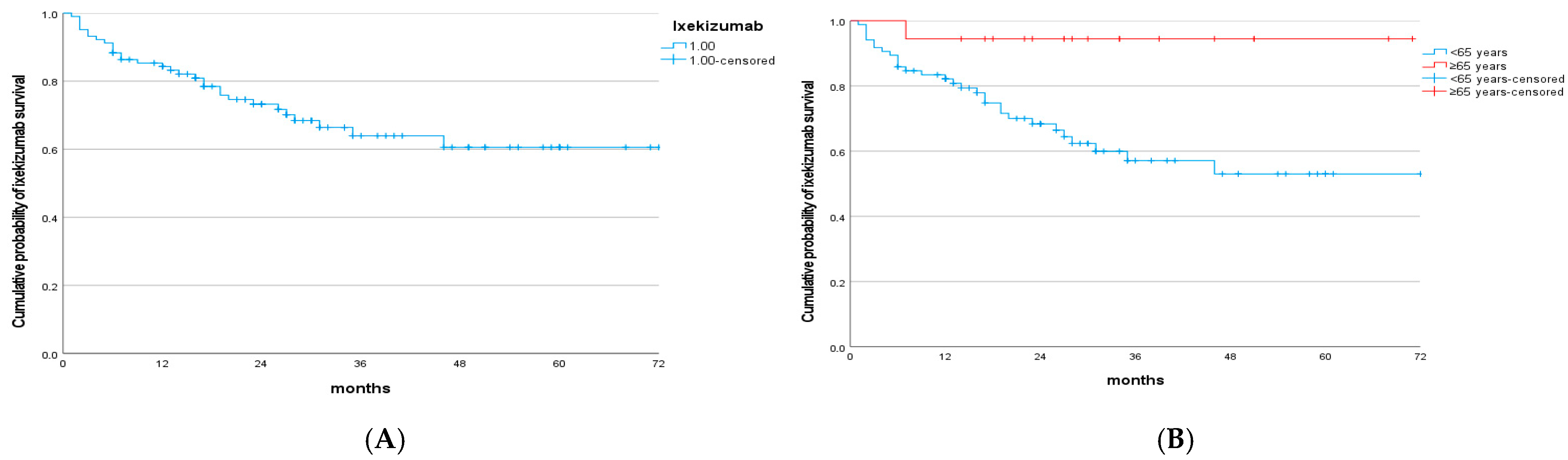
3.2. Drug Survival
3.3. Univariate Analysis
3.4. Adverse Effects
3.5. Patients Discontinuing Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dogra, S.; Mahajan, R. Psoriasis: Epidemiology, clinical features, co-morbidities, and clinical scoring. Indian Dermatol. Online J. 2016, 7, 471–480. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Krueger, J.G.; Lebwohl, M.G. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp. Dermatol. 2018, 27, 409–417. [Google Scholar] [CrossRef]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Levrero, P.; Carusso, R.; Morales, C.; Arretche, V.; Nicola, A.; Fossati, M.; Cueto, M.; Dorado, N.; García, C.; et al. Psoriasis vulgar moderada y severa: Opciones terapéuticas (tratamientos convencionales). Arch. Med. Interna 2013, 35, 93–100. [Google Scholar]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Challenges and future trends in the treatment of psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef]
- Mrowietz, U.; Lauffer, F.; Sondermann, W.; Gerdes, S.; Sewerin, P. Psoriasis as a systemic disease. Dtsch. Arztebl. Int. 2024, 121, 467–472. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Lin, W.; Lu, L.; Su, J.; Chen, X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct. Target. Ther. 2023, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Sbidian, E.; Chaimani, A.; Garcia-Doval, I.; Doney, L.; Dressler, C.; Hua, C. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 1, CD011535. [Google Scholar] [CrossRef]
- Azevedo, A.; Torres, T. Clinical efficacy and safety of ixekizumab for treatment of psoriasis. Actas Dermosifiliogr. 2017, 108, 305–314. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Taltz (Ixekizumab) Injection, for Subcutaneous Use: Initial U.S. Approval 2016 [Internet]; FDA: Silver Spring, MD, USA, 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/125521Orig1s000Approv.pdf (accessed on 20 July 2025).
- European Medicines Agency (EMA). Taltz: EPAR–Product Information [Internet]; EMA: Amsterdam, The Netherlands, 2016; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/taltz (accessed on 20 July 2025).
- Dávila-Seijo, P.; Dauden, E.; Carretero, G.; Ferrandiz, C.; Vanaclocha, F.; Gómez-García, F.-J.; Herrera-Ceballos, E.; De la Cueva-Dobao, P.; Belinchón, I.; Sánchez-Carazo, J.-L.; et al. Survival of Classic and Biological Systemic Drugs in Psoriasis: Results of the BIOBADADERM Registry and Critical Analysis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1942–1950. [Google Scholar] [CrossRef]
- Borrás-Blasco, J.; Valcuende-Rosique, A.; Cornejo-Uixeda, S. Letter to the editor regarding “Efficacy, drug survival, safety and metabolic parameters of ixekizumab in patients with moderate-to-severe psoriasis in China: A two-year real-world study”. Int. Immunopharmacol. 2025, 164, 115370. [Google Scholar] [CrossRef] [PubMed]
- Becher, G.; Conner, S.; Ingram, J.A.; Stephen, K.E.; McInnes, A.C.; Heald, A.H.; Riley, P.A.; Davies, M.; Domenech, A.; Kasujee, I. A Retrospective Real-World Study of the Effectiveness and Tolerability of Tildrakizumab in UK Adults with Moderate-to-Severe Chronic Plaque Psoriasis. Dermatol. Ther. 2022, 12, 2343–2354. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Siegel, M.P.; Bagel, J.; Boh, E.E.; Buell, M.; Cooper, K.D.; Duffin, K.C.; Eichenfield, L.F.; Garg, A.; Gelfand, J.M.; et al. From the Medical Board of the National Psoriasis Foundation: Treatment targets for plaque psoriasis. J. Am. Acad. Dermatol. 2017, 76, 290–298. [Google Scholar] [CrossRef]
- Caldarola, G.; Chiricozzi, A.; Megna, M.; Dapavo, P.; Giunta, A.; Burlando, M.; Malagoli, P.; Dini, V.; Mariani, M.; Fabbrocini, G.; et al. Real-life experience with ixekizumab in plaque psoriasis: A multi-center, retrospective, 3-year study. Expert Opin. Biol. Ther. 2023, 23, 365–370. [Google Scholar] [CrossRef]
- Ntawuyamara, E.; Deng, B.; Liang, Y. Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study. Sci. Rep. 2025, 15, 15528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yiu, Z.Z.N.; Becher, G.; Kirby, B.; Laws, P.; Reynolds, N.J.; Smith, C.H.; Warren, R.B.; Griffiths, C.E.M.; BADBIR Study Group; Browne, F.; et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022, 158, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Burlando, M.; Salvi, I.; Castelli, R.; Herzum, A.; Cozzani, E.; Parodi, A. Long-term clinical efficacy and safety of ixekizumab for psoriatic patients: A single-center experience. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4060–4064. [Google Scholar] [PubMed]
- Mastorino, L.; Dapavo, P.; Burzi, L.; Rosset, F.; Giunipero di Corteranzo, I.; Leo, F.; Verrone, A.; Stroppiana, E.; Ortoncelli, M.; Ribero, S.; et al. Drug survival, effectiveness and safety of ixekizumab for moderate-to-severe psoriasis up to 5 years. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 568–575. [Google Scholar] [CrossRef]
- Saeki, H.; Nakagawa, H.; Ishii, T.; Morisaki, Y.; Aoki, T.; Berclaz, P.Y.; Heffernan, M. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1148–1155. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Burge, R.; Malatestinic, W.N.; Zhu, B.; Zhao, Y.; McCormack, J.; Kimel, M.; Merola, J.F. Ixekizumab real-world effectiveness at 24 weeks in patients with psoriasis: Data from the United States Taltz customer support program. Dermatol. Ther. 2023, 13, 1831–1846. [Google Scholar] [CrossRef]
- Lockshin, B.; Harrison, R.W.; McLean, R.R.; Crabtree, M.M.; Konicek, B.W.; Zhu, B.; Malatestinic, W.N.; Atiya, B.; Murage, M.J.; Burge, R.T. Outcomes in ixekizumab patients following exposure to secukinumab and other biologics in the CorEvitas Psoriasis Registry. Dermatol. Ther. 2022, 12, 2797–2815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Mu, Z.; Zhao, Y.; Zhang, J.; Cai, L. Efficacy, drug survival, safety and metabolic parameters of ixekizumab in patients with moderate-to-severe psoriasis in China: A two-year real-world study. Int. Immunopharmacol. 2024, 143 Pt 2, 113474. [Google Scholar] [CrossRef]
- Bucur, S.; Serban, E.D.; Ileanu, B.V.; Costache, R.S.; Nicolescu, A.C.; Constantin, T.; Costache, D.O.; Constantin, M.-M. Effectiveness and drug survival of ixekizumab and secukinumab in patients with moderate to severe plaque psoriasis: Real-world data from Bucharest, Romania. Psoriasis 2024, 14, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Strober, B.; Schrader, A.; Li, A.H.; Eckmann, T.; Zhu, B.; Malatestinic, W.N.; Birt, J.; Feely, M.; Blauvelt, A. Six-month real-world study to assess the effectiveness of ixekizumab after switching from IL-23 inhibitors and other biologic therapies: The CorEvitas Psoriasis Registry. Drugs Real World Outcomes 2024, 11, 451–464. [Google Scholar] [CrossRef]
- Pinter, A.; Eyerich, K.; Costanzo, A.; Garrelts, A.; Schuster, C.; Mert, C.; Lampropoulou, A.; Fotiou, K.; Maul, J.-T.; Papp, K.A. Association of disease duration and PASI response rates at week 12 in patients with moderate-to-severe plaque psoriasis receiving biologics in the real-world psoriasis study of health outcomes (PSoHO). J. Dermatol. Treat. 2024, 35, 2350227. [Google Scholar] [CrossRef]
- Pinter, A.; Costanzo, A.; Khattri, S.; Smith, S.D.; Carrascosa, J.M.; Tada, Y.; Riedl, E.; Reich, A.; Brnabic, A.; Haustrup, N.; et al. Comparative effectiveness and durability of biologics in clinical practice: Month 12 outcomes from the international, observational psoriasis study of health outcomes (PSoHO). Dermatol. Ther. 2024, 14, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhdanava, M.; Fitzgerald, T.; Pilon, D.; Teneralli, R.E.; Shah, A.; Diaz, L.; Lefebvre, P.; Feldman, S.R. Comparative analysis of persistence and remission with guselkumab versus secukinumab and ixekizumab in the United States. J. Dermatol. Treat. 2024, 35, 2349658. [Google Scholar] [CrossRef]
- Li, Y.; Lv, C.; Dang, L.; Lin, B.; Tao, J.; Zhang, C.; Zhou, X.; Ma, H.; Lu, Y.; Chen, R.; et al. Effectiveness of ixekizumab in Chinese patients with moderate-severe plaque psoriasis with special area involvement: Subanalysis of a prospective, multicenter, observational real-world study. Dermatol. Ther. 2024, 14, 907–918. [Google Scholar] [CrossRef]
- Armstrong, A.; González-Cantero, A.; Khattri, S.; Muzy, G.; Malatestinic, W.N.; Lampropoulou, A.; Feely, M.; See, S.K.; Mert, C.; Blauvelt, A. Comparing achievement of National Psoriasis Foundation treatment targets among patients with plaque psoriasis treated with ixekizumab versus other biologics in clinical and real-world studies. Dermatol. Ther. 2024, 14, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.; Gargiulo, L.; Ibba, L.; Cortese, A.; Toso, F.; Orsini, D.; Lora, V.; Frascione, P.; Sena, P.; Carugno, A.; et al. Effectiveness of ixekizumab for the treatment of moderate-to-severe plaque psoriasis with involvement of difficult-to-treat areas: A 52-week multicenter retrospective study. J. Dermatol. 2024, 51, 839–843. [Google Scholar] [CrossRef]
- Mucherino, S.; Rafaniello, C.; Serino, M.; Zinzi, A.; Trama, U.; Capuano, A.; Menditto, E.; Orlando, V. Drug utilization and measurement of medication adherence: A real-world study of psoriasis in Italy. Pharmaceutics 2023, 15, 2647. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Megna, M.; Giunta, A.; Carrera, C.G.; Dapavo, P.; Balato, A.; Malagoli, P.; Mazzoccoli, S.; Parodi, A.; Sabatino, S.; et al. Ixekizumab is effective in the long-term management in moderate-to-severe plaque psoriasis: Results from an Italian retrospective cohort study (the LOTIXE study). J. Dermatol. Treat. 2023, 34, 2246606. [Google Scholar] [CrossRef]
- Wang, C.; Torisu-Itakura, H.; Hanada, T.; Matsuo, T.; Cai, Z.; Osaga, S.; Aranishi, T. Treatment persistence of interleukin-17 inhibitor class drugs among patients with psoriasis in Japan: A retrospective database study. J. Dermatol. Treat. 2023, 34, 2229465. [Google Scholar] [CrossRef]
- Reich, A.; Reed, C.; Schuster, C.; Robert, C.; Treuer, T.; Lubrano, E. Real-world evidence for ixekizumab in the treatment of psoriasis and psoriatic arthritis: Literature review 2016–2021. J. Dermatol. Treat. 2023, 34, 2160196. [Google Scholar] [CrossRef]
- Thomas, S.E.; Barenbrug, L.; Hannink, G.; Seyger, M.M.B.; de Jong, E.M.G.J.; van den Reek, J.M.P.A. Drug survival of IL-17 and IL-23 inhibitors for psoriasis: A systematic review and meta-analysis. Drugs 2024, 84, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Sewerin, P.; Schuster, C.; Ng, K.J.; Papadimitropoulos, M.; Gadagamma, S.; Nuñez, M.; Lampropoulou, A. Real-world evidence for ixekizumab in the treatment of psoriasis, psoriatic arthritis, and axial spondyloarthritis: Systematic literature review 2022–2023. Adv. Ther. 2025, 42, 4224–4254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gulliver, W.; Penney, M.; Power, R.; Gulliver, S.; Montmayeur, S.; Burge, R. Moderate-to-severe plaque psoriasis patients treated with ixekizumab: Early real world outcomes and adverse events. J. Dermatol. Treat. 2022, 33, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Lynde, C.; Turchin, I.; Avadisian, M.; Labelle, M.; Papp, K.A. Real-world, long-term treatment patterns of commonly used biologics in Canadian patients with moderate-to-severe chronic plaque psoriasis. J. Dermatol. 2022, 49, 95–105. [Google Scholar] [CrossRef]
- Kishimoto, M.; Komine, M.; Kamiya, K.; Sugai, J.; Mieno, M.; Ohtsuki, M. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J. Dermatol. 2020, 47, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kojanova, M.; Hugo, J.; Velackova, B.; Cetkovska, P.; Fialova, J.; Dolezal, T.; Tichy, M.; Gkalpakiotis, S. Efficacy, safety, and drug survival of patients with psoriasis treated with IL-17 inhibitors—Brodalumab, ixekizumab, and secukinumab: Real-world data from the Czech Republic BIOREP registry. J. Dermatol. Treat. 2022, 33, 2827–2837. [Google Scholar] [CrossRef]
- Lee, E.B.; Pithadia, D.J.; Reynolds, K.A.; Reddy, S.P.; Egeberg, A.; Wu, J.J. Real-world drug survival of ixekizumab for psoriasis. J. Am. Acad. Dermatol. 2019, 81, 270–272. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.J.; Zhong, X.Y.; Yu, Y.Y.; Yu, N.; Wang, Y.; Yi, X.-M.; Ding, Y.-F.; Shi, Y.-L. Drug Survival Outcomes Associated with the Real-World Use of Ixekizumab, Secukinumab, Guselkumab, and Adalimumab for the Treatment of Plaque Psoriasis in China: A 52-Week Single-Center Retrospective Study. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2245–2252. Available online: https://www.dovepress.com/drug-survival-outcomes-associated-with-the-real-world-use-of-ixekizuma-peer-reviewed-fulltext-article-CCID (accessed on 21 December 2024). [CrossRef]
- Lunder, T.; Zorko, M.S.; Kolar, N.K.; Suhodolcan, A.B.; Marovt, M.; Leskovec, N.K.; Marko, P.B. Drug survival of biological therapy is showing class effect: Updated results from Slovenian National Registry of psoriasis. Int. J. Dermatol. 2019, 58, 631–641. [Google Scholar] [CrossRef]
- Lockshin, B.; Cronin, A.; Harrison, R.W.; McLean, R.R.; Anatale-Tardiff, L.; Burge, R.; Zhu, B.; Malatestinic, W.N.; Atiya, B.; Murage, M.J.; et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: The Corrona Psoriasis Registry. Dermatol. Ther. 2021, 34, e14808. [Google Scholar] [CrossRef]
- Schots, L.; Soenen, R.; Blanquart, B.; Thomas, D.; Lambert, J. Blocking interleukin-17 in psoriasis: Real-world experience from the PsoPlus cohort. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Graier, T.; Salmhofer, W.; Jonak, C.; Weger, W.; Kölli, C.; Gruber, B.; Sator, P.; Prillinger, K.; Mlynek, A.; Schütz-Bergmayr, M.; et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: A time-period-adjusted registry analysis. Br. J. Dermatol. 2021, 184, 1094–1105. [Google Scholar] [CrossRef]
- Torres, T.; Puig, L.; Vender, R.; Yeung, J.; Carrascosa, J.M.; Piaserico, S.; Gisondi, P.; Lynde, C.; Ferreira, P.; Bastos, P.M.; et al. Drug survival of interleukin (IL)-17 and IL-23 inhibitors for the treatment of psoriasis: A retrospective multi-country, multicentric cohort study. Am. J. Clin. Dermatol. 2022, 23, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Tsuruta, N.; Yamaguchi, K.; Ohata, C.; Ohyama, B.; Katayama, E.; Sugita, K.; Kuwashiro, M.; Hashimoto, A.; Yonekura, K.; et al. Survival rates of systemic interventions for psoriasis in the Western Japan Psoriasis Registry: A multicenter retrospective study. J. Dermatol. 2023, 50, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Balato, A.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; Dapavo, P.; Dini, V.; et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for moderate-to-severe plaque psoriasis: A retrospective multicenter real-world experience on 5932 treatment courses—IL PSO (Italian landscape psoriasis). Front. Immunol. 2023, 14, 1341708. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.; Zhdanava, M.; Pilon, D.; Shah, A.; Hilts, A.; Lefebvre, P.; Feldman, S.R. Long-term psoriasis control with guselkumab, adalimumab, secukinumab, or ixekizumab in the USA. Dermatol. Ther. 2023, 13, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Galache Osuna, C.; Gómez-Vila, B.; Aubán Pariente, J.; Vázquez Losada, B.; Gómez de Castro, C.; Requena López, S.; Velázquez, Á.d.D.; García, L.P.; Fernández, L.O.; Diez, S.G.; et al. Ustekinumab drug survival in patients with psoriasis: A retrospective study of real clinical practice. Medicina 2020, 56, 584. [Google Scholar] [CrossRef]
- Gómez-de Castro, C.; Mir-Bonafé, M.; Arias-Martínez, A.; Martínez-Camblor, P.; Díaz-Coto, S.; Santos-Juanes, J. Comment on “Baseline patients’ characteristics as predictors for therapeutic survival and response in patients with psoriasis on biological treatments”. Australas. J. Dermatol. 2019, 60, e258–e259. [Google Scholar] [CrossRef]
- Palacios-García, L.; Gómez-de-Castro, C.; Mir-Bonafé, M.; Calzón, C.; Galache, C.; Santos-Juanes, J. Comment on “Secukinumab drug survival in patients with psoriasis: A multicenter, real-world, retrospective study”. J. Am. Acad. Dermatol. 2019, 81, e81–e82. [Google Scholar] [CrossRef]
- Ting, S.; Lowe, P.; Smith, A.; Fernández-Peñas, P. Drug survival of biologics in psoriasis: An Australian multicentre retrospective study. Australas. J. Dermatol. 2024, 65, 350–357. [Google Scholar] [CrossRef]
- Chabra, S.; Birt, J.; Bolce, R.; Lisse, J.; Malatestinic, W.N.; Zhu, B.; Kimel, M.; McCormack, J.; Stefan, M.; Cragun, W.C. Satisfaction with the injection experience of a new, citrate-free formulation of ixekizumab. Adv. Ther. 2024, 41, 1672–1684. [Google Scholar] [CrossRef]
- Malagoli, P.; Dapavo, P.; Pavia, G.; Amoruso, F.; Argenziano, G.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; Dini, V.; et al. Real-life long-term efficacy and safety of ixekizumab in moderate-to-severe psoriasis: A 192 weeks multicentric retrospective study—IL PSO (Italian landscape psoriasis). Dermatol. Ther. 2022, 35, e15608. [Google Scholar] [CrossRef]
- Osuna, C.G.; García, S.R.; Martín, J.C.; Jiménez, V.G.; López, F.V.; Santos-Juanes, J. Use of biological treatments in elderly patients with skin psoriasis in the real world. Life 2021, 11, 1348. [Google Scholar] [CrossRef]
- Hacınecipoğlu, F.; Çelik, G.; Kartal, S.P. Efficacy and safety of IL-17 and IL-23 inhibitors in elderly patients with plaque psoriasis: A real-world study. J. Dermatol. 2025, 52, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
| Total Patients (n = 103) | <65 y (n = 85) | ≥65 y (n = 18) | p | |
|---|---|---|---|---|
| Sex (male), n (%) | 46 (44.7%) | 39 (45.9%) | 7 (38.9%) | 0.779 |
| Age at start of biologic treatment (years), mean ± SD | 51.1 ± 14.3 | 46.85 ± 11.76 | 71.22 ± 4.58 | 0.001 |
| Positive family history of psoriasis (yes), n (%) | 67 (65%) | 58 (68.2%) | 9 (50%) | 0.229 |
| Onset before 40 years of age (%) | 82 (79.6%) | 75 (88.2%) | 11 (61.1%) | 0.014 |
| Duration of treatment (months); mean | 52.5 (IC95% = 46.01–58.99) | 48.30 (IC = 40.89–55.71) | 67.44 (IC = 60.67–74.20) | 0.003 |
| Initial PASI | 13.2 ± 6.1 | 14.4 ± 7.3 | 12.8 ± 5.8 | 0.32 |
| Comorbidities, n (%) | ||||
| Obesity (BMI ≥ 30) | 47 (45.6%) | 41 (48.2%) | 6 (33.3) | 0.372 |
| Diabetes mellitus | 19 (18.4%) | 13 (15.3%) | 6 (33.3%) | 0.145 |
| Arterial hypertension | 46 (44.7%) | 33 (38.8%) | 13 (72.2%) | 0.020 |
| Dyslipidemia | 44 (42.7%) | 32 (37.6%) | 12 (66.7%) | 0.046 |
| Arthritis | 59 (57.3%) | 49 (57.6) | 10 (55.6%) | 1.000 |
| Prior treatments with biologics (%) | 99 (96.1%) | 82 (96.5%) | 17 (94.4%) | 1.000 |
| One biologic | 37 (35.9%) | 30 (35.3%) | 7 (38.9%) | |
| Two biologics | 39 (37.9%) | 33 (38.8%) | 6 (33.3%) | 0.115 |
| Three biologics | 16 (15.5%) | 15 (17.6%) | 1(5.6%) | |
| Four biologics | 6 (5.8%) | 4 (4.7%) | 2 (11.1%) | |
| Five Biologics | 1 (1%) | 0 | 1 (5.6%) |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Univariate Analysis | p-Value | HR (95% CI) | p-Value | HR (95% CI) |
| Age at treatment initiation ≥ 65 years | 0.039 | 0.123 (0.017–0.902) | 0.023 | 0.079 (0.009–0.700) |
| Age at psoriasis onset ≥ 40 years | 0.238 | 0.986 (0.964–1.009) | 0.534 | 1.475 (0.432–5.034) |
| Female sex | 0.145 | 1.729 (0.827–3.612) | 0.054 | 2.436 (0.986–6.019) |
| Obesity (BMI ≥ 30) | 0.444 | 1.317 (0.650–2.667) | 0.546 | 1.271 (0.583–2775) |
| Arthritis (yes) | 0.512 | 0.789 (0.388–1.604) | 0.287 | 0.654 (0.299–1.431) |
| Hypertension (yes) | 0.277 | 0.671 (0.327–1.377) | 0.578 | 0.774 (0.313–1.913) |
| Dyslipidemia (yes) | 0.540 | 1.248 (0.615–2.529) | 0.104 | 1.975 (0.869–4.489) |
| Family history of psoriasis (yes) | 0.591 | 1.230 (0.578–2.616) | 0.762 | 0.880 (0.383–2.019) |
| Diabetes (yes) | 0.868 | 0.927 (0.380–2.262) | 0.796 | 0.869 (0.299–2.524) |
| >1 prior biologic | 0.869 | 0.939 (0.445–1.983) | 0.813 | 1.105 (0.481–2.019) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noval-Martín, I.; Santos-Juanes, J.; Álvarez-Losada, I.; Palacios-García, L.; Lozano-Blazquez, A.; García-Jimenez, V.; Galache Osuna, C.; Galache, R.S.-J. The Age Factor in Ixekizumab Survival: Older Patients Show Higher Long-Term Treatment Survival. Medicina 2025, 61, 1827. https://doi.org/10.3390/medicina61101827
Noval-Martín I, Santos-Juanes J, Álvarez-Losada I, Palacios-García L, Lozano-Blazquez A, García-Jimenez V, Galache Osuna C, Galache RS-J. The Age Factor in Ixekizumab Survival: Older Patients Show Higher Long-Term Treatment Survival. Medicina. 2025; 61(10):1827. https://doi.org/10.3390/medicina61101827
Chicago/Turabian StyleNoval-Martín, Inés, Jorge Santos-Juanes, Irene Álvarez-Losada, Laura Palacios-García, Ana Lozano-Blazquez, Virginia García-Jimenez, Cristina Galache Osuna, and Raquel Santos-Juanes Galache. 2025. "The Age Factor in Ixekizumab Survival: Older Patients Show Higher Long-Term Treatment Survival" Medicina 61, no. 10: 1827. https://doi.org/10.3390/medicina61101827
APA StyleNoval-Martín, I., Santos-Juanes, J., Álvarez-Losada, I., Palacios-García, L., Lozano-Blazquez, A., García-Jimenez, V., Galache Osuna, C., & Galache, R. S.-J. (2025). The Age Factor in Ixekizumab Survival: Older Patients Show Higher Long-Term Treatment Survival. Medicina, 61(10), 1827. https://doi.org/10.3390/medicina61101827







