Current and Emerging Approaches in the Management of Severe Ocular Surface Disease
Abstract
1. Introduction
2. Current and Emerging Management Strategies
2.1. Topical
2.2. Plasma Rich in Growth Factors [PRGF]
2.3. Punctal Plugs
2.4. Therapeutic Contact Lenses
2.5. Heat, Light, and Low-Level Light Therapy
2.6. Surgical and Transplantation
2.7. Gene Therapy
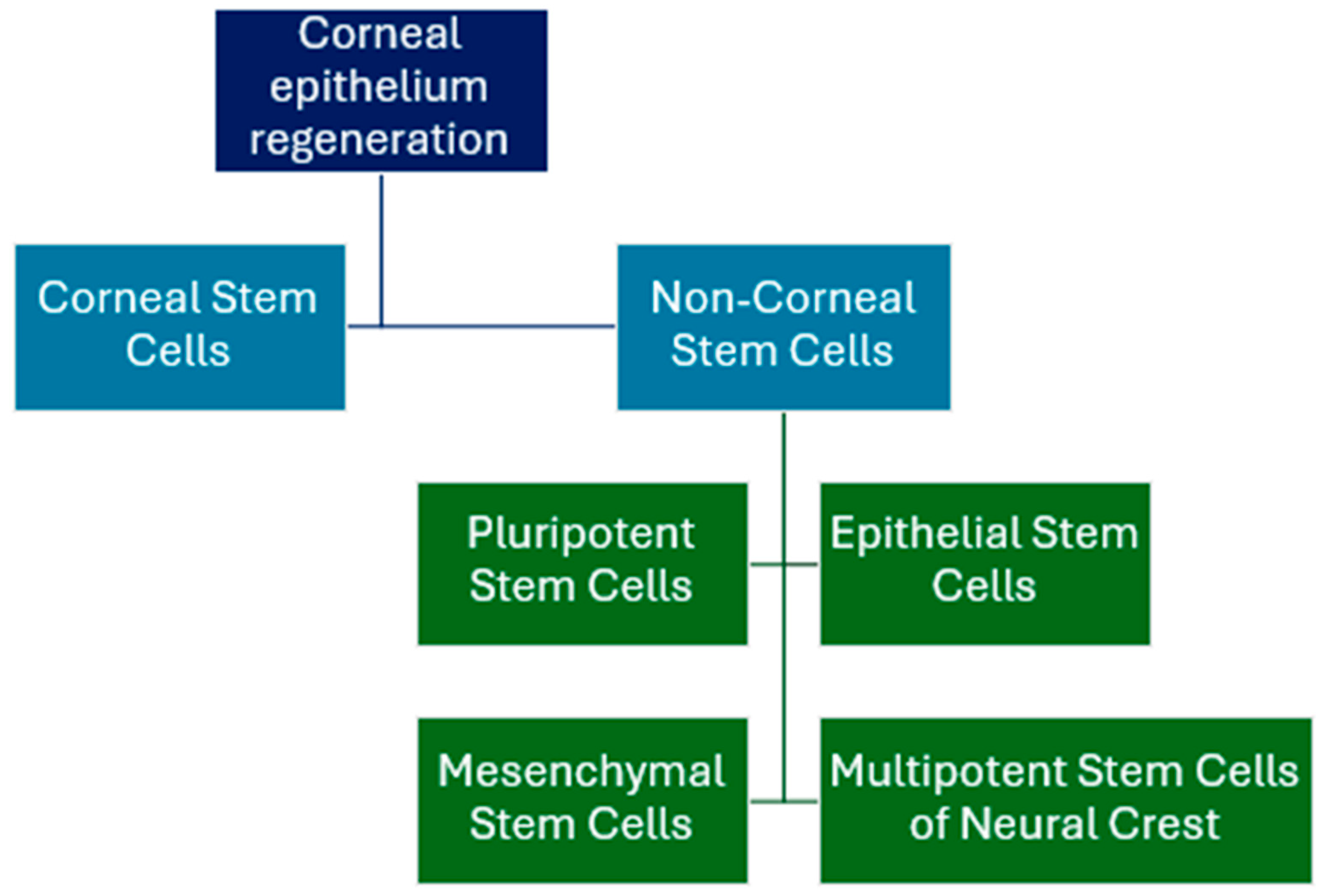
2.8. Cell-Based Therapies
2.9. Amniotic Membrane and Amniotic Drops
2.10. Egg Shell Membrane
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sotozono, C.; Inatomi, T.; Nakamura, T.; Ueta, M.; Imai, K.; Fukuoka, H.; Komai, S.; Ishida, G.; Kitazawa, K.; Yokoi, N.; et al. Oral mucosal epithelial transplantation and limbal-rigid contact lens: A therapeutic modality for the treatment of severe ocular surface disorders. Cornea 2020, 39, S19–S27. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Benítez-Del-Castillo, J.; Loya-Garcia, D.; Inomata, T.; Iyar, G.; Liang, L.; Pult, H.; Sabater, A.L.; Starr, C.E.; Vehof, J.; et al. TFOS DEWS III Diagnostic Methodology. Am. J. Ophthalmol. 2025, 279, 387–450. [Google Scholar] [CrossRef] [PubMed]
- Galor, A.; Britten-Jones, A.C.; Feng, Y.; Ferrari, G.; Goldblum, D.; Gupta, P.K.; Merayo-Lloves, J.; Na, K.S.; Naroo, S.A.; Nichols, K.K.; et al. TFOS Lifestyle: Impact of lifestyle challenges on the ocular surface. Ocul. Surf. 2023, 28, 262–303. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Gutierrez, A.K.; Baer, A.N.; Albayda, J.; Manno, R.L.; Haque, U.; Lipson, E.J.; Bleich, K.B.; Shah, A.A.; Naidoo, J.; et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann. Rheum. Dis. 2017, 76, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.T.; Elia, M.; Materin, M.A.; Sznol, M.; Chow, J. Cyclosporine for dry eye associated with nivolumab: A case pro-gressing to corneal perforation. Cornea 2016, 35, 399–401. [Google Scholar] [CrossRef]
- Vishnevskia-Dai, V.; Rozner, L.; Berger, R.; Jaron, Z.; Elyashiv, S.; Markel, G.; Zloto, O. Ocular side effects of novel anti-cancer biological therapies. Sci. Rep. 2021, 11, 787. [Google Scholar] [CrossRef]
- Quesada, J.M.; Lloves, J.M.; Delgado, D.V. Ocular chemical burns in the workplace: Epidemiological characteristics. Burns 2019, 46, 1212–1218. [Google Scholar] [CrossRef]
- Dua, H.S.; Ting, D.S.J.; Al Saadi, A.; Said, D.G. Chemical eye injury: Pathophysiology, assessment and management. Eye 2020, 34, 2001–2019. [Google Scholar] [CrossRef] [PubMed]
- Kunert, K.S.; Tisdale, A.S.; Gipson, I.K. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch. Ophthalmol. 2002, 120, 330–337. [Google Scholar] [CrossRef]
- Periman, L.M.; Mah, F.S.; Karpecki, P.M. A review of the mechanism of action of cyclosporine A: The role of cyclosporine A in dry eye disease and recent formulation developments. Clin. Ophthalmol. 2020, 2020, 4187–4200. [Google Scholar] [CrossRef]
- Pflugfelder, S.C. Antiinflammatory therapy for dry eye. Am. J. Ophthalmol. 2004, 137, 337–342. [Google Scholar] [CrossRef]
- Anitua, E.; De la Fuente, M.; Muruzabal, F.; Riestra, A.; Merayo-Lloves, J.; Orive, G. Plasma rich in growth factors [PRGF] eye drops stimulates scarless regeneration compared to autologous serum in the ocular surface stromal fibroblasts. Exp. Eye Res. 2015, 135, 118–126. [Google Scholar] [CrossRef]
- Jones, L.; Jongkhajornpong, J.P.; Markoulli, M.; Karpecki, P.; Akpek, E.K.; Basu, S.; Bitton, E.; Chen, W.; Dhaliwal, D.K.; Dogru, M.; et al. TFOS DEWS III Management and Therapy Report. Am. J. Ophthalmol. 2025, 279, 289–386. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y.; et al. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Sridhar, U.; Gokhale, N.; Doddigarla, V.R.; Sharma, S.; Basu, S. Autologous serum eye drops in dry eye disease: Preferred practice pattern guidelines. Indian J. Ophthalmol. 2023, 71, 1357–1363. [Google Scholar] [CrossRef]
- Fox, R.I.; Chan, R.; Michelson, J.B.; Belmont, J.B.; Michelson, P.E. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis Rheum. 1984, 27, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Janus, J.; Chmielewska, K.; Antoniewicz-Papis, J. Allogeneic serum-based eye drops may give better results than autolo-gous drops in Sjögren’s syndrome dry eye. Transfus. Apher. Sci. 2024, 63, 103991. [Google Scholar] [CrossRef]
- Hassan, A.; Balal, S.; Cook, E.; Dehbi, H.M.; Pardhan, S.; Bourne, R.; Ahmad, S.; Sharma, A. Finger-prick autologous blood (FAB) eye drops for dry eye disease: Single masked multi-Centre randomised controlled trial. Clin. Ophthalmol. 2022, 16, 3973. [Google Scholar] [CrossRef] [PubMed]
- Than, J.; Balal, S.; Wawrzynski, J.; Nesaratnam, N.; Saleh, G.M.; Moore, J.; Patel, A.; Shah, S.; Sharma, B.; Kumar, B.; et al. Fingerprick autologous blood: A novel treatment for dry eye syndrome. Eye 2017, 31, 1655–1663. [Google Scholar] [CrossRef]
- Ralph, R.A.; Doane, M.G.; Dohlman, C.H. Clinical experience with a mobile ocular perfusion pump. Arch. Ophthalmol. 1975, 93, 1039–1043. [Google Scholar] [CrossRef]
- López-Plandolit, S.; Morales, M.C.; Freire, V.; Grau, A.E.; Durán, J.A. Efficacy of plasma rich in growth factors for the treat-ment of dry eye. Cornea 2011, 30, 1312–1317. [Google Scholar] [CrossRef]
- Soifer, M.; Tovar, A.; Wang, M.; Mousa, H.M.; Yennam, S.; Sabater, A.L.; Pflugfelder, S.C.; Perez, V.L. A multicenter report of the use of plasma rich in growth factors [PRGF] for the treatment of patients with ocular surface diseases in North America. Ocul. Surf. 2022, 25, 40–48. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; Tayebba, A.; Riestra, A.; Perez, V.L.; Merayo-Lloves, J.; Orive, G. Autologous serum and plasma rich in growth factors in ophthalmology: Preclinical and clinical studies. Acta Ophthalmol. 2015, 93, e605–e614. [Google Scholar] [CrossRef] [PubMed]
- Jongkhajornpong, P.; Lekhanont, K.; Rattanasiri, S.; Numthavaj, P.; McKay, G.; Attia, J.; Thakkinstian, A. Efficacy of 100% autologous platelet-rich plasma and 100% autologous serum in dry eye disease: A randomised controlled trial. BMJ Open Ophthalmol. 2024, 9, e001857. [Google Scholar] [CrossRef]
- Freeman, J.M. The punctum plug: Evaluation of a new treatment for the dry eye. Trans. Sect. Ophthalmol. Am. Acad. Ophthal-mol. Otolaryngol. 1975, 79, OP874-9. [Google Scholar]
- Kumar, S.V. Role of punctal plugs as a primarily treatment modality in moderate to severe dry eye. Int. J. Clin. Trials 2020, 7, 72–76. [Google Scholar] [CrossRef]
- Chi, S.L.; Acquah, K.F.; Richard, M.J.; Lee, P.P.; Sloan, F.A. Longitudinal evidence on punctal plug use in an elderly popula-tion. Ophthalmic Plast. Reconstr. Surg. 2012, 28, 289–293. [Google Scholar]
- Jehangir, N.; Bever, G.; Mahmood, S.J.; Moshirfar, M. Comprehensive review of the literature on existing punctal plugs for the management of dry eye disease. J. Ophthalmol. 2016, 2016, 9312340. [Google Scholar] [CrossRef]
- Best, A.L.; Labetoulle, M.; Legrand, M.; M’garrech, M.; Barreau, E.; Rousseau, A. Punctal and canalicular plugs: Indications, efficacy and safety. J. Français D’ophtalmologie 2019, 42, e95–e104. [Google Scholar] [CrossRef] [PubMed]
- Baxter, S.A.; Laibson, P.R. Punctal plugs in the management of dry eyes. Ocul. Surf. 2004, 2, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Mansour, K.; Leonhardt, C.J.; Kalk, W.W.; Bootsma, H.; Bruin, K.J.; Blanksma, L.J. Lacrimal punctum occlusion in the treat-ment of severe keratoconjunctivitis sicca caused by Sjögren syndrome: A uniocular evaluation. Cornea 2007, 26, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Burgess, P.I.; Koay, P.; Clark, P. SmartPlug versus silicone punctal plug therapy for dry eye: A prospective randomized trial. Cornea 2008, 27, 391–394. [Google Scholar] [CrossRef]
- Wang, Y.; Carreno-Galeano, J.T.; Singh, R.B.; Dana, R.; Yin, J. Long-term outcomes of punctal cauterization in the management of ocular surface diseases. Cornea 2021, 40, 168–171. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Eslani, M.; Haq, Z.; Shirzadeh, E.; Huvard, M.J.; Djalilian, A.R. Current and upcoming therapies for ocu-lar surface chemical injuries. Ocul. Surf. 2017, 15, 48–64. [Google Scholar] [CrossRef]
- Shi, D.-N.; Song, H.; Ding, T.; Qiu, W.-Q.; Wang, W. Evaluation of the safety and efficacy of therapeutic bandage contact lenses on post-cataract surgery patients. Int. J. Ophthalmol. 2018, 11, 230–234. [Google Scholar] [CrossRef]
- Chen, X.; Yuan, R.; Sun, M.; Chen, X.; Lin, S.; Ye, J.; Chen, C. Efficacy of an ocular bandage contact lens for the treatment of dry eye after phacoemulsification. BMC Ophthalmol. 2019, 19, 13. [Google Scholar] [CrossRef]
- Thomas, M.; Shorter, E.; Joslin, C.E.; McMahon, T.J.; Cortina, M.S. Contact lens use in patients with Boston keratoprosthesis type 1: Fitting, management, and complications. Eye Contact Lens. 2015, 41, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Kammerdiener, L.L.; Speiser, J.L.; Aquavella, J.V.; Harissi-Dagher, M.; Dohlman, C.H.; Chodosh, J.; Ciolino, J.B. Protective effect of soft contact lenses after Boston keratoprosthesis. Br. J. Ophthalmol. 2016, 100, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Tan, D.T.; Chan, W.K. Therapeutic use of Bausch & Lomb PureVision contact lenses. CLAO J. 2001, 27, 179–185. [Google Scholar]
- Sharma, A.; Kaur, R.; Kumar, S.; Gupta, P.; Pandav, S.; Patnaik, B.; Gupta, A. Fibrin glue versus N-butyl-2-cyanoacrylate in corneal perforations. Ophthalmology 2003, 110, 291–298. [Google Scholar] [CrossRef]
- Jhanji, V.; Young, A.L.; Mehta, J.S.; Sharma, N.; Agarwal, T.; Vajpayee, R.B. Management of corneal perforation. Surv. Oph-thalmol. 2011, 56, 522–538. [Google Scholar] [CrossRef]
- Rana, M.; Savant, V. A brief review of techniques used to seal corneal perforation using cyanoacrylate tissue adhesive. Contact Lens Anterior Eye 2013, 36, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Burke, M.T.; Carbia, B.E.; Plummer, C.; Chauhan, A. Extended drug delivery by contact lenses for glaucoma therapy. J. Control. Release 2012, 162, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma Therapy by Extended Release of Timolol from Nanoparticle Loaded Silicone-Hydrogel Contact Lenses. J. Control. Release Off. J. Control. Release Soc. 2013, 165, 82–89. [Google Scholar] [CrossRef]
- Mondal, H.; Kim, H.J.; Mohanto, N.; Jee, J.P. A review on dry eye disease treatment: Recent Progress, diagnostics, and future perspectives. Pharmaceutics 2023, 15, 990. [Google Scholar] [CrossRef]
- Finis, D.; Hayajneh, J.; König, C.; Borrelli, M.; Schrader, S.; Geerling, G. Evaluation of an automated thermodynamic treat-ment (LipiFlow®) system for meibomian gland dysfunction: A prospective, randomized, observer-masked trial. Ocul. Surf. 2014, 12, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.L.; Jung, J.; Gonzales, D.D.; Shacterman, S.; Afshari, N.; Cheng, L. Comparison of manual versus automated thermal lid therapy with expression for meibomian gland dysfunction in patients with dry eye disease. Sci. Rep. 2024, 14, 22287. [Google Scholar] [CrossRef]
- Demolin, L.; Es-Safi, M.; Soyfoo, M.S.; Motulsky, E. Intense Pulsed Light Therapy in the Treatment of Dry Eye Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3039. [Google Scholar] [CrossRef]
- Xue, A.L.; Wang, M.T.M.; Ormonde, S.E.; Craig, J. Randomised double-masked placebo-controlled trial of the cumulative treatment efficacy profile of intense pulsed light therapy for meibomian gland dysfunction. Ocul. Surf. 2020, 18, 286–297. [Google Scholar] [CrossRef]
- Toyos, R.; Desai, N.R.; Toyos, M.; Dell, S.J. Intense pulsed light improves signs and symptoms of dry eye disease due to meibomian gland dysfunction: A randomized controlled study. PLoS ONE 2022, 17, e0270268. [Google Scholar] [CrossRef]
- Suwal, A.; Hao, J.-L.; Zhou, D.-D.; Liu, X.-F.; Suwal, R.; Lu, C.-W. Use of Intense Pulsed Light to Mitigate Meibomian Gland Dysfunction for Dry Eye Disease. Int. J. Med. Sci. 2020, 17, 1385–1392. [Google Scholar] [CrossRef]
- Papageorgiou, P.; Clayton, W.; Norwood, S.; Chopra, S.; Rustin, M. Treatment of rosacea with intense pulsed light: Signifi-cant improvement and long-lasting results. Br. J. Dermatol. 2008, 159, 628–632. [Google Scholar] [CrossRef]
- Fishman, H.A.; Periman, L.M.; Shah, A.A. Real-Time Video Microscopy of In Vitro Demodex Death by Intense Pulsed Light. Photobiomodul. Photomed. Laser Surg. 2020, 38, 472–476. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Azar, D.T.; Baudouin, C.; Bitton, E.; Chen, W.; Hafezi, F.; Hamrah, P.; Hogg, R.E.; Horwath-Winter, J.; Kon-tadakis, G.A.; et al. TFOS Lifestyle: Impact of elective medications and procedures on the ocular surface. Ocul. Surf. 2023, 29, 331–385. [Google Scholar] [CrossRef]
- Wladis, E.J.; Aakalu, V.K.; Foster, J.A.; Freitag, S.K.; Sobel, R.K.; Tao, J.P.; Yen, M.T. Intense pulsed light for meibomian gland disease: A report by the American Academy of Ophthalmology. Ophthalmology 2020, 127, 1227–1233. [Google Scholar] [CrossRef]
- Messmer, E.M. Pathophysiology of dry eye disease and novel therapeutic targets. Exp. Eye Res. 2022, 217, 108944. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.B.; Marta, A.; Ramalhão, J.P.; Marques, J.H.; Barbosa, I. Pulsed light therapy in the management of dry eye disease: Current perspectives. Clin. Ophthalmol. 2022, 16, 3883. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Li, L.; Wang, H.; Zhao, C.; Ke, L.; Sen, D.; Qi, M.; Li, S.; Wang, M.; Zeng, Q. Adverse events of intense pulsed light combined with meibomian gland expression versus meibomian gland expression in the treatment of meibomian gland dysfunction. Lasers Surg. Med. 2021, 53, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, H.; Kim, S.; Cho, K.J. Effect of low-level light therapy in patients with dry eye: A prospective, randomized, observer-masked trial. Sci. Rep. 2022, 12, 3575. [Google Scholar] [CrossRef]
- Ballesteros-Sanchez, A.; Gargallo-Martinez, B.; Sánchez-González, M.C.; Sanchez-Gonzalez, J.M. Intense pulse light com-bined with low-level light therapy in dry eye disease: A systematic review. Eye Contact Lens 2023, 49, 8–13. [Google Scholar] [CrossRef]
- Marques, J.H.; Marta, A.; Baptista, P.M.; Almeida, D.; José, D.; Sousa, P.J.; Barbosa, I. Low-level light therapy in association with intense pulsed light for meibomian gland dysfunction. Clin. Ophthalmol. 2022, 16, 4003. [Google Scholar] [CrossRef] [PubMed]
- Gurnani, B.; Kaur, K. Current Approach in Surgical Management of Dry Eyes–Dry Eye Review II. TNOA J. Ophthalmic Sci. Res. 2021, 59, 241–249. [Google Scholar] [CrossRef]
- Kim, B.; Lee, Y.; Son, H.S.; Choi, C.Y. Clinical outcomes of conjunctivochalasis treatment with a new ophthalmic radiofre-quency device. BMC Ophthalmol. 2024, 24, 302. [Google Scholar] [CrossRef]
- Dhillon, H.K.; Bahadur, H.; Raj, A. A comparative study of tarsorrhaphy and amniotic membrane transplantation in the healing of persistent corneal epithelial defects. Indian J. Ophthalmol. 2020, 68, 29–33. [Google Scholar]
- Prischmann, J.; Sufyan, A.; Ting, J.Y.; Ruffin, C.; Perkins, S.W. Dry eye symptoms and chemosis following blepharoplasty: A 10-year retrospective review of 892 cases in a single-surgeon series. JAMA Facial Plast. Surg. 2013, 15, 39–46. [Google Scholar] [CrossRef]
- Hollander, M.H.; Pott, J.W.R.; Delli, K.; Vissink, A.; Schepers, R.H.; Jansma, J. Impact of upper blepharoplasty, with or without orbicularis oculi muscle removal, on tear film dynamics and dry eye symptoms: A randomized controlled trial. Acta Ophthalmol. 2022, 100, 564–571. [Google Scholar] [CrossRef]
- Rodrigues, C.; Carvalho, F.; Marques, M. Upper eyelid blepharoplasty: Surgical techniques and results—Systematic review and meta-analysis. Aesthetic Plast. Surg. 2023, 47, 1870–1883. [Google Scholar] [CrossRef]
- Zemba, M.; Stamate, A.C.; Tataru, C.P.; Branisteanu, D.C.; Balta, F. Conjunctival flap surgery in the management of ocular surface disease. Exp. Ther. Med. 2020, 20, 3412–3416. [Google Scholar] [CrossRef]
- Singh, S.; Basu, S.; Geerling, G. Salivary gland transplantation for dry eye disease: Indications, techniques, and outcomes. Ocul. Surf. 2022, 26, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Bhalekar, S.; Amescua, G.; Singh, S.; Basu, S. Minor salivary gland transplantation for severe dry eye disease due to cicatrising conjunctivitis: Multicentre long-term outcomes of a modified technique. Br. J. Ophthalmol. 2021, 105, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Anguela, X.M.; High, K.A. Entering the modern era of gene therapy. Annu. Rev. Med. 2019, 70, 273–288. [Google Scholar] [CrossRef]
- Amador, C.; Shah, R.; Ghiam, S.; Kramerov, A.A.; Ljubimov, A.V. Gene Therapy in the Anterior Eye Segment. Curr. Gene Ther. 2022, 22, 104–131. [Google Scholar] [CrossRef]
- Trousdale, M.D.; Zhu, Z.; Stevenson, D.; Schechter, J.E.; Ritter, T.; Mircheff, A.K. Expression of TNF inhibitor gene in the lacrimal gland promotes recovery of tear production and tear stability and reduced immunopathology in rabbits with in-duced autoimmune dacryoadenitis. J. Autoimmune Dis. 2005, 2, 6. [Google Scholar] [CrossRef]
- Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Velasco-García, A.M.; Aloy-Reverté, C.; Vilarrodona, A.; Casaroli-Marano, R.P. Xenofree generation of limbal stem cells for ocular surface advanced cell therapy. Stem Cell Res. Ther. 2019, 10, 374. [Google Scholar] [CrossRef]
- Bandeira, F.; Goh, T.W.; Setiawan, M.; Yam, G.H.F.; Mehta, J.S. Cellular therapy of corneal epithelial defect by adipose mesenchymal stem cell-derived epithelial progenitors. Stem Cell Res. Ther. 2020, 11, 14. [Google Scholar] [CrossRef]
- Nosrati, H.; Alizadeh, Z.; Nosrati, A.; Ashrafi-Dehkordi, K.; Banitalebi-Dehkordi, M.; Sanami, S.; Khodaei, M. Stem cell-based therapeutic strategies for corneal epithelium regeneration. Tissue Cell 2021, 68, 101470. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.P.; Singh, S.; Thacker, M.; Pati, F.; Vemuganti, G.K.; Basu, S.; Singh, V. Newer approaches to dry eye therapy: Nano-technology, regenerative medicine, and tissue engineering. Indian J. Ophthalmol. 2023, 71, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Walkden, A. Amniotic membrane transplantation in ophthalmology: An updated perspective. Clin. Ophthalmol. 2020, 2020, 2057–2072. [Google Scholar] [CrossRef]
- Hopkinson, A.; Britchford, E.R.; Sidney, L.E. Preparation of dried amniotic membrane for corneal repair. In Corneal Regener-ation Methods and Protocols; Ahearne, M., Ed.; Humana: New York, NY, USA, 2020; pp. 143–157. [Google Scholar]
- Davis, J.S., II. Skin grafting at the johns hopkins hospital. Ann. Surg. 1909, 50, 542–549. [Google Scholar] [CrossRef] [PubMed]
- De Rötth, A. Plastic repair of conjunctival defects with fetal membranes. Arch. Ophthalmol. 1940, 23, 522–525. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, D.; Maharana, P.K.; Kriplani, A.; Velpandian, T.; Pandey, R.M.; Vajpayee, R.B. Comparison of Amniotic Membrane Transplantation and Umbilical Cord Serum in Acute Ocular Chemical Burns: A Randomized Controlled Trial. Am. J. Ophthalmol. 2016, 168, 157–163. [Google Scholar] [CrossRef]
- Tabatabaei, S.A.; Soleimani, M.; Behrouz, M.J.; Torkashvand, A.; Anvari, P.; Yaseri, M. A randomized clinical trial to evalu-ate the usefulness of amniotic membrane transplantation in bacterial keratitis healing. Ocul. Surf. 2017, 15, 218–226. [Google Scholar] [CrossRef]
- Tandon, R.; Gupta, N.; Kalaivani, M.; Sharma, N.; Titiyal, J.S.; Vajpayee, R.B. Amniotic membrane transplantation as an ad-junct to medical therapy in acute ocular burns. Br. J. Ophthalmol. 2011, 95, 199–204. [Google Scholar] [CrossRef]
- Kjaergaard, N.; Helmig, R.B.; Schonheyder, H.C.; Uldbjerg, N.; Hansen, E.S.; Madsen, H. Chorioamniotic membranes con-stitute a competent barrier to group b streptococcus in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 83, 165–169. [Google Scholar] [CrossRef]
- Kjaergaard, N.; Hein, M.; Hyttel, L.; Helmig, R.; Schønheyder, H.; Uldbjerg, N.; Madsen, H. Antibacterial properties of hu-man amnion and chorion in vitro. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 94, 224–229. [Google Scholar] [CrossRef]
- Gabric, N.; Mravicic, I.; Dekaris, I.; Karaman, Z.; Mitrovic, S. Human amniotic membrane in the reconstruction of the ocular surface. Doc. Ophthalmol. 1999, 98, 273–283. [Google Scholar] [CrossRef]
- Sangwan, V.S.; Basu, S. Antimicrobial properties of amniotic membrane. Br. J. Ophthalmol. 2011, 95, 1. [Google Scholar] [CrossRef] [PubMed]
- Sanders, F.W.B.; Huang, J.; Alió Del Barrio, J.L.; Hamada, S.; McAlinden, C. Amniotic membrane transplantation: Structural and biological properties, tissue preparation, application and clinical indications. Eye 2023, 38, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Travé-Huarte, S.; Wolffsohn, J.S. Bilateral Sutureless Application of Human Dehydrated Amniotic Membrane with a Spe-cialised Bandage Contact Lens for Moderate-to-Severe Dry Eye Disease: A Prospective Study with 1-Month Follow-Up. Clin. Ophthalmol. 2024, 2024, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.B.; Sheha, H.; Tighe, S.; Janik, S.B.; Bowden, F.W.; Chokshi, A.R.; Singer, M.A.; Nanda, S.; Qazi, M.A.; Dierker, D.; et al. Treatment outcomes in the DRy eye amniotic membrane [DREAM] study. Clin. Ophthalmol. 2018, 2018, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.S.; Kim, K.Y.; Lee, Y.W.; Han, S.B.; Choi, C.Y. Clinical outcomes and indications of in-office sutureless dried gamma ray-sterilized human amniotic membrane transplantation with bandage contact lenses in various ocular surface disorders. Cornea 2024, 43, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.; Janik, S.B.; Bowden, F.W.; Chokshi, A.; Singer, M.A.; Tighe, S.; Mead, O.G.; Nanda, S.; Qazi, M.; Dierker, D.; et al. Association of treatment duration and clinical outcomes in dry eye treatment with sutureless cryopreserved amniotic membrane. Clin. Ophthalmol. 2023, 17, 2697–2703. [Google Scholar] [CrossRef]
- Kheirkhah, A.; Johnson, D.A.; Paranjpe, D.R.; Raju, V.K.; Casas, V.; Tseng, S.C.G. Temporary sutureless amniotic membrane patch for acute alkaline burns. Arch. Ophthalmol. 2008, 126, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Sheha, H.; Liang, L.Y.; Li, J.J.; Tseng, S.C.G. Sutureless amniotic membrane transplantation for severe bacterial keratitis. Cornea 2009, 28, 1118–1123. [Google Scholar] [CrossRef]
- Suri, K.; Kosker, M.; Raber, I.M.; Hammersmith, K.M.; Nagra, P.K.; Ayres, B.D.; Halfpenny, C.P.; Rapuano, C.J. Sutureless amniotic membrane prokera for ocular surface disorders: Short-term results. Article. Eye 2013, 39, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Morkin, M.I.; Hamrah, P. Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. Ocul. Surf. 2018, 16, 132–138. [Google Scholar] [CrossRef]
- Maqsood, S.; Elsawah, K.; Dhillon, N.; Soliman, S.; Laginaf, M.; Lodhia, V.; Lake, D.; Hamada, S.; Elalfy, M. Management of persistent corneal epithelial defects with human amniotic membrane-derived dry matrix. Clin. Ophthalmol. 2021, 15, 2231–2238. [Google Scholar] [CrossRef]
- Mimouni, M.; Trinh, T.; Sorkin, N.; Cohen, E.; Santaella, G.; Rootman, D.S.; Slomovic, A.R.; Chan, C.C. Sutureless dehydrat-ed amniotic membrane for persistent epithelial defects. Euro J. Ophthalmol. 2022, 32, 875–879. [Google Scholar] [CrossRef]
- Voigt, J. Cost utility analysis of cryopreserved amniotic membrane versus topical cyclosporine for the treatment of moder-ate to severe dry eye syndrome. Cost. Eff. Resour. Alloc. 2020, 18, 56. [Google Scholar] [CrossRef]
- Kolomeyer, A.M.; Do, B.K.; Tu, Y.; Chu, D.S. Placement of ProKera in the management of ocular manifestations of acute Stevens-Johnson syndrome in an outpatient. Eye Contact Lens. 2013, 39, e7–e11. [Google Scholar] [CrossRef]
- Cheng, A.M.; Zhao, D.; Chen, R.; Yin, H.Y.; Tighe, S.; Sheha, H.; Casas, V.; Tseng, S.C. Accelerated restoration of ocular sur-face health in dry eye disease by self-retained cryopreserved amniotic membrane. Ocul. Surf. 2016, 14, 56–63. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]
- John, T.; Tighe, S.; Sheha, H.; Hamrah, P.; Salem, Z.M.; Cheng, A.M.S.; Wang, M.X.; Rock, N.D. Corneal Nerve Regeneration after Self-Retained Cryopreserved Amniotic Membrane in Dry Eye Disease. J. Ophthalmol. 2017, 2017, 6404918. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.L.; Barreales, S.; Sabater-Cruz, N.; Martinez-Conesa, E.M.; Vilarrodona, A.; Casaroli-Marano, R.P. Amniotic mem-brane extract eye drops: A new approach to severe ocular surface pathologies. Cell Tissue Bank. 2022, 23, 473–481. [Google Scholar] [CrossRef]
- Sabater-Cruz, N.; Figueras-Roca, M.; Ferrán-Fuertes, M.; Agustí, E.; Martínez-Conesa, E.M.; Pérez-Rodríguez, M.L.; Vilarrodona, A.; Casaroli-Marano, R.P. Amniotic membrane extract eye drops for ocular surface diseases: Use and clinical outcome in real-world practice. Int. Ophthalmol. 2021, 41, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Agusti, E.; Martinez-Conesa, E.M.; Perez, M.L.; Sabater-Cruz, N.; Casaroli-Marano, R.P.; Vilarrodona, A. 30 A new step on amniotic membrane extract eye drops [AMEED] development for the treatment of severe ocular surface pathologies. BMJ Open Ophthalmol. 2022, 7, A13. [Google Scholar] [CrossRef]
- Nam, J.W.; Kim, J.; Yoon, H.J.; Yoon, K.C. Effects of amniotic membrane extract eye drops on persistent epithelial defects of the cornea. J. Korean Ophthalmol. Soc. 2021, 62, 1340–1347. [Google Scholar] [CrossRef]
- Mensah, R.A.; Salim, K.; Peszko, K.; Diop, S.; Wong, T.H.; Chau, D.Y. The chicken eggshell membrane: A versatile, sustaina-ble, biological material for translational biomedical applications. Biomed. Mater. 2023, 18, 042001. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, C.; Arena, S.; Scaloni, A.; Guerrier, L.; Boschetti, E.; Mendieta, M.E.; Citterio, A.; Righetti, P.G. Exploring the chicken egg white proteome with combinatorial peptide ligand libraries. J. Proteome Res. 2008, 7, 3461–3474. [Google Scholar] [CrossRef]
- Wong, M.; Hendrix, M.J.; von der Mark, K.; Little, C.; Stern, R. Collagen in the egg shell membranes of the hen. Dev. Biol. 1984, 104, 28–36. [Google Scholar] [CrossRef]
- Leach, R.M., Jr. Biochemistry of the organic matrix of the eggshell. Poult. Sci. 1982, 61, 2040–2047. [Google Scholar] [CrossRef]
- Rønning, S.B.; Berg, R.S.; Høst, V.; Veiseth-Kent, E.; Wilhelmsen, C.R.; Haugen, E.; Suso, H.P.; Barham, P.; Schmidt, R.; Pedersen, M.E. Processed eggshell membrane powder is a promising biomaterial for use in tissue engineering. Int. J. Mol. Sci. 2020, 21, 8130. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Suso, H.P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C 2019, 95, 192–203. [Google Scholar] [CrossRef]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Hou, W.; Zhou, Y. Osteopontin in bone metabolism and bone diseases. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e919159. [Google Scholar] [CrossRef] [PubMed]
- Scatena, M.; Liaw, L.; Giachelli, C.M. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler.Thromb. Vasc. Biol. 2007, 27, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, H.; Panitch, A. Glycosaminoglycans in tissue engineering: A review. Biomolecules 2020, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.S.; Hewlings, S. The effect of oral hydrolyzed eggshell membrane on the appearance of hair, skin, and nails in healthy middle-aged adults: A randomized double-blind placebo-controlled clinical trial. J. Cosmet. Dermatol. 2020, 19, 1463–1472. [Google Scholar] [CrossRef]
- Mensah, R.A.; Jo, S.B.; Kim, H.; Park, S.M.; Patel, K.D.; Cho, K.J.; Cook, M.T.; Kirton, S.B.; Hutter, V.; Sidney, L.E.; et al. The eggshell membrane: A potential biomaterial for corneal wound healing. J. Biomater. Appl. 2021, 36, 912–929. [Google Scholar] [CrossRef]
- Coover, D. The use of egg membrane in ophthalmic surgery. Ophthal. Record 1899, 8, 222–224. [Google Scholar]
- Choi, J.; Lee, J.; Shin, M.E.; Been, S.; Lee, D.H.; Khang, G. Eggshell membrane/gellan gum composite hydrogels with in-creased degradability, biocompatibility, and anti-swelling properties for effective regeneration of retinal pigment epithe-lium. Polymers 2020, 12, 2941. [Google Scholar] [CrossRef] [PubMed]
- Briggs, E.; Mensah, R.A.; Patel, K.D.; Mandakhbayar, N.E.; Sharifulden, N.S.; Erdogan, Z.K.; Silva, L.V.B.; Salim, K.; Kim, H.W.; Nguyen, L.T.; et al. Therapeutic application of an Ag-nanoparticle-PNIPAAm-modified eggshell membrane construct for dermal regeneration and reconstruction. Pharmaceutics 2022, 14, 2162. [Google Scholar] [CrossRef] [PubMed]
- Mensah, R.A.; Cook, M.T.; Kirton, S.B.; Hutter, V.; San Chau, D.Y. A drug-incorporated-microparticle-eggshell-membrane-scaffold (DIMES) dressing: A novel biomaterial for localised wound regeneration. Eur. J. Pharm. Biopharm. 2023, 190, 258–269. [Google Scholar] [CrossRef]
- Mensah, R.A.; Trotta, F.; Briggs, E.; Sharifulden, N.S.; Silva, L.V.B.; Keskin-Erdogan, Z.; Diop, S.; Kureshi, A.K.; Chau, D.Y. A sustainable, green-processed, Ag-nanoparticle-incorporated eggshell-derived biomaterial for wound-healing applications. J. Funct. Biomater. 2023, 14, 450. [Google Scholar] [CrossRef] [PubMed]
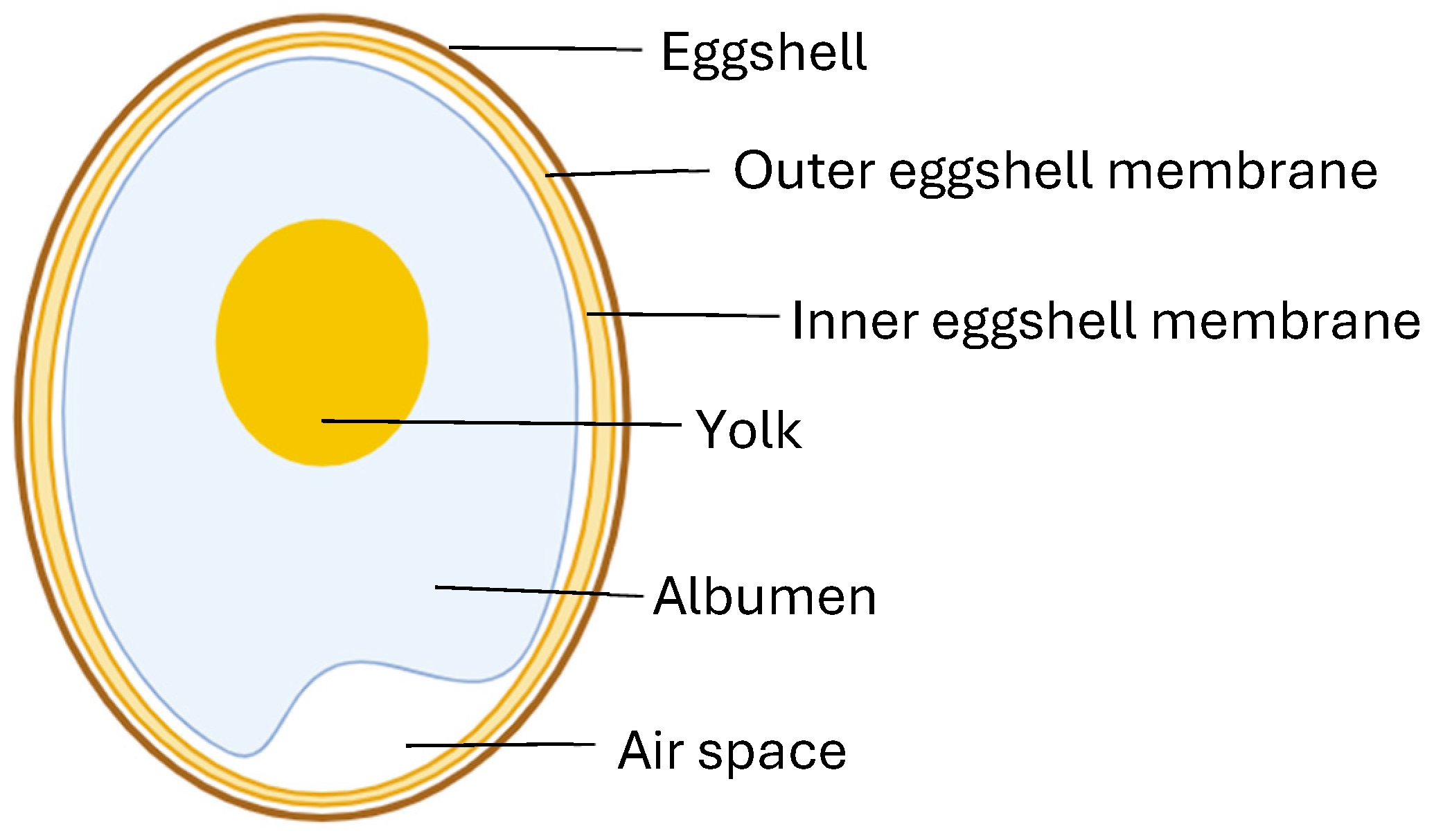
| ESM Component | Benefits of Molecules in Tissue Engineering | References | |
|---|---|---|---|
| Collagen | - Acts as a scaffold to facilitate cell seeding and adherence - Type V collagen maintains structural integrity and tensile strength of the ESM | [113,114] | |
| Fibronectin | - Facilitates cellular adhesion, growth, migration, and repair | [115] | |
| Osteopontin | - Has an immunomodulatory effect on the host by regulating cytokine release and macrophage recruitment | [116,117] | |
| Glycosaminoglycans | Hyaluronic acid | - Maintains viscoelasticity via water retention, promotes ECM secretion, reduces inflammation, and is involved in every step of wound healing | [118] |
| Heparin | - Has a high affinity to growth factors, which allows easy modification of ESM to promote growth and healing | ||
| Chondroitin sulfate | - Promotes cellular adhesion and induces cellular differentiation | ||
| Keratan sulfate | - Aids the control of charge and ion gradients needed for cellular adhesion, proliferation, and differentiation | ||
| Amino Acids | - Amino acids found in high abundance in the ESM include proline, cysteine, glycine, glutamine, and asparagine - Important for collagen and protein synthesis needed to maintain the ECM matrix - Impacts metabolic and physiologic processes such as cellular proliferation which can act as an energy substrate | [119] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhallu, S.K.; Pritchard, M.J.; Chau, D.Y.S.; Kirton, S.B. Current and Emerging Approaches in the Management of Severe Ocular Surface Disease. Medicina 2025, 61, 1819. https://doi.org/10.3390/medicina61101819
Dhallu SK, Pritchard MJ, Chau DYS, Kirton SB. Current and Emerging Approaches in the Management of Severe Ocular Surface Disease. Medicina. 2025; 61(10):1819. https://doi.org/10.3390/medicina61101819
Chicago/Turabian StyleDhallu, Sandeep K., Molly J. Pritchard, David Y. S. Chau, and Stewart B. Kirton. 2025. "Current and Emerging Approaches in the Management of Severe Ocular Surface Disease" Medicina 61, no. 10: 1819. https://doi.org/10.3390/medicina61101819
APA StyleDhallu, S. K., Pritchard, M. J., Chau, D. Y. S., & Kirton, S. B. (2025). Current and Emerging Approaches in the Management of Severe Ocular Surface Disease. Medicina, 61(10), 1819. https://doi.org/10.3390/medicina61101819







