Abstract
Background and Objectives: Ixekizumab is a human monoclonal antibody targeting interleukin-17A, approved for the treatment of moderate-to-severe plaque psoriasis. Given its demonstrated efficacy and safety in clinical trials, this study aimed to evaluate the real-world drug survival of Ixekizumab and identify clinical predictors of treatment discontinuation. Materials and Methods: A retrospective, observational, hospital-based study was conducted in the Department of Dermatology at the Central University Hospital of Asturias (HUCA). Patients with moderate-to-severe plaque psoriasis who initiated treatment with Ixekizumab (Taltz®) between 8 June 2017 and 10 October 2024, were included. Demographic data, comorbidities, age at disease onset, family history, PASI score, and previous treatments were recorded. Drug survival was assessed using Kaplan–Meier survival curves and the log-rank test. Predictors of discontinuation were analyzed using univariate and multivariate Cox proportional hazards models. Results: A total of 103 patients (55.3% women) were included. Drug survival rates were 85% at one year, 73% at two years, and 61% at four years, with a mean treatment duration of 52.5 months (95% CI: 46.01–58.99). Multivariate analysis showed that patients under the age of 65 had a significantly higher risk of treatment discontinuation (hazard ratio: 1.813; p < 0.05). The most common reason for discontinuation was secondary treatment failure (45.16%). Ixekizumab demonstrated sustained drug survival in a real-world setting, with rates falling within the mid-to-upper range reported in the literature. Older age (>65 years) was associated with greater treatment persistence, highlighting a potential influence of age on long-term therapeutic adherence.
1. Introduction
Psoriasis is a chronic, inflammatory, immune-mediated proliferative disease associated with T-cell dysfunction. It primarily affects the skin, nails, and joints, although it is now considered a systemic condition due to its frequent association with cardiovascular, metabolic, and neuropsychiatric comorbidities, among others [1,2]. Clinically, psoriasis is classified as mild, moderate, or severe. According to data from Europe and the United States, approximately 80% of patients present with mild forms of the disease. In moderate-to-severe cases, psoriasis has a significant impact on quality of life, with both physical and psychological consequences [1].
The most common clinical presentation of psoriasis is the so-called plaque psoriasis (psoriasis vulgaris), accounting for approximately 85–90% of all cases. It is characterized by well-demarcated erythematous plaques covered with whitish or silvery scales, typically distributed symmetrically. The most frequently affected sites include the scalp, elbows, and knees—particularly the extensor surfaces—as well as the umbilical area and sacral region, although lesions may appear on any part of the body. The disease follows a chronic course, marked by recurrent flares and periods of remission [3].
Current therapeutic options include topical treatments, systemic therapies, and phototherapy [4,5,6]. Treatment selection is determined by various factors, including clinical severity—typically classified as mild or moderate-to-severe—comorbidities, and the presence of psoriatic arthritis [6]. The introduction of biologic therapies has represented a major shift in the therapeutic goals for psoriasis. According to current guidelines, biologic agents may be used as first-line treatment for moderate-to-severe psoriasis, demonstrating greater efficacy compared to conventional systemic therapies such as methotrexate [7,8].
The pathophysiology of psoriasis involves a complex interplay between cytokines, specific receptors, and intracellular signaling molecules that ultimately result in an inflammatory cascade. Biologic therapies act by selectively targeting key molecules involved in the disease process. The most relevant signaling pathways in plaque psoriasis include tumor necrosis factor-alpha (TNF-α), interleukin-23 (IL-23), and interleukin-17 (IL-17) [9]. The IL-17 family comprises structurally related cytokines, from IL-17A to IL-17F. Among these, IL-17A, IL-17C, and IL-17F are most prominently expressed in psoriatic lesions. IL-17A, secreted by activated T-helper cells, acts primarily on keratinocytes, stimulating the production of proinflammatory cytokines, chemokines, and other mediators that recruit neutrophils, macrophages, and lymphocytes, and activate local fibroblasts. Furthermore, IL-17 has been linked to increased keratinocyte proliferation and mitotic activity [9].
Ixekizumab is a humanized IgG4 monoclonal antibody that selectively binds to IL-17A with high specificity and affinity thereby neutralizing its biological activity and disrupting the proinflammatory cascade typical of psoriasis [10,11]. Although IL-17C and IL-17F are found at higher concentrations in psoriatic lesions, IL-17A is considered the most biologically active cytokine of the group and a central mediator of inflammation and tissue damage in plaque psoriasis [9]. Ixekizumab was approved by the U.S. Food and Drug Administration (FDA) in March 2016, and subsequently by the European Medicines Agency (EMA), for the treatment of moderate-to-severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. The recommended dosing regimen includes a 160 mg initial dose administered subcutaneously (as two 80 mg injections) at week 0, followed by 80 mg every two weeks through week 12. From week 16 onwards, a maintenance dose of 80 mg every four weeks is indicated [12,13].
Drug survival refers to the duration over which a treatment remains effective, safe, and acceptable to the patient [14]. This metric not only reflects therapeutic effectiveness and tolerability but also encompasses patient satisfaction and adherence. Given that drug survival may be influenced by both clinical efficacy and patient behavior (i.e., adherence), future studies should incorporate adherence measures to provide a more accurate assessment of real-world treatment outcomes. This consideration is particularly relevant, as non-adherence can confound the interpretation of drug survival data and lead to overly optimistic estimates of treatment effectiveness and safety [15].
The aim of this study was to assess the long-term drug survival of ixekizumab and to identify clinical predictors of treatment discontinuation in patients with plaque psoriasis managed at the Dermatology Department of HUCA.
2. Materials and Methods
We performed a retrospective, single-center study in the Department of Dermatology at the Central University Hospital of Asturias (HUCA), including patients treated with ixekizumab between 8 June 2017, and 10 October 2024. The study protocol was approved by the Ethics and Research Committee of the Principality of Asturias, Spain (Ref. 2024-432).
A total of 103 patients who received standard doses of ixekizumab (Taltz®) for plaque psoriasis were included. Clinical and demographic baseline data were retrieved from electronic medical records. The following variables were collected: sex, age, weight, height, family history of psoriasis (at least one affected first-degree relative), age at disease onset, prior exposure to biologics (categorized as biologic-naïve or previously treated), and presence of psoriatic arthritis confirmed by a rheumatologist.
Comorbidities such as hypertension, diabetes mellitus, and dyslipidemia were also recorded. These were identified through medical history, patient report, or active treatment with antihypertensive, antidiabetic, or lipid-lowering agents. Patients with systolic/diastolic blood pressure > 135/85 mmHg at consultation were also classified as hypertensive. Dyslipidemia was defined as triglycerides > 150 mg/dL, total cholesterol > 200 mg/dL, or LDL cholesterol > 160 mg/dL. Body mass index (BMI) was calculated as weight in kilograms divided by height squared in meters (kg/m2), with obesity defined as BMI ≥ 30 kg/m2, following World Health Organization (WHO) criteria.
Drug survival was defined as the time from treatment initiation to permanent discontinuation. Treatment response was classified as primary failure when PASI 75 was not achieved at week 16, and as secondary failure when loss of PASI 75 occurred after an initial response beyond week 16. Uncontrolled joint disease was defined as persistence of prior symptoms or new-onset arthritis during follow-up.
Statistical Analysis
Data were summarised and statistical analyses performed with IBM SPSS version 27.0 (IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation for continuous variables, and the number and percentage for categorical variables. Group differences for qualitative variables were investigated with the chi-square test. Survival curves were derived using the Kaplan–Meier estimator and compared using the long-rank test. Cox proportional hazard regression models were used for multivariate analyses, and unadjusted and adjusted hazard ratios (HR) were both used to summarize the studied differences. 95% confidence intervals (95% CIs) are also provided. The proportionality of the risks was checked beforehand using the Schoenfeld residual.
We selected the following variables as possible predictors: sex, age of onset of psoriasis, age at treatment initiation, family history, presence of obesity, arthritis, arterial hypertension, diabetes and dyslipidemia, and previous use of biological drugs (>1). Group differences were considered statistically significant for values of p < 0.05.
The final sample size was sufficient to enable hazard ratios >1.75, proportional differences >25% and standardized differences in means >0.5 to be considered significant (Type I error = 0.05, Type II error = 0.2).
3. Results
3.1. Patient Characteristics
The study cohort comprised 103 patients, all identified as of White ethnicity. Baseline characteristics are detailed in Table 1. The differences between the two groups are that patients aged ≥ 65 years had a higher age at treatment initiation, a later age at psoriasis onset, a longer mean duration of ixekizumab therapy, and a higher prevalence of comorbidities such as hypertension and dyslipidemia.

Table 1.
Baseline characteristics of patients.
3.2. Drug Survival
The survival of the drug is shown in Figure 1.
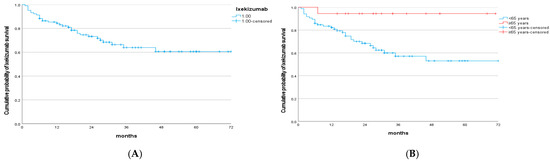
Figure 1.
(A) Kaplan–Meier curve of ixekizumab survival. (B) Kaplan–Meier curve of ixekizumab survival according to age.
Overall ixekizumab survival was 85% at year 1, 73% at year 2, and 61% from year 4 through year 6. The mean drug survival was 52.5 months (95% CI, 46.01–58.99). Using the log-rank test, statistically significant differences were observed according to treatment initiation at age < 65 years (p = 0.014) (Figure 1B). However, no significant differences in drug survival were found with respect to the presence of arthritis (p = 0.509), obesity (p = 0.441), prior biologic exposure (p = 0.868), or female sex (p = 0.139). Likewise, no significant differences were observed for hypertension (p = 0.680) or dyslipidemia (p = 868).
3.3. Univariate Analysis
In the univariate analysis, statistically significant differences were observed only for age at treatment initiation with ixekizumab (≥65 years; p = 0.039). Patients who started treatment at ≥65 years had a significantly lower risk of discontinuation compared with those who initiated therapy before the age of 65 (HR = 0.123; 95% CI, 0.017–0.902), indicating that discontinuation was more frequent among younger patients (Table 2).

Table 2.
Univariate and multivariate analysis of predictors of discontinuation with p-values and 95% confidence intervals.
3.4. Adverse Effects
Adverse events were recorded in seven patients, including oral aphthae, urticaria, diarrhea (three cases), recurrent oropharyngeal candidiasis, and vitiligo. In addition, two deaths were reported, attributable to hepatic cirrhosis and oropharyngeal carcinoma, respectively.
3.5. Patients Discontinuing Treatment
At the end of the study, 72 out of the 103 patients who initiated treatment with ixekizumab remained on therapy (69.9%). A total of 31 patients (30.1%) discontinued treatment due to the following reasons: primary failure in 8 patients (25.80%), secondary failure in 14 patients (45.16%), the occurrence of adverse events in 7 patients (22.58%) and during follow-up two deaths occurred, both unrelated to the ixekizumab: oropharyngeal carcinoma and the other to liver cirrhosis (6.45%). In the group aged >65 years, only one patient discontinued treatment due to secondary failure.
4. Discussion
In this study, we report a series of patients treated with ixekizumab in a real-world clinical practice setting. The interpretation of our findings must consider the inherent difficulty of comparing results across studies, largely due to heterogeneity in methodological approaches, differences in publication timelines, and variability in the clinical variables included in each analysis. Such factors represent significant challenges for drawing robust, generalizable conclusions. Indeed, the degree of variability can be so pronounced that, even within the same study, drug survival has been shown to differ between recruiting hospital centers [16].
Although numerous studies have investigated ixekizumab use in real-world settings [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] relatively few have specifically evaluated treatment survival [41,42,43,44,45,46,47,48,49,50,51,52,53,54]. This underscores the relevance of our work, as it provides additional evidence on long-term persistence and the reasons for discontinuation, contributing to a better understanding of ixekizumab performance beyond clinical trial conditions.
The demographic characteristics of our cohort are comparable to those described in a review of studies published between 2016 and 2021 [10]. The mean age of our patients was 51.1 years, within the range reported in that review, where mean ages varied between 45 and 53.6 years. These findings are also consistent with those published by Caldarola et al. [18], Gargiulo [53], and Gottlieb [24]. In our clinical practice, the mean age of patients treated with ixekizumab was slightly higher than that observed in our previous series with other biologic agents, such as ustekinumab (47.9 years) [55], adalimumab (45.9 years) [56], and secukinumab (50.5 years) [57]. In our cohort, women accounted for the majority of patients (55.3%), a finding that contrasts with most published series, in which male predominance has been consistently reported [20,22,48,53].
A distinctive feature of our cohort is the remarkably high proportion of patients with prior exposure to biologic therapies (96.1%), substantially exceeding the figures reported in previously published studies: Caldarola (56.86%) [18], Gargiulo (51.7%) [53], Gottlieb (48.6%) [24], and Fitzgerald (58.2%) [54]. This finding is largely explained by the therapeutic policy currently implemented in our region. According to the protocol established by the Commission for the Rational Use of Medicines of the Principality of Asturias (CURMP), adalimumab biosimilars are prioritized as the first-line biologic option for moderate-to-severe plaque psoriasis. Consequently, patients eligible for subsequent lines of therapy, such as ixekizumab, are predominantly biologic-experienced, which may influence both treatment survival and the comparability of our results with those of other cohorts.
In our cohort, the probability of continuing treatment with ixekizumab was 85% at one year, 73% at two years, and 61% between the fourth and sixth years. These data reflect a high drug survival during the first year, although slightly lower than that reported in some studies, such as Caldarola (92.11%) [18] and Gargiulo (95.6%) [53]. Our results are comparable to those of Ting (87.2%) [58] and Lockshin (81%) [48], and considerably higher than those reported by Zhdanava (63.4% at one year) [31]. At four years of follow-up, our data show a survival rate slightly lower than that described by Torres (71%) [51]. At five years, our findings are similar to those reported by Ting (59.4%) [58] and Mastorino, who observed a continuation rate of 66% [22]. However, our four-year rates are considerably lower than those reported by Gargiulo, who documented a survival of 82.6% at the same time point [53]. These differences at four years may be attributed to the high proportion of patients with prior exposure to biologic therapies in our cohort. In the literature, there is no clear consensus regarding the impact of previous biologic use on drug survival. However, several studies suggest a potential association, both for biologic agents in general [20] and specifically for the interleukin-17 inhibitor class [50]. In particular, the study by Gargiulo highlights this factor as relevant to the long-term continuation of ixekizumab treatment [53].
One factor that may have positively contributed to treatment adherence in our cohort is the change in ixekizumab formulation from citrate-containing to citrate-free. This new formulation was preferred and well tolerated by most patients who switched from the original presentation, which may have facilitated treatment continuation. Similarly, patients who initiated therapy directly with the citrate-free formulation also showed good acceptance, consistent with the findings reported by Chabra et al. [59]. These observations suggest that factors such as formulation tolerability may play a relevant role in long-term drug survival, beyond the clinical and demographic variables traditionally considered.
In our study, no patient clinical or demographic variables, nor disease-related characteristics, were found to significantly influence ixekizumab drug survival, with the exception of older age. Specifically, gender showed no association with treatment discontinuation, in line with the findings of Schots [49] and Malagoli [60], but in contrast with those of Graier, who reported a higher risk of discontinuation in women [50].
Regarding prior exposure to biologic therapies, our analysis specifically compared patients with exposure to a single biologic versus those who had received multiple biologic treatments. Although several studies have demonstrated that prior biologic exposure is associated with an increased risk of discontinuation [18,20,50,51,53], our results are consistent with other reports showing no significant influence of this factor [43,49,60]. In our analysis, obesity was not associated with an increased likelihood of treatment discontinuation. However, Caldarola et al. [18] reported that a body weight ≥ 90 kg was linked to reduced drug survival. Similarly, Mastorino [22] and Torres [51] observed decreased treatment effectiveness in patients with higher mean BMI values.
Taken together, these discrepancies highlight the lack of consensus in the literature and underscore the need for larger, multicenter studies with stratified analyses to better elucidate the impact of demographic, clinical, and therapeutic variables on long-term drug survival.
However, statistically significant differences were observed in the univariate analysis with respect to age, showing greater treatment survival in patients who initiated ixekizumab at ≥65 years. This finding contrasts with the results of a previous study from our group, in which other biologic treatments—but not ixekizumab, due to the very small number of patients—showed higher survival in older patients without reaching statistical significance [61], similar to what has been more recently reported [62]. We were unable to identify a clear explanation for the higher drug survival observed in patients aged >65 years. Although previous studies have suggested that biologics may be more frequently prescribed in elderly patients with fewer comorbidities, in our cohort patients aged >65 years actually presented a higher prevalence of all comorbidities. We also considered the potential influence of treatment behavior on drug survival. Older patients may show greater compliance and disease awareness, partly due to their experience managing multiple chronic conditions that require strict adherence to therapy. This behavioral factor, although not directly determined by age itself, could indirectly enhance treatment persistence and may help explain the higher survival observed in patients aged ≥65 years in our cohort.It should be noted that the observed effect of age (≥65 years) on treatment survival is based on a small subgroup (n = 18) with wide confidence intervals. Therefore, this finding should be interpreted as exploratory rather than definitive.
Of the 103 patients who initiated treatment with ixekizumab, 31 discontinued therapies during follow-up (30.1%). The overall discontinuation rate in our cohort was slightly higher than that reported in studies by Caldarola (22.54%) [18] and Lockshin (22.00%) [25]. With regard to discontinuations due to adverse events, our series showed a rate of 7%, which is consistent with the findings of Chiricozzi (4%) [36] and Malagoli (6%) [60], but markedly lower than the rate reported by Mastorino (32.8%) [22]. This variability may reflect differences in patient selection, safety monitoring, and reporting criteria across studies.
Our study has several limitations that should be considered when interpreting the results. First, it is a retrospective study and therefore subject to the inherent biases of this design. Second, the study was conducted in a single center, which may also limit the generalizability of the results. Third, treatment selection was not randomized but rather based on individual clinical criteria in the context of routine practice, which may introduce selection bias. Fourth, the sample size was limited, and a substantial proportion of patients were still receiving treatment at the study cutoff date, which could affect estimates of drug survival. Fifth, the recent availability of new biologic therapies may have led to a greater tendency to switch treatments after shorter treatment periods in recent years. Sixth, the small sample size in the subgroup of patients older than 65 years may limit the robustness of the results, and therefore these findings must be taken with caution. Finally, the concomitant use of topical therapies was not accounted for in the analysis. This study provides real-world evidence on ixekizumab use with up to six years of follow-up, contributing information beyond the controlled setting of clinical trials. The systematic collection of data within a single tertiary hospital ensured consistency in patient management and follow-up. In addition, the analysis of drug survival and reasons for discontinuation offers insights that may support clinical decision-making and health policy.
5. Conclusions
In conclusion, our study shows that ixekizumab, in a cohort with a high proportion of non-naïve patients, demonstrates drug survival rates within the upper range of those reported in the literature. Survival was higher among patients aged over 65 years. This finding, which has not been previously described, suggests that ixekizumab is an effective long-term treatment option even in heavily pretreated populations. Nonetheless, Future studies should aim to validate these findings in larger, multicenter cohorts and explore predictive factors of treatment persistence, including clinical characteristics, biomarkers, and comorbidities. Comparative analyses with other biologics and evaluations of long-term safety and quality-of-life outcomes would also provide valuable insights.
Author Contributions
Conceptualization, J.S.-J., C.G.O. and R.S.-J.G.; methodology, J.S.-J., C.G.O. and R.S.-J.G.; software, L.P.-G. and I.Á.-L.; validation, J.S.-J., and R.S.-J.G. formal analysis, I.N.-M. and I.Á.-L.; investigation, I.N.-M., L.P.-G., I.Á.-L. and V.G.-J.; resources, I.N.-M.; data curation, I.N.-M., L.P.-G., I.Á.-L., V.G.-J. and A.L.-B.; writing—original draft preparation, I.N.-M. and R.S.-J.G.; writing—review and editing, J.S.-J. and C.G.O.; visualization, I.N.-M., L.P.-G. and R.S.-J.G.; supervision, C.G.O.; project administration, J.S.-J. and A.L.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee Ethics and Research Committee of the Principality of Asturias, Spain ((Ref. 2024-432) and 31 October 2024.
Informed Consent Statement
Patient consent was waived due to it being exempted by the ethics committee.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dogra, S.; Mahajan, R. Psoriasis: Epidemiology, clinical features, co-morbidities, and clinical scoring. Indian Dermatol. Online J. 2016, 7, 471–480. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Krueger, J.G.; Lebwohl, M.G. Systemic immune mechanisms in atopic dermatitis and psoriasis with implications for treatment. Exp. Dermatol. 2018, 27, 409–417. [Google Scholar] [CrossRef]
- Christophers, E. Psoriasis—Epidemiology and clinical spectrum. Clin. Exp. Dermatol. 2001, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.; Levrero, P.; Carusso, R.; Morales, C.; Arretche, V.; Nicola, A.; Fossati, M.; Cueto, M.; Dorado, N.; García, C.; et al. Psoriasis vulgar moderada y severa: Opciones terapéuticas (tratamientos convencionales). Arch. Med. Interna 2013, 35, 93–100. [Google Scholar]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef]
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, M. Challenges and future trends in the treatment of psoriasis. Int. J. Mol. Sci. 2023, 24, 13313. [Google Scholar] [CrossRef]
- Mrowietz, U.; Lauffer, F.; Sondermann, W.; Gerdes, S.; Sewerin, P. Psoriasis as a systemic disease. Dtsch. Arztebl. Int. 2024, 121, 467–472. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Lin, W.; Lu, L.; Su, J.; Chen, X. Signaling pathways and targeted therapies for psoriasis. Signal Transduct. Target. Ther. 2023, 8, 437. [Google Scholar] [CrossRef] [PubMed]
- Sbidian, E.; Chaimani, A.; Garcia-Doval, I.; Doney, L.; Dressler, C.; Hua, C. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst. Rev. 2020, 1, CD011535. [Google Scholar] [CrossRef]
- Azevedo, A.; Torres, T. Clinical efficacy and safety of ixekizumab for treatment of psoriasis. Actas Dermosifiliogr. 2017, 108, 305–314. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Taltz (Ixekizumab) Injection, for Subcutaneous Use: Initial U.S. Approval 2016 [Internet]; FDA: Silver Spring, MD, USA, 2016. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/125521Orig1s000Approv.pdf (accessed on 20 July 2025).
- European Medicines Agency (EMA). Taltz: EPAR–Product Information [Internet]; EMA: Amsterdam, The Netherlands, 2016; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/taltz (accessed on 20 July 2025).
- Dávila-Seijo, P.; Dauden, E.; Carretero, G.; Ferrandiz, C.; Vanaclocha, F.; Gómez-García, F.-J.; Herrera-Ceballos, E.; De la Cueva-Dobao, P.; Belinchón, I.; Sánchez-Carazo, J.-L.; et al. Survival of Classic and Biological Systemic Drugs in Psoriasis: Results of the BIOBADADERM Registry and Critical Analysis. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1942–1950. [Google Scholar] [CrossRef]
- Borrás-Blasco, J.; Valcuende-Rosique, A.; Cornejo-Uixeda, S. Letter to the editor regarding “Efficacy, drug survival, safety and metabolic parameters of ixekizumab in patients with moderate-to-severe psoriasis in China: A two-year real-world study”. Int. Immunopharmacol. 2025, 164, 115370. [Google Scholar] [CrossRef] [PubMed]
- Becher, G.; Conner, S.; Ingram, J.A.; Stephen, K.E.; McInnes, A.C.; Heald, A.H.; Riley, P.A.; Davies, M.; Domenech, A.; Kasujee, I. A Retrospective Real-World Study of the Effectiveness and Tolerability of Tildrakizumab in UK Adults with Moderate-to-Severe Chronic Plaque Psoriasis. Dermatol. Ther. 2022, 12, 2343–2354. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Siegel, M.P.; Bagel, J.; Boh, E.E.; Buell, M.; Cooper, K.D.; Duffin, K.C.; Eichenfield, L.F.; Garg, A.; Gelfand, J.M.; et al. From the Medical Board of the National Psoriasis Foundation: Treatment targets for plaque psoriasis. J. Am. Acad. Dermatol. 2017, 76, 290–298. [Google Scholar] [CrossRef]
- Caldarola, G.; Chiricozzi, A.; Megna, M.; Dapavo, P.; Giunta, A.; Burlando, M.; Malagoli, P.; Dini, V.; Mariani, M.; Fabbrocini, G.; et al. Real-life experience with ixekizumab in plaque psoriasis: A multi-center, retrospective, 3-year study. Expert Opin. Biol. Ther. 2023, 23, 365–370. [Google Scholar] [CrossRef]
- Ntawuyamara, E.; Deng, B.; Liang, Y. Cutaneous and systemic improvements in psoriasis patients after different biologic treatments in a real-world longitudinal prospective study. Sci. Rep. 2025, 15, 15528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yiu, Z.Z.N.; Becher, G.; Kirby, B.; Laws, P.; Reynolds, N.J.; Smith, C.H.; Warren, R.B.; Griffiths, C.E.M.; BADBIR Study Group; Browne, F.; et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022, 158, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Burlando, M.; Salvi, I.; Castelli, R.; Herzum, A.; Cozzani, E.; Parodi, A. Long-term clinical efficacy and safety of ixekizumab for psoriatic patients: A single-center experience. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 4060–4064. [Google Scholar] [PubMed]
- Mastorino, L.; Dapavo, P.; Burzi, L.; Rosset, F.; Giunipero di Corteranzo, I.; Leo, F.; Verrone, A.; Stroppiana, E.; Ortoncelli, M.; Ribero, S.; et al. Drug survival, effectiveness and safety of ixekizumab for moderate-to-severe psoriasis up to 5 years. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 568–575. [Google Scholar] [CrossRef]
- Saeki, H.; Nakagawa, H.; Ishii, T.; Morisaki, Y.; Aoki, T.; Berclaz, P.Y.; Heffernan, M. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1148–1155. [Google Scholar] [CrossRef]
- Gottlieb, A.B.; Burge, R.; Malatestinic, W.N.; Zhu, B.; Zhao, Y.; McCormack, J.; Kimel, M.; Merola, J.F. Ixekizumab real-world effectiveness at 24 weeks in patients with psoriasis: Data from the United States Taltz customer support program. Dermatol. Ther. 2023, 13, 1831–1846. [Google Scholar] [CrossRef]
- Lockshin, B.; Harrison, R.W.; McLean, R.R.; Crabtree, M.M.; Konicek, B.W.; Zhu, B.; Malatestinic, W.N.; Atiya, B.; Murage, M.J.; Burge, R.T. Outcomes in ixekizumab patients following exposure to secukinumab and other biologics in the CorEvitas Psoriasis Registry. Dermatol. Ther. 2022, 12, 2797–2815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Mu, Z.; Zhao, Y.; Zhang, J.; Cai, L. Efficacy, drug survival, safety and metabolic parameters of ixekizumab in patients with moderate-to-severe psoriasis in China: A two-year real-world study. Int. Immunopharmacol. 2024, 143 Pt 2, 113474. [Google Scholar] [CrossRef]
- Bucur, S.; Serban, E.D.; Ileanu, B.V.; Costache, R.S.; Nicolescu, A.C.; Constantin, T.; Costache, D.O.; Constantin, M.-M. Effectiveness and drug survival of ixekizumab and secukinumab in patients with moderate to severe plaque psoriasis: Real-world data from Bucharest, Romania. Psoriasis 2024, 14, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.; Strober, B.; Schrader, A.; Li, A.H.; Eckmann, T.; Zhu, B.; Malatestinic, W.N.; Birt, J.; Feely, M.; Blauvelt, A. Six-month real-world study to assess the effectiveness of ixekizumab after switching from IL-23 inhibitors and other biologic therapies: The CorEvitas Psoriasis Registry. Drugs Real World Outcomes 2024, 11, 451–464. [Google Scholar] [CrossRef]
- Pinter, A.; Eyerich, K.; Costanzo, A.; Garrelts, A.; Schuster, C.; Mert, C.; Lampropoulou, A.; Fotiou, K.; Maul, J.-T.; Papp, K.A. Association of disease duration and PASI response rates at week 12 in patients with moderate-to-severe plaque psoriasis receiving biologics in the real-world psoriasis study of health outcomes (PSoHO). J. Dermatol. Treat. 2024, 35, 2350227. [Google Scholar] [CrossRef]
- Pinter, A.; Costanzo, A.; Khattri, S.; Smith, S.D.; Carrascosa, J.M.; Tada, Y.; Riedl, E.; Reich, A.; Brnabic, A.; Haustrup, N.; et al. Comparative effectiveness and durability of biologics in clinical practice: Month 12 outcomes from the international, observational psoriasis study of health outcomes (PSoHO). Dermatol. Ther. 2024, 14, 1479–1493. [Google Scholar] [CrossRef] [PubMed]
- Zhdanava, M.; Fitzgerald, T.; Pilon, D.; Teneralli, R.E.; Shah, A.; Diaz, L.; Lefebvre, P.; Feldman, S.R. Comparative analysis of persistence and remission with guselkumab versus secukinumab and ixekizumab in the United States. J. Dermatol. Treat. 2024, 35, 2349658. [Google Scholar] [CrossRef]
- Li, Y.; Lv, C.; Dang, L.; Lin, B.; Tao, J.; Zhang, C.; Zhou, X.; Ma, H.; Lu, Y.; Chen, R.; et al. Effectiveness of ixekizumab in Chinese patients with moderate-severe plaque psoriasis with special area involvement: Subanalysis of a prospective, multicenter, observational real-world study. Dermatol. Ther. 2024, 14, 907–918. [Google Scholar] [CrossRef]
- Armstrong, A.; González-Cantero, A.; Khattri, S.; Muzy, G.; Malatestinic, W.N.; Lampropoulou, A.; Feely, M.; See, S.K.; Mert, C.; Blauvelt, A. Comparing achievement of National Psoriasis Foundation treatment targets among patients with plaque psoriasis treated with ixekizumab versus other biologics in clinical and real-world studies. Dermatol. Ther. 2024, 14, 933–952. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.; Gargiulo, L.; Ibba, L.; Cortese, A.; Toso, F.; Orsini, D.; Lora, V.; Frascione, P.; Sena, P.; Carugno, A.; et al. Effectiveness of ixekizumab for the treatment of moderate-to-severe plaque psoriasis with involvement of difficult-to-treat areas: A 52-week multicenter retrospective study. J. Dermatol. 2024, 51, 839–843. [Google Scholar] [CrossRef]
- Mucherino, S.; Rafaniello, C.; Serino, M.; Zinzi, A.; Trama, U.; Capuano, A.; Menditto, E.; Orlando, V. Drug utilization and measurement of medication adherence: A real-world study of psoriasis in Italy. Pharmaceutics 2023, 15, 2647. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Megna, M.; Giunta, A.; Carrera, C.G.; Dapavo, P.; Balato, A.; Malagoli, P.; Mazzoccoli, S.; Parodi, A.; Sabatino, S.; et al. Ixekizumab is effective in the long-term management in moderate-to-severe plaque psoriasis: Results from an Italian retrospective cohort study (the LOTIXE study). J. Dermatol. Treat. 2023, 34, 2246606. [Google Scholar] [CrossRef]
- Wang, C.; Torisu-Itakura, H.; Hanada, T.; Matsuo, T.; Cai, Z.; Osaga, S.; Aranishi, T. Treatment persistence of interleukin-17 inhibitor class drugs among patients with psoriasis in Japan: A retrospective database study. J. Dermatol. Treat. 2023, 34, 2229465. [Google Scholar] [CrossRef]
- Reich, A.; Reed, C.; Schuster, C.; Robert, C.; Treuer, T.; Lubrano, E. Real-world evidence for ixekizumab in the treatment of psoriasis and psoriatic arthritis: Literature review 2016–2021. J. Dermatol. Treat. 2023, 34, 2160196. [Google Scholar] [CrossRef]
- Thomas, S.E.; Barenbrug, L.; Hannink, G.; Seyger, M.M.B.; de Jong, E.M.G.J.; van den Reek, J.M.P.A. Drug survival of IL-17 and IL-23 inhibitors for psoriasis: A systematic review and meta-analysis. Drugs 2024, 84, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Puig, L.; Sewerin, P.; Schuster, C.; Ng, K.J.; Papadimitropoulos, M.; Gadagamma, S.; Nuñez, M.; Lampropoulou, A. Real-world evidence for ixekizumab in the treatment of psoriasis, psoriatic arthritis, and axial spondyloarthritis: Systematic literature review 2022–2023. Adv. Ther. 2025, 42, 4224–4254. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gulliver, W.; Penney, M.; Power, R.; Gulliver, S.; Montmayeur, S.; Burge, R. Moderate-to-severe plaque psoriasis patients treated with ixekizumab: Early real world outcomes and adverse events. J. Dermatol. Treat. 2022, 33, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Gooderham, M.J.; Lynde, C.; Turchin, I.; Avadisian, M.; Labelle, M.; Papp, K.A. Real-world, long-term treatment patterns of commonly used biologics in Canadian patients with moderate-to-severe chronic plaque psoriasis. J. Dermatol. 2022, 49, 95–105. [Google Scholar] [CrossRef]
- Kishimoto, M.; Komine, M.; Kamiya, K.; Sugai, J.; Mieno, M.; Ohtsuki, M. Drug survival of biologic agents for psoriatic patients in a real-world setting in Japan. J. Dermatol. 2020, 47, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kojanova, M.; Hugo, J.; Velackova, B.; Cetkovska, P.; Fialova, J.; Dolezal, T.; Tichy, M.; Gkalpakiotis, S. Efficacy, safety, and drug survival of patients with psoriasis treated with IL-17 inhibitors—Brodalumab, ixekizumab, and secukinumab: Real-world data from the Czech Republic BIOREP registry. J. Dermatol. Treat. 2022, 33, 2827–2837. [Google Scholar] [CrossRef]
- Lee, E.B.; Pithadia, D.J.; Reynolds, K.A.; Reddy, S.P.; Egeberg, A.; Wu, J.J. Real-world drug survival of ixekizumab for psoriasis. J. Am. Acad. Dermatol. 2019, 81, 270–272. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.J.; Zhong, X.Y.; Yu, Y.Y.; Yu, N.; Wang, Y.; Yi, X.-M.; Ding, Y.-F.; Shi, Y.-L. Drug Survival Outcomes Associated with the Real-World Use of Ixekizumab, Secukinumab, Guselkumab, and Adalimumab for the Treatment of Plaque Psoriasis in China: A 52-Week Single-Center Retrospective Study. Clin. Cosmet. Investig. Dermatol. 2022, 15, 2245–2252. Available online: https://www.dovepress.com/drug-survival-outcomes-associated-with-the-real-world-use-of-ixekizuma-peer-reviewed-fulltext-article-CCID (accessed on 21 December 2024). [CrossRef]
- Lunder, T.; Zorko, M.S.; Kolar, N.K.; Suhodolcan, A.B.; Marovt, M.; Leskovec, N.K.; Marko, P.B. Drug survival of biological therapy is showing class effect: Updated results from Slovenian National Registry of psoriasis. Int. J. Dermatol. 2019, 58, 631–641. [Google Scholar] [CrossRef]
- Lockshin, B.; Cronin, A.; Harrison, R.W.; McLean, R.R.; Anatale-Tardiff, L.; Burge, R.; Zhu, B.; Malatestinic, W.N.; Atiya, B.; Murage, M.J.; et al. Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: The Corrona Psoriasis Registry. Dermatol. Ther. 2021, 34, e14808. [Google Scholar] [CrossRef]
- Schots, L.; Soenen, R.; Blanquart, B.; Thomas, D.; Lambert, J. Blocking interleukin-17 in psoriasis: Real-world experience from the PsoPlus cohort. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Graier, T.; Salmhofer, W.; Jonak, C.; Weger, W.; Kölli, C.; Gruber, B.; Sator, P.; Prillinger, K.; Mlynek, A.; Schütz-Bergmayr, M.; et al. Biologic drug survival rates in the era of anti-interleukin-17 antibodies: A time-period-adjusted registry analysis. Br. J. Dermatol. 2021, 184, 1094–1105. [Google Scholar] [CrossRef]
- Torres, T.; Puig, L.; Vender, R.; Yeung, J.; Carrascosa, J.M.; Piaserico, S.; Gisondi, P.; Lynde, C.; Ferreira, P.; Bastos, P.M.; et al. Drug survival of interleukin (IL)-17 and IL-23 inhibitors for the treatment of psoriasis: A retrospective multi-country, multicentric cohort study. Am. J. Clin. Dermatol. 2022, 23, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Yanase, T.; Tsuruta, N.; Yamaguchi, K.; Ohata, C.; Ohyama, B.; Katayama, E.; Sugita, K.; Kuwashiro, M.; Hashimoto, A.; Yonekura, K.; et al. Survival rates of systemic interventions for psoriasis in the Western Japan Psoriasis Registry: A multicenter retrospective study. J. Dermatol. 2023, 50, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, L.; Ibba, L.; Malagoli, P.; Balato, A.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; Dapavo, P.; Dini, V.; et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for moderate-to-severe plaque psoriasis: A retrospective multicenter real-world experience on 5932 treatment courses—IL PSO (Italian landscape psoriasis). Front. Immunol. 2023, 14, 1341708. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.; Zhdanava, M.; Pilon, D.; Shah, A.; Hilts, A.; Lefebvre, P.; Feldman, S.R. Long-term psoriasis control with guselkumab, adalimumab, secukinumab, or ixekizumab in the USA. Dermatol. Ther. 2023, 13, 1053–1068. [Google Scholar] [CrossRef] [PubMed]
- Galache Osuna, C.; Gómez-Vila, B.; Aubán Pariente, J.; Vázquez Losada, B.; Gómez de Castro, C.; Requena López, S.; Velázquez, Á.d.D.; García, L.P.; Fernández, L.O.; Diez, S.G.; et al. Ustekinumab drug survival in patients with psoriasis: A retrospective study of real clinical practice. Medicina 2020, 56, 584. [Google Scholar] [CrossRef]
- Gómez-de Castro, C.; Mir-Bonafé, M.; Arias-Martínez, A.; Martínez-Camblor, P.; Díaz-Coto, S.; Santos-Juanes, J. Comment on “Baseline patients’ characteristics as predictors for therapeutic survival and response in patients with psoriasis on biological treatments”. Australas. J. Dermatol. 2019, 60, e258–e259. [Google Scholar] [CrossRef]
- Palacios-García, L.; Gómez-de-Castro, C.; Mir-Bonafé, M.; Calzón, C.; Galache, C.; Santos-Juanes, J. Comment on “Secukinumab drug survival in patients with psoriasis: A multicenter, real-world, retrospective study”. J. Am. Acad. Dermatol. 2019, 81, e81–e82. [Google Scholar] [CrossRef]
- Ting, S.; Lowe, P.; Smith, A.; Fernández-Peñas, P. Drug survival of biologics in psoriasis: An Australian multicentre retrospective study. Australas. J. Dermatol. 2024, 65, 350–357. [Google Scholar] [CrossRef]
- Chabra, S.; Birt, J.; Bolce, R.; Lisse, J.; Malatestinic, W.N.; Zhu, B.; Kimel, M.; McCormack, J.; Stefan, M.; Cragun, W.C. Satisfaction with the injection experience of a new, citrate-free formulation of ixekizumab. Adv. Ther. 2024, 41, 1672–1684. [Google Scholar] [CrossRef]
- Malagoli, P.; Dapavo, P.; Pavia, G.; Amoruso, F.; Argenziano, G.; Bardazzi, F.; Burlando, M.; Carrera, C.G.; Damiani, G.; Dini, V.; et al. Real-life long-term efficacy and safety of ixekizumab in moderate-to-severe psoriasis: A 192 weeks multicentric retrospective study—IL PSO (Italian landscape psoriasis). Dermatol. Ther. 2022, 35, e15608. [Google Scholar] [CrossRef]
- Osuna, C.G.; García, S.R.; Martín, J.C.; Jiménez, V.G.; López, F.V.; Santos-Juanes, J. Use of biological treatments in elderly patients with skin psoriasis in the real world. Life 2021, 11, 1348. [Google Scholar] [CrossRef]
- Hacınecipoğlu, F.; Çelik, G.; Kartal, S.P. Efficacy and safety of IL-17 and IL-23 inhibitors in elderly patients with plaque psoriasis: A real-world study. J. Dermatol. 2025, 52, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).