Comparison of the Effects of Sugammadex and Pyridostigmine on Postoperative Nausea and Vomiting and the Recovery Profile in Pediatric Patients Undergoing Strabismus Surgery: A Prospective, Double-Blind, Observational Study
Abstract
1. Introduction
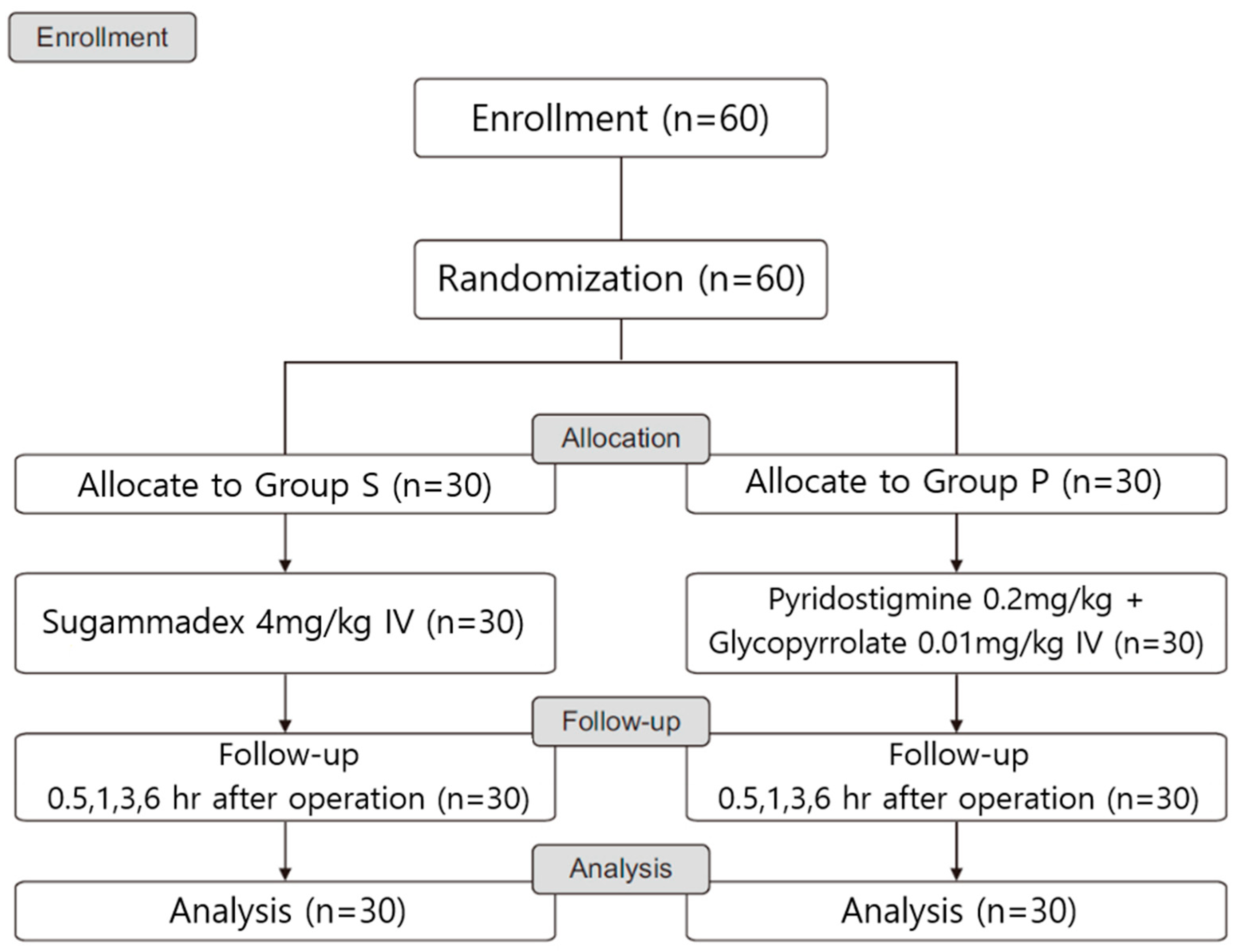
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Inclusion and Exclusion Criteria
2.3. Anesthesia, NMBRDs, PONV, Heart Rate and Covariates
- (1)
- Age, gender, height, weight, body mass index, ASA-PS, and anesthesia time.
- (2)
- The primary endpoint was the frequency estimation of PONV using the BARF scale—PONV between 30 min and 1 h after surgery was measured in the PACU and between 3 and 6 h after surgery in the ward.
- (3)
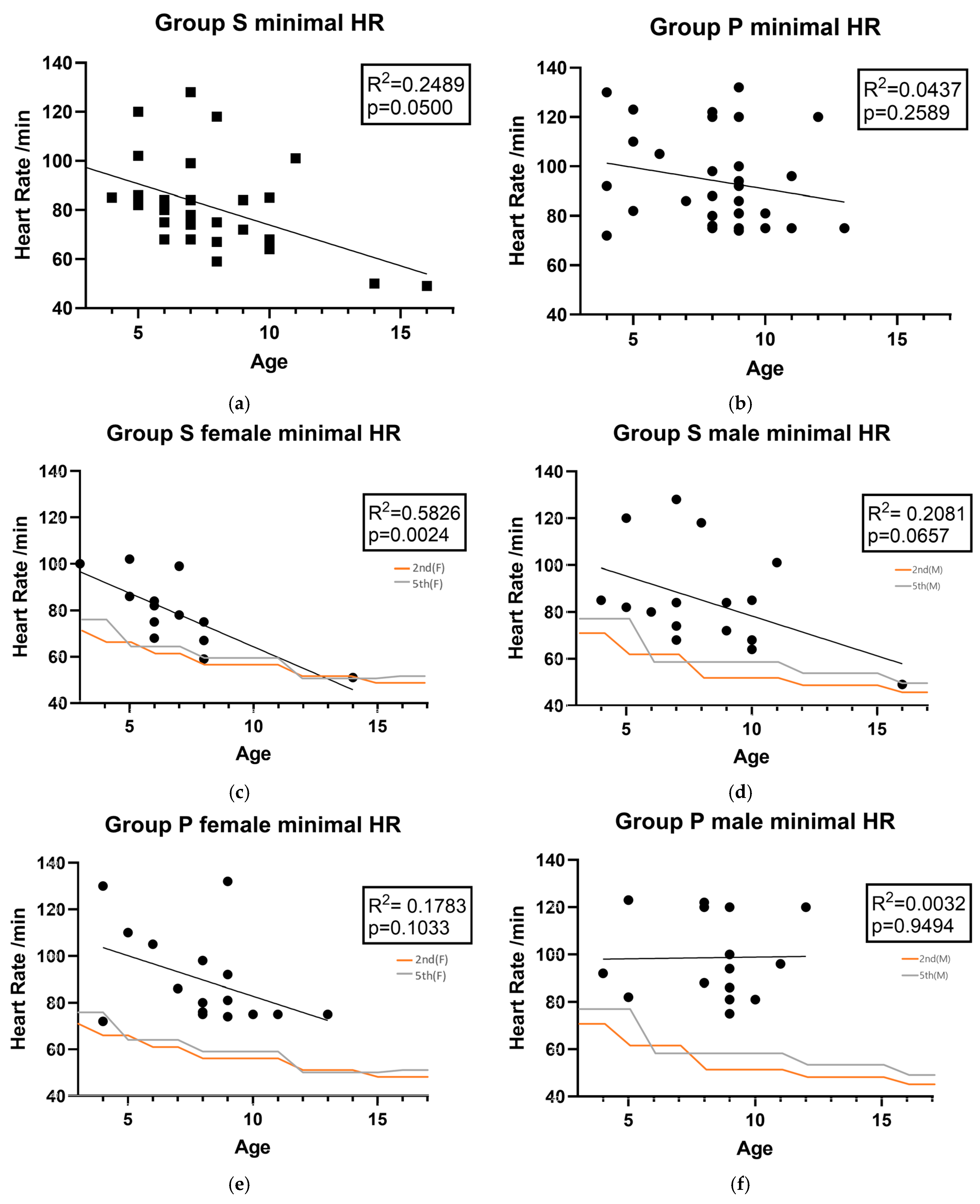
- The secondary endpoints were the recovery time to TOF > 0.9 from the NMBRD administration, change in heart rate (HR) after NMBRD administration, and the difference between the basal and maximal change in the HR after NMBRD administration, as measured by an anesthetist blinded to the NMBRD.
2.4. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. The Incidences of PONV
3.3. The Recovery Profile of Sugammadex and Pyridostigmine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| ASA-PS | Anesthesiologists Physical Status |
| BARF | Baxter Animated Retching Faces |
| GD | Gastric Decompression |
| HR | Heart Rate |
| NMB | Neuromuscular Blockade |
| NMBRD | Neuromuscular Blockade Reversal Drug |
| PACU | Post-anesthesia Care Unit |
| PONV | Postoperative Nausea and Vomiting |
| TOF | Train-of-four |
References
- Ames, W.A.; Machovec, K. An update on the management of PONV in a pediatric patient. Best Pr. Pract. Res. Clin. Anaesthesiol. 2020, 34, 749–758. [Google Scholar] [CrossRef]
- Joshi, G.P.; Abdelmalak, B.B.; Weigel, W.A.; Harbell, M.W.; Kuo, C.I.; Soriano, S.G.; Stricker, P.A.; Tipton, T.; Grant, M.D.; Marbella, A.M.; et al. 2023 American Society of Anesthesiologists Practice Guidelines for Preoperative Fasting: Carbohydrate-containing Clear Liquids with or without Protein, Chewing Gum, and Pediatric Fasting Duration—A Modular Update of the 2017 American Society of Anesthesiologists Practice Guidelines for Preoperative Fasting. Anesthesiology 2023, 138, 132–151. [Google Scholar]
- Weibel, S.; Schaefer, M.S.; Raj, D.; Rücker, G.; Pace, N.L.; Schlesinger, T.; Meybohm, P.; Kienbaum, P.; Eberhart, L.H.J.; Kranke, P. Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: An abridged Cochrane network meta-analysis. Anaesthesia 2021, 76, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Gupta, R.; Gupta, L.K. Sugammadex versus Neostigmine for Reversal of Neuromuscular Blockade in Adults and Children: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Drug Saf. 2024, 19, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, M.S.; Choi, J.W.; Kim, H.; Kwon, Y.S.; Lee, J.J. Comparison of the Effects of Sugammadex, Neostigmine, and Pyridostigmine on Postoperative Nausea and Vomiting: A Propensity Matched Study of Five Hospitals. J. Clin. Med. 2020, 9, 3477. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Ravi, K.; Camilleri, M.; Andrews, C.; Szarka, L.A.; Low, P.A.; Zinsmeister, A.R.; Bharucha, A.E. Effect of Neostigmine on Gastroduodenal Motility in Patients With Suspected Gastrointestinal Motility Disorders. Neurogastroenterol. Motil. 2015, 27, 1761–1768. [Google Scholar] [CrossRef]
- Lewis, S.M.B.L.; Heitkemper, M.M.; Harding, M.; Kwong, J.; Roberts, D. Medical Surgical Nursing: Assessment and Management of Clinical Problems; Elsevier: St. Louis, MO, USA, 2017. [Google Scholar]
- Jung, K.T.; Kim, S.H.; Kim, D.J.; Kim, S.H.; An, T.H. Effect of gastric decompression on postoperative vomiting in pediatric patients undergoing strabismus surgery: A randomized controlled study. Anesth. Pain Med. 2020, 15, 66–72. [Google Scholar] [CrossRef]
- Watcha, M.F.; Lee, A.D.; Medellin, E.; Felberg, M.T.; Bidani, S.A. Clinical Use of the Pictorial Baxter Retching Faces Scale for the Measurement of Postoperative Nausea in Children. Anesth. Analg. 2019, 128, 1249–1255. [Google Scholar] [CrossRef]
- Alsuhebani, M.; Sims, T.; Hansen, J.K.; Hakim, M.; Walia, H.; Miller, R.; Tumin, D.; Tobias, J.D. Heart rate changes following the administration of sugammadex in children: A prospective, observational study. J. Anesth. 2020, 34, 238–242. [Google Scholar] [CrossRef]
- Saarel, E.V.; Granger, S.; Kaltman, J.R.; Minich, L.L.; Tristani-Firouzi, M.; Kim, J.J.; Ash, K.; Tsao, S.S.; Berul, C.I.; Stephenson, E.A.; et al. Electrocardiograms in Healthy North American Children in the Digital Age. Circ. Arrhythmia Electrophysiol. 2018, 11, e005808. [Google Scholar] [CrossRef]
- Bom, A.; Hope, F.; Rutherford, S.; Thomson, K. Preclinical pharmacology of sugammadex. J. Crit. Care 2009, 24, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Kim, J.H.; Hwang, S.M.; Choi, J.W.; Kang, S.S. Comparison of the Effect of Sugammadex and Pyridostigmine on Postoperative Catheter-Related Bladder Discomfort: A Retrospective Matched Cohort Analysis. Medicina 2022, 58, 590. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Choi, G.J.; Kang, H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C.; Oh, J.; Park, Y.H. Effects of sugammadex vs. pyridostigmine-glycopyrrolate on post-operative nausea and vomiting: Propensity score matching. Acta Anaesthesiol. Scand. 2017, 61, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ure, D.; James, K.S.; McNeill, M.; Booth, J.V. Glycopyrrolate reduces nausea during spinal anaesthesia for caesarean section without affecting neonatal outcome. Br. J. Anaesth. 1999, 82, 277–279. [Google Scholar] [CrossRef]
- Hurford, W.E.; Eckman, M.H.; Welge, J.A. Data and meta-analysis for choosing sugammadex or neostigmine for routine reversal of rocuronium block in adult patients. Data Brief 2020, 32, 106241. [Google Scholar] [CrossRef]
- Mat, N.; Yeoh, C.N.; Maaya, M.; Zain, J.M.; Ooi, J.S.M. Effects of Sugammadex and Neostigmine on Post-operative Nausea and Vomiting in ENT Surgery. Front. Med. 2022, 9, 905131. [Google Scholar] [CrossRef]
- Kranke, P.; Eberhart, L.H.; Toker, H.; Roewer, N.; Wulf, H.; Kiefer, P. A prospective evaluation of the POVOC score for the prediction of postoperative vomiting in children. Anesth. Analg. 2007, 105, 1592–1597. [Google Scholar] [CrossRef]
- Eberhart, L.H.J.; Geldner, G.; Kranke, P.; Morin, A.M.; Schauffelen, A.; Treiber, H.; Wulf, H. The development and validation of a risk score to predict the probability of postoperative vomiting in pediatric patients. Anesth. Analg. 2004, 99, 1630–1637. [Google Scholar] [CrossRef]
- Bourdaud, N.; Devys, J.M.; Bientz, J.; Lejus, C.; Hebrard, A.; Tirel, O.; Lecoutre, D.; Sabourdin, N.; Nivoche, Y.; Baujard, C.; et al. Development and validation of a risk score to predict the probability of postoperative vomiting in pediatric patients: The VPOP score. Paediatr. Anaesth. 2014, 24, 945–952. [Google Scholar] [CrossRef]
- Nathan, N. Management of Postoperative Nausea and Vomiting: The 4th Consensus Guidelines. Anesth. Analg. 2020, 131, 410. [Google Scholar] [CrossRef]
- Voss, T.; Wang, A.; DeAngelis, M.; Speek, M.; Saldien, V.; Hammer, G.B.; Wrishko, R.; Herring, W.J. Sugammadex for reversal of neuromuscular blockade in pediatric patients: Results from a phase IV randomized study. Paediatr. Anaesth. 2022, 32, 436–445. [Google Scholar] [CrossRef]
- Messerer, B.; Stijic, M.; Sandner-Kiesling, A.; Brillinger, J.M.; Helm, J.; Scheer, J.; Strohmeier, C.S.; Avian, A. Is PONV still a problem in pediatric surgery: A prospective study of what children tell us. Front. Pediatr. 2023, 11, 1241304. [Google Scholar] [CrossRef]
- Kovac, A.L. Pathophysiology and Risk Factors for Postoperative Nausea and Vomiting in Adults and Children. BJA Educ. 2025, 25, 234–239. [Google Scholar] [CrossRef]
- Carvalho, E.V.G.; Caldas, S.M.C.; Costa, D.F.P.P.M.D.; Gomes, C.M.G.P. Bradycardia in a pediatric population after sugammadex administration: Case series. Braz. J. Anesthesiol. 2023, 73, 101–103. [Google Scholar] [CrossRef]
- Vaswani, Z.G.; Smith, S.M.; Zapata, A.; Gottlieb, E.A.; Sheeran, P.W. Bradycardic Arrest in a Child with Complex Congenital Heart Disease Due to Sugammadex Administration. J. Pediatr. Pharmacol. Ther. 2023, 28, 667–670. [Google Scholar] [CrossRef]
- Kang, M.; Ragan, B.G.; Park, J.H. Issues in outcomes research: An overview of randomization techniques for clinical trials. J. Athl. Train. 2008, 43, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Mraovic, B.; Timko, N.J.; Choma, T.J. Comparison of recovery after sugammadex or neostigmine reversal of rocuronium in geriatric patients undergoing spine surgery: A randomized controlled trial. Croat. Med. J. 2021, 62, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yoon, S.W.; Choi, G.J.; Park, Y.-H.; Kang, H.; Baek, C.W.; Jung, Y.H.; Woo, Y.C. The Effects of Glycopyrrolate as Premedication on Post-Operative Nausea and Vomiting: A Propensity Score Matching Analysis. Open Anesth. J. 2019, 13, 93–99. [Google Scholar] [CrossRef][Green Version]
| Variable | Group S (n = 30) | Group P (n = 30) | p-Value |
|---|---|---|---|
| Age (years) | 7.60 ± 2.8 (6.5–8.7) | 8.07 ± 2.3 (7.2–8.9) | 0.484 |
| Sex (M/F) | (17/13) | (14/16) | 0.605 |
| BMI | 18.9 ± 3.9 (17.4–20.4) | 18.3 ± 4.8 (16.5–20.1) | 0.592 |
| ASA-PS Class (I/II) | (28/2) | (26/4) | 0.671 |
| Anesthesia Time (min) | 64.3 ± 12.2 (59.8–68.9) | 62.9 ± 11.2 (62.9–71.3) | 0.364 |
| Time After Emergence | Group S (n = 30) | Group P (n = 30) | p-Value |
|---|---|---|---|
| 0.5 h | 4/30 (13.3%) | 4/30 (13.3%) | 1.000 |
| 1 h | 2/30 (6.7%) | 1/30 (3.3%) | 0.554 |
| 3 h | 0 | 0 | - |
| 6 h | 0 | 0 | - |
| Variables | Group S (n = 30) | Group P (n = 30) | p-Value |
|---|---|---|---|
| TOF > 0.9 time (s) | 62.7 ± 27.0 (50.9–74.5) | 125.9 ± 47.6 (105.3–147.1) | <0.001 |
| Heart rate variation (%) | 13.9 ± 11.2 | 0.3 ± 11.7 | <0.001 |
| Changes over-limit HR (n) | (3/0) | (1/0) |
| Changes in HR | Group S (n = 30) | Group P (n = 30) | p-Value |
|---|---|---|---|
| Baseline (before induction) | 100.1 ± 15.7 (94.3–106.0) | 102.5 ± 11.2 (98.3–106.7) | 0.504 |
| Before HR NMBRD use | 95.1 ± 17.9 (88.4–101.8) | 95.0 ± 16.9 (88.7–101.3) | 0.994 |
| After HR NMBRD use | 81.8 ± 19.2 (74.7–89.0) | 94.0 ± 19.3 (86.8–101.2) | 0.018 |
| PACU (30 min after NMBRD) | 107.0 ± 15.0 (101.4–112.6) | 103.1 ± 10.1 (99.3–106.8) | 0.240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Jong, H.S.; Ha, E.G.; Cho, S.Y.; Jung, K.T.; Kim, D.J. Comparison of the Effects of Sugammadex and Pyridostigmine on Postoperative Nausea and Vomiting and the Recovery Profile in Pediatric Patients Undergoing Strabismus Surgery: A Prospective, Double-Blind, Observational Study. Medicina 2025, 61, 1826. https://doi.org/10.3390/medicina61101826
Kim SH, Jong HS, Ha EG, Cho SY, Jung KT, Kim DJ. Comparison of the Effects of Sugammadex and Pyridostigmine on Postoperative Nausea and Vomiting and the Recovery Profile in Pediatric Patients Undergoing Strabismus Surgery: A Prospective, Double-Blind, Observational Study. Medicina. 2025; 61(10):1826. https://doi.org/10.3390/medicina61101826
Chicago/Turabian StyleKim, Se Hun, Hwa Song Jong, Eun Gyo Ha, Su Yeon Cho, Ki Tae Jung, and Dong Joon Kim. 2025. "Comparison of the Effects of Sugammadex and Pyridostigmine on Postoperative Nausea and Vomiting and the Recovery Profile in Pediatric Patients Undergoing Strabismus Surgery: A Prospective, Double-Blind, Observational Study" Medicina 61, no. 10: 1826. https://doi.org/10.3390/medicina61101826
APA StyleKim, S. H., Jong, H. S., Ha, E. G., Cho, S. Y., Jung, K. T., & Kim, D. J. (2025). Comparison of the Effects of Sugammadex and Pyridostigmine on Postoperative Nausea and Vomiting and the Recovery Profile in Pediatric Patients Undergoing Strabismus Surgery: A Prospective, Double-Blind, Observational Study. Medicina, 61(10), 1826. https://doi.org/10.3390/medicina61101826






