Oncological Safety of Intrauterine Manipulator Use in Laparoscopic Hysterectomy for Endometrial Cancer: A Propensity Score-Matched Analysis
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Patient Characteristics of the Entire Cohort
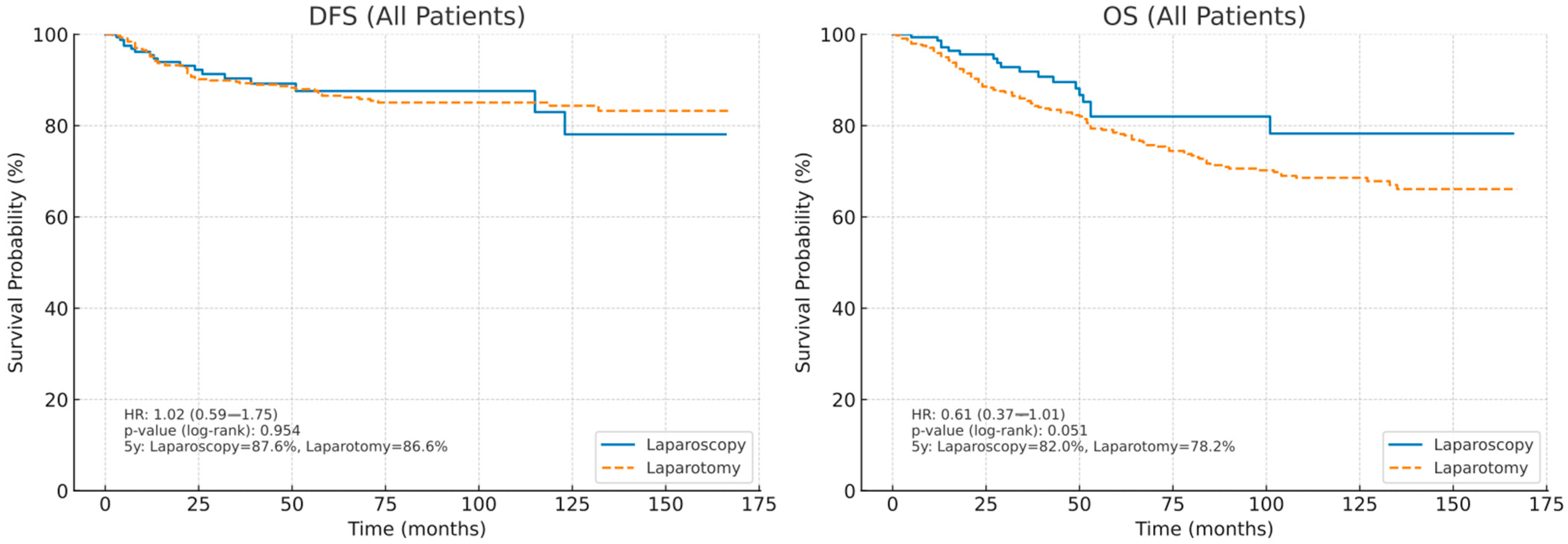
3.2. Survival Outcomes of the Entire Cohort
3.3. Patient Characteristics After Propensity Score Matching
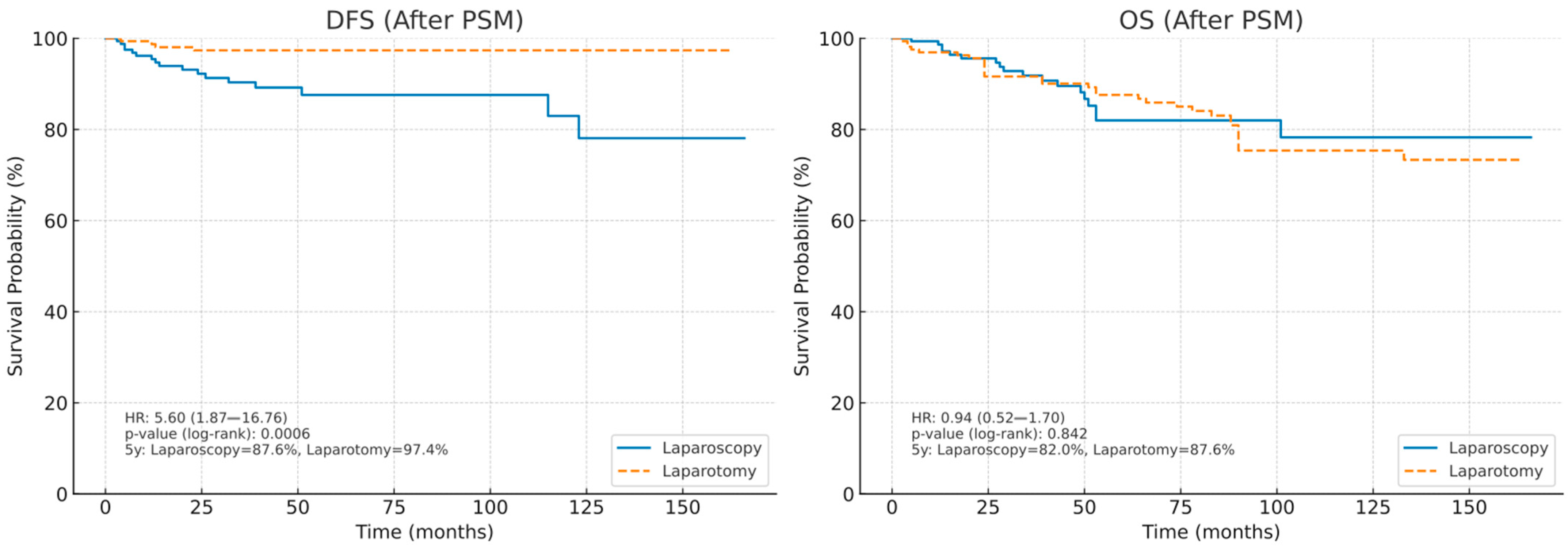
3.4. Survival Outcomes After Propensity Score Matching
3.5. Univariate and Multivariate Analysis of DFS
3.6. Univariate and Multivariate Analysis of OS
4. Discussion
- Macroscopic dissemination. The insertion and manipulation of the device, whether balloon-based or not, may compromise the integrity of the myometrium, leading to iatrogenic uterine rupture and direct exposure of malignant tissue to the peritoneal cavity and surgical field. In addition, tumor fragments shed into the vagina during surgery may potentially spread into the abdominal cavity with gas insufflation following colpotomy.
- Microscopic dissemination. The use of an IUM markedly increases intrauterine pressure, producing global distension in line with Pascal’s principle. This effect is amplified by the sustained pressure required for uterine mobilization and colpotomy. The elevated intrauterine pressure may facilitate the passive migration of malignant cells through the myometrium into the fallopian tubes and lymphovascular spaces. Such changes in pressure dynamics may also alter the tumor microenvironment, favoring intraoperative hematogenous spread of tumor cells [10].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CA-125 | Cancer antigen 125 |
| CT | Computed tomography |
| DFS | Disease-free survival |
| EC | Endometrial cancer |
| FIGO | International Federation of Obstetrics and Gynecology |
| IUM | Intrauterine manipulator |
| LACC | Laparoscopic Approach to Cervical Cancer |
| LVSI | Lymphovascular space invasion |
| MI | Myometrial Invasion |
| OS | Overall survival |
| PET/CT | Positron emission tomography combined with CT |
| PSM | Propensity Score Matching |
| RECIST | Response Evaluation Criteria In Solid Tumors |
References
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Cibula, D.; Colombo, N.; Creutzberg, C.L.; Ledermann, J.; Mirza, M.R.; Vergote, I.; Abu-Rustum, N.R.; Bosse, T.; et al. ESGO-ESTRO-ESP guidelines for the management of patients with endometrial carcinoma: Update 2025. Lancet Oncol. 2025, 26, 423–435. [Google Scholar] [CrossRef]
- Janda, M.; Gebski, V.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; McCartney, A.; Nascimento, M.; Neesham, D.; et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): A randomised trial. Lancet Oncol. 2010, 11, 772–780. [Google Scholar] [CrossRef]
- Kornblith, A.B.; Huang, H.Q.; Walker, J.L.; Spirtos, N.M.; Rotmensch, J.; Cella, D. Quality of life of patients with endometrial cancer undergoing laparoscopic International Federation of Gynecology and Obstetrics staging compared with laparotomy: A Gynecologic Oncology Group study. J. Clin. Oncol. 2009, 27, 5337–5342. [Google Scholar] [CrossRef]
- Mourits, M.J.; Bijen, C.B.; Arts, H.J.; ter Brugge, H.G.; van der Sijde, R.; Paulsen, L.; Wijma, J.; Bongers, M.Y.; Post, W.J.; van der Zee, A.G.; et al. Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: A randomised trial. Lancet Oncol. 2010, 11, 763–771. [Google Scholar] [CrossRef]
- Walker, J.L.; Piedmonte, M.R.; Spirtos, N.M.; Eisenkop, S.M.; Schlaerth, J.B.; Mannel, R.S.; Barakat, R.; Pearl, M.L.; Sharma, S.K. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012, 30, 695–700. [Google Scholar] [CrossRef]
- NCCN. Clinical Practice Guidelines in Oncology (Nccn Guidelines) Version 3. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 1 August 2025).
- van den Haak, L.; Alleblas, C.; Nieboer, T.E.; Rhemrev, J.P.; Jansen, F.W. Efficacy and safety of uterine manipulators in laparoscopic surgery: A review. Arch. Gynecol. Obstet. 2015, 292, 1003–1011. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Padilla-Iserte, P.; Lago, V.; Tauste, C.; Díaz-Feijoo, B.; Gil-Moreno, A.; Oliver, R.; Coronado, P.; Martín-Salamanca, M.B.; Pantoja-Garrido, M.; Marcos-Sanmartin, J.; et al. Impact of uterine manipulator on oncological outcome in endometrial cancer surgery. Am. J. Obstet. Gynecol. 2021, 224, 65.e1–65.e11. [Google Scholar] [CrossRef] [PubMed]
- Machida, H.; Hom, M.S.; Adams, C.L.; Eckhardt, S.E.; Garcia-Sayre, J.; Mikami, M.; Matsuo, K. Intrauterine Manipulator Use During Minimally Invasive Hysterectomy and Risk of Lymphovascular Space Invasion in Endometrial Cancer. Int. J. Gynecol. Cancer. 2018, 28, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, H.S.; Lee, K.B.; Yoo, C.W.; Park, S.Y.; Seo, S.S. Does the use of a uterine manipulator with an intrauterine balloon in total laparoscopic hysterectomy facilitate tumor cell spillage into the peritoneal cavity in patients with endometrial cancer? Int. J. Gynecol. Cancer 2008, 18, 1145–1149. [Google Scholar] [CrossRef]
- Guntupalli, S.R.; Zighelboim, I.; Kizer, N.T.; Zhang, Q.; Powell, M.A.; Thaker, P.H.; Goodfellow, P.J.; Mutch, D.G. Lymphovascular space invasion is an independent risk factor for nodal disease and poor outcomes in endometrioid endometrial cancer. Gynecol. Oncol. 2012, 124, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, Y.; Kosan, B.; Yalcin, S.; Abay, M.; Ozerkan, K. The Impact of Lymphovascular Space Invasion on Recurrence and Survival in FIGO Stage I Node-Negative Endometrioid Endometrial Cancer. J. Clin. Med. 2025, 14, 6535. [Google Scholar] [CrossRef]
- Siegenthaler, F.; Johann, S.; Imboden, S.; Samartzis, N.; Ledermann-Liu, H.; Sarlos, D.; Eberhard, M.; Mueller, M.D. Prospective Multicenter Trial Assessing the Impact of Positive Peritoneal Cytology Conversion on Oncological Outcome in Patients with Endometrial Cancer Undergoing Minimally Invasive Surgery with the use of an Intrauterine Manipulator: Positive Peritoneal Cytology Conversion and Its Association with Oncological Outcome in Endometrial Cancer. Ann. Surg. Oncol. 2022, 29, 8320–8333. [Google Scholar] [CrossRef]
- Sonoda, Y.; Zerbe, M.; Smith, A.; Lin, O.; Barakat, R.R.; Hoskins, W.J. High incidence of positive peritoneal cytology in low-risk endometrial cancer treated by laparoscopically assisted vaginal hysterectomy. Gynecol. Oncol. 2001, 80, 378–382. [Google Scholar] [CrossRef]
- Krizova, A.; Clarke, B.A.; Bernardini, M.Q.; James, S.; Kalloger, S.E.; Boerner, S.L.; Mulligan, A.M. Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: A blinded, retrospective review. Am. J. Surg. Pathol. 2011, 35, 15–26. [Google Scholar] [CrossRef]
- Uccella, S.; Bonzini, M.; Malzoni, M.; Fanfani, F.; Palomba, S.; Aletti, G.; Corrado, G.; Ceccaroni, M.; Seracchioli, R.; Shakir, F.; et al. The effect of a uterine manipulator on the recurrence and mortality of endometrial cancer: A multi-centric study by the Italian Society of Gynecological Endoscopy. Am. J. Obstet. Gynecol. 2017, 216, 592e1–592e11. [Google Scholar] [CrossRef]
- Marcos-Sanmartín, J.; López Fernández, J.A.; Sánchez-Payá, J.; Piñero-Sánchez, Ó.C.; Román-Sánchez, M.J.; Quijada-Cazorla, M.A.; Candela-Hidalgo, M.A.; Martínez-Escoriza, J.C. Does the Type of Surgical Approach and the Use of Uterine Manipulators Influence the Disease-Free Survival and Recurrence Rates in Early-Stage Endometrial Cancer? Int. J. Gynecol. Cancer. 2016, 26, 1722–1726. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, Y.; Lin, S.; Cao, C.; Wu, P.; Gao, P.; Zhi, W.; Peng, T.; Gui, L.; Wu, P. The effects of uterine manipulators in minimally invasive hysterectomy for endometrial cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2020, 46, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Gueli Alletti, S.; Perrone, E.; Fedele, C.; Cianci, S.; Pasciuto, T.; Chiantera, V.; Uccella, S.; Ercoli, A.; Vizzielli, G.; Fagotti, A.; et al. A Multicentric Randomized Trial to Evaluate the ROle of Uterine MANipulator on Laparoscopic/Robotic HYsterectomy for the Treatment of Early-Stage Endometrial Cancer: The ROMANHY Trial. Front. Oncol. 2021, 10, 720894. [Google Scholar] [CrossRef] [PubMed]
- Zorzato, P.C.; Uccella, S.; Biancotto, G.; Bosco, M.; Festi, A.; Franchi, M.; Garzon, S. Intrauterine manipulator during hysterectomy for endometrial cancer: A systematic review and meta-analysis of oncologic outcomes. Am. J. Obstet. Gynecol. 2024, 230, 185–198. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Delair, D.; Soslow, R.A.; Gardner, G.J.; Barakat, R.R.; Leitao, M.M. Tumoral displacement into fallopian tubes in patients undergoing robotically assisted hysterectomy for newly diagnosed endometrial cancer. Int. J. Gynecol. Pathol. 2013, 32, 188–192. [Google Scholar] [CrossRef]
- Eoh, K.J.; Kim, Y.N.; Nam, E.J.; Kim, S.W.; Kim, Y.T. Clinical Relevance of Uterine Manipulation on Oncologic Outcome in Robot-Assisted versus Open Surgery in the Management of Endometrial Cancer. J. Clin. Med. 2023, 12, 1950. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Gebski, V.; Davies, L.C.; Forder, P.; Brand, A.; Hogg, R.; Jobling, T.W.; Land, R.; Manolitsas, T.; Nascimento, M.; et al. Effect of Total Laparoscopic Hysterectomy vs Total Abdominal Hysterectomy on Disease-Free Survival Among Women With Stage I Endometrial Cancer: A Randomized Clinical Tria l. JAMA 2017, 28, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Vizzielli, G.; Taliento, C.; Bernardi, G.; Martinello, R.; Cianci, S.; Riemma, G.; Scambia, G.; Greco, P. Influence of uterine manipulator on oncological outcome in minimally invasive surgery of endometrial cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Laparoscopy (n = 168) | Laparotomy (n = 444) | p a Value |
|---|---|---|---|
| Age, y, median (range), n, (%) | 62.0 (27.0–85.0) | 63.5 (37.0–93.0) | 0.047 |
| BMI, kg/m2, median (range) | 35.0 (18.0–54.0) | 33.9 (18.4–65.3) | 0.035 |
| Pre-operative CA-125, (U/mL), median (range) | 15.0 (3.0–243.0) | 17.0 (2.8–455.0) | 0.042 |
| Tumor size, cm, median (range) | 3.5 (0.2–10.0) | 4.0 (0.2–15.3) | <0.001 |
| Histological types, n, (%) | 0.004 | ||
| Endometrioid | 156 (92.9%) | 356 (80.2%) | |
| Serous | 3 (1.8%) | 35 (7.9%) | |
| Mucinous | 0 (0.0%) | 6 (1.4%) | |
| Clear cell | 2 (1.2%) | 15 (3.4%) | |
| Carcinosarcoma | 4 (2.4%) | 18 (4.1%) | |
| Others b | 3 (1.8%) | 14 (3.2%) | |
| FIGO stage c, n, (%) | 0.002 | ||
| IA | 127 (75.6%) | 268 (60.4%) | |
| IB | 21 (12.5%) | 66 (14.9%) | |
| II | 7 (4.2%) | 28 (6.3%) | |
| III | 13 (7.7%) | 82 (18.5%) | |
| FIGO grade | <0.001 | ||
| 1 | 103 (61.3%) | 150 (33.8%) | |
| 2 | 41 (24.4%) | 148 (33.3%) | |
| 3 | 24 (14.3%) | 146 (32.9%) | |
| Depth of myometrial invasion, n, (%) | <0.001 | ||
| None | 25 (14.9%) | 36 (8.1%) | |
| <50% | 115 (68.5%) | 278 (62.6%) | |
| ≥50% | 28 (16.7%) | 130 (29.3%) | |
| LVSI, n, (%) | 0.019 | ||
| Absent | 137 (81.5%) | 319 (71.8%) | |
| Present | 31 (18.5%) | 125 (28.2%) | |
| Pelvic Lymphadenectomy, n, (%) | 0.003 | ||
| Yes | 124 (73.8%) | 378 (85.1%) | |
| No | 44 (26.2%) | 65 (14.6%) | |
| Para-aortic Lymphadenectomy, n, (%) | <0.001 | ||
| Yes | 53 (31.5%) | 326 (73.4%) | |
| No | 115 (68.5%) | 116 (26.1%) | |
| Pelvic LN harvested, median (range) | 20.0 (2.0–54.0) | 22.0 (2.0–82.0) | 0.067 |
| Para-aortic LN harvested, median (range) | 13.0 (1.0–45.0) | 18.0 (1.0–51.0) | <0.001 |
| Peritoneal washing cytology, n, (%) | 0.717 | ||
| Negative | 166 (98.8%) | 436 (98.2%) | |
| Positive | 1 (0.6%) | 2 (0.5%) | |
| Unknown | 1 (0.6%) | 6 (1.4%) | |
| Adjuvant treatment, n, (%) | <0.001 | ||
| None | 65 (38.7%) | 105 (23.6%) | |
| Chemotherapy | 4 (2.4%) | 23 (5.2%) | |
| Radiotherapy | 75 (44.6%) | 193 (43.5%) | |
| Chemotherapy and radiotherapy | 24 (14.3%) | 123 (27.7%) | |
| Recurrence, n, (%) | 0.394 | ||
| Yes | 17 (10.1%) | 58 (13.1%) | |
| No | 151 (89.9%) | 386 (86.9%) | |
| Recurrence site, n, (%) | 0.613 | ||
| Local | 7 (41.1%) | 25 (43.1%) | |
| Distant | 6 (35.2%) | 27 (46.5%) | |
| Local+Distant | 4 (23.7%) | 6 (10.4%) |
| Characteristics | Laparoscopy (n = 168) | Laparotomy (n = 168) | p Value a |
|---|---|---|---|
| Age, y, median (range), n, (%) | 62.0 (27.0–85.0) | 63.0 (37.0–85.0) | 0.712 |
| BMI, kg/m2, median (range) | 35.0 (18.0–54.0) | 35.5 (18.4–65.3) | 0.086 |
| Pre-operative CA-125, (U/mL), median (range) | 15.0 (3.0–243.0) | 16.0 (2.8–397.0) | 0.171 |
| Tumor size, cm, median (range) | 3.5 (0.2–10.0) | 3.7 (0.5–15.3) | 0.643 |
| Histological types, n, (%) | 0.210 | ||
| Endometrioid | 156 (92.9%) | 158 (94.0%) | |
| Serous | 3 (1.8%) | 6 (3.6%) | |
| Mucinous | 0 (0.0%) | 2 (1.2%) | |
| Clear cell | 2 (1.2%) | 0 (0.0%) | |
| Carcinosarcoma | 4 (2.4%) | 1 (0.6%) | |
| Others b | 3 (1.8%) | 1 (0.6%) | |
| FIGO stage c, n, (%) | 0.655 | ||
| IA | 127 (75.6%) | 128 (76.2%) | |
| IB | 21 (12.5%) | 22 (13.1%) | |
| II | 7 (4.2%) | 3 (1.8%) | |
| III | 13 (7.7%) | 15 (9%) | |
| FIGO grade | 0.002 | ||
| 1 | 103 (61.3%) | 72 (42.9%) | |
| 2 | 41 (24.4%) | 57 (33.9%) | |
| 3 | 24 (14.3%) | 39 (23.2%) | |
| Depth of myometrial invasion, n, (%) | 0.064 | ||
| None | 25 (14.9%) | 11 (6.5%) | |
| <50% | 115 (68.5%) | 114 (67.9%) | |
| ≥50% | 28 (16.7%) | 43 (25.6%) | |
| LVSI, n, (%) | 0.056 | ||
| Absent | 137 (81.5%) | 119 (70.8%) | |
| Present | 31 (18.5%) | 49 (29.2%) | |
| Pelvic Lymphadenectomy, n, (%) | 0.084 | ||
| Yes | 124 (73.8%) | 137 (81.5%) | |
| No | 44 (26.2%) | 31 (18.5%) | |
| Para-aortic Lymphadenectomy, n, (%) | <0.001 | ||
| Yes | 53 (31.5%) | 125 (74.4%) | |
| No | 115 (68.5%) | 43 (25.6%) | |
| Pelvic LN harvested, median (range) | 20.0 (2.0–54.0) | 27.0 (10.0–82.0) | <0.001 |
| Para-aortic LN harvested, median (range) | 13.0 (1.0–45.0) | 18.0 (2.0–44.0) | <0.001 |
| Peritoneal washing cytology, n, (%) | 0.606 | ||
| Negative | 166 (98.8%) | 167 (99.4%) | |
| Positive | 1 (0.6%) | 0 (0.0%) | |
| Unknown | 1 (0.6%) | 1 (0.6%)4%) | |
| Adjuvant treatment, n, (%) | 0.013 | ||
| None | 65 (38.7%) | 41 (24.4%) | |
| Chemotherapy | 4 (2.4%) | 4 (2.4%) | |
| Radiotherapy | 75 (44.6%) | 104 (61.9%) | |
| Chemotherapy and radiotherapy | 24 (14.3%) | 19 (11.3%) | |
| Recurrence, n, (%) | 0.028 | ||
| Yes | 17 (10.1%) | 10 (6.0%) | |
| No | 151 (89.9%) | 158 (94.0%) | |
| Recurrence site, n, (%) | 0.514 | ||
| Local | 7 (41.1%) | 5 (50%) | |
| Distant | 6 (35.2%) | 2 (20%) | |
| Local+Distant | 4 (23.7%) | 3 (30%) |
| Variable | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value |
|---|---|---|---|---|
| Age | 1.01 (0.99–1.04) | 0.21 | – | – |
| BMI | 1.02 (0.96–1.06) | 0.25 | – | – |
| Pre-operative CA-125 | 1.00 (0.99–1.01) | 0.33 | – | – |
| Tumor size | 1.03 (0.98–1.08) | 0.22 | – | – |
| Histologic type | ||||
| Endometrioid | 1.00 (–) | – | – | |
| Non-endometrioid | 3.10 (1.80–5.35) | <0.001 | 3.57 (1.80–7.07) | <0.001 |
| FIGO Stage | ||||
| I (Ref.) | 1.00 (–) | – | – | – |
| II | 1.40 (0.70–2.80) | 0.30 | – | – |
| III | 2.85 (1.60–5.10) | <0.001 | 3.06 (1.59–5.87) | <0.001 |
| Grade | ||||
| 1 (Ref.) | 1.00 (–) | – | – | – |
| 2 | 1.65 (0.90–3.01) | 0.11 | – | – |
| 3 | 2.20 (1.20–4.05) | 0.01 | 2.63 (1.10–6.27) | 0.03 |
| Positive cytology | 0.95 (0.30–3.05) | 0.93 | – | – |
| LVSI, Present | 1.50 (1.01–2.25) | 0.04 | 1.24 (0.72–2.13) | 0.44 |
| Depth of myometrial invasion, ≥50 | 1.15 (0.75–1.75) | 0.50 | – | – |
| Methods of surgery, Laparotomy | 0.60 (0.40–0.95) | 0.03 | 0.51 (0.29–0.92) | 0.03 |
| Adjuvant treatment | ||||
| None (Ref.) | 1.00 (–) | – | – | – |
| Radiotherapy | 1.50 (0.80–2.80) | 0.20 | – | – |
| Chemotherapy | 2.40 (1.20–4.80) | 0.01 | 2.69 (0.77–9.35) | 0.12 |
| Chemoradiotherapy | 1.35 (0.70–2.70) | 0.35 | – | – |
| Variable | Univariate HR (95% CI) | p Value | Multivariate HR (95% CI) | p Value |
|---|---|---|---|---|
| Age | 1.02 (0.99–1.06) | 0.18 | – | – |
| BMI | 1.01 (0.97–1.08) | 0.19 | – | – |
| Pre-operative CA-125 | 1.00 (0.99–1.01) | 0.40 | – | – |
| Tumor size | 1.04 (0.98–1.11) | 0.20 | – | – |
| Histologic type | ||||
| Endometrioid | 1.00 (–) | – | – | |
| Non-endometrioid | 4.50 (2.20–9.10) | <0.001 | 5.12 (2.26–11.58) | <0.001 |
| FIGO Stage | ||||
| I (Ref.) | 1.00 (–) | – | – | – |
| II | 1.40 (0.60–3.30) | 0.40 | – | – |
| III | 3.00 (1.50–6.00) | <0.001 | 2.98 (1.41–6.31) | <0.001 |
| Grade | ||||
| 1 (Ref.) | 1.00 (–) | – | – | – |
| 2 | 1.50 (0.70–3.30) | 0.29 | – | – |
| 3 | 3.10 (1.50–6.40) | <0.001 | 4.51 (1.27–15.99) | 0.02 |
| Positive cytology | 1.10 (0.30–3.90) | 0.90 | – | – |
| LVSI, Present | 1.35 (0.75–2.45) | 0.31 | – | – |
| Depth of myometrial invasion, ≥50 | 1.25 (0.70–2.20) | 0.45 | – | – |
| Methods of surgery, Laparotomy | 0.75 (0.40–1.40) | 0.36 | – | – |
| Adjuvant treatment | ||||
| None (Ref.) | 1.00 (–) | – | – | – |
| Radiotherapy | 1.40 (0.70–3.00) | 0.30 | – | – |
| Chemotherapy | 1.70 (0.80–3.70) | 0.16 | – | – |
| Chemoradiotherapy | 1.20 (0.50–2.80) | 0.65 | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalcin, Y.; Kosan, B.; Yalcin, S.; Ozerkan, K. Oncological Safety of Intrauterine Manipulator Use in Laparoscopic Hysterectomy for Endometrial Cancer: A Propensity Score-Matched Analysis. Medicina 2025, 61, 1820. https://doi.org/10.3390/medicina61101820
Yalcin Y, Kosan B, Yalcin S, Ozerkan K. Oncological Safety of Intrauterine Manipulator Use in Laparoscopic Hysterectomy for Endometrial Cancer: A Propensity Score-Matched Analysis. Medicina. 2025; 61(10):1820. https://doi.org/10.3390/medicina61101820
Chicago/Turabian StyleYalcin, Yakup, Bahadir Kosan, Serenat Yalcin, and Kemal Ozerkan. 2025. "Oncological Safety of Intrauterine Manipulator Use in Laparoscopic Hysterectomy for Endometrial Cancer: A Propensity Score-Matched Analysis" Medicina 61, no. 10: 1820. https://doi.org/10.3390/medicina61101820
APA StyleYalcin, Y., Kosan, B., Yalcin, S., & Ozerkan, K. (2025). Oncological Safety of Intrauterine Manipulator Use in Laparoscopic Hysterectomy for Endometrial Cancer: A Propensity Score-Matched Analysis. Medicina, 61(10), 1820. https://doi.org/10.3390/medicina61101820





