Timing Matters: A Randomized Controlled Trial Comparing Preoperative and Postoperative Erector Spinae Plane Block for Analgesia in Laparoscopic Cholecystectomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size and Power Calculation
2.3. Participants
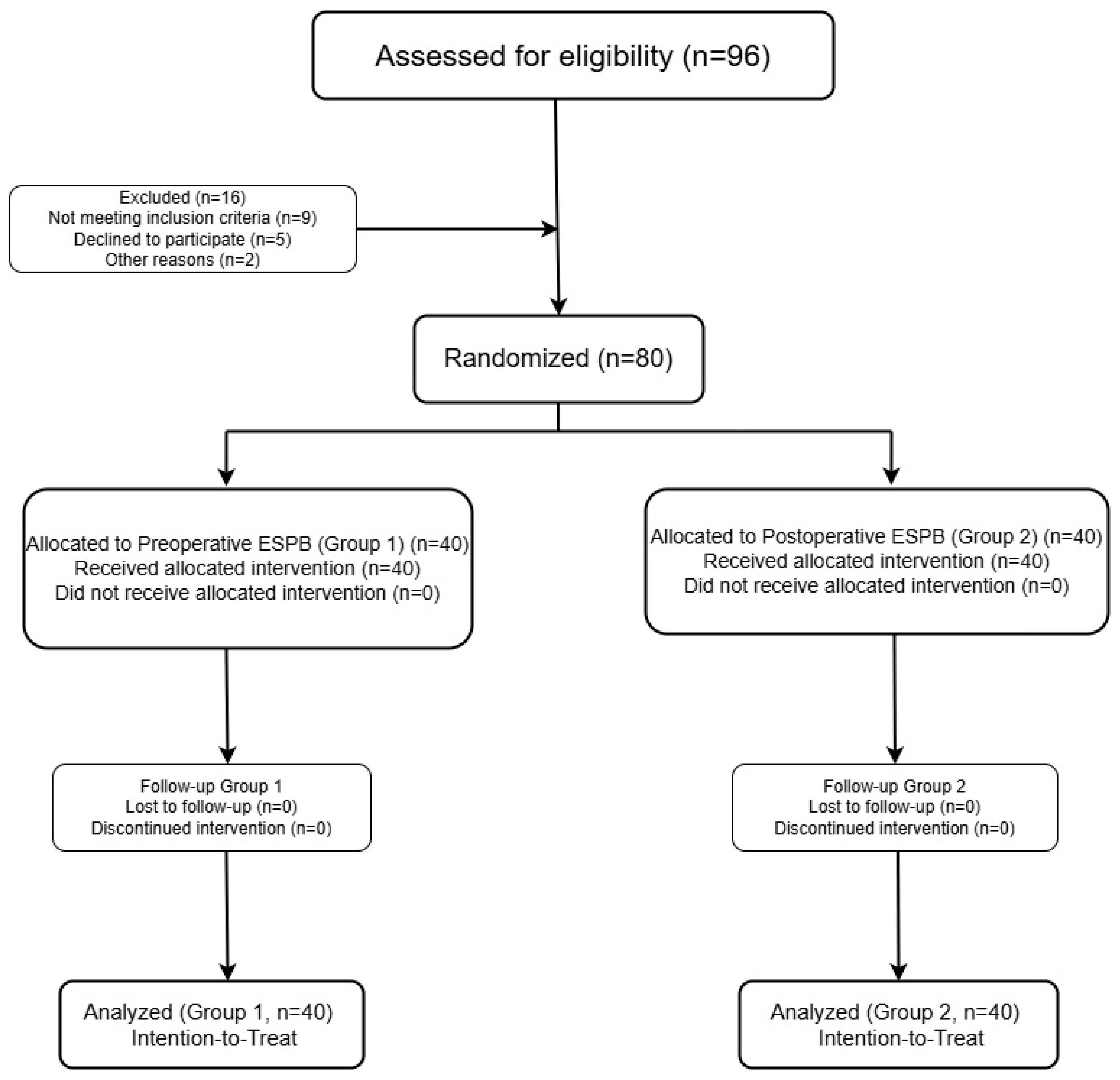
2.4. Randomization and Blinding
- Group 1 (n = 40): received bilateral ESPB in the preoperative period prior to induction of anesthesia,
- Group 2 (n = 40): received bilateral ESPB postoperatively prior to extubation.
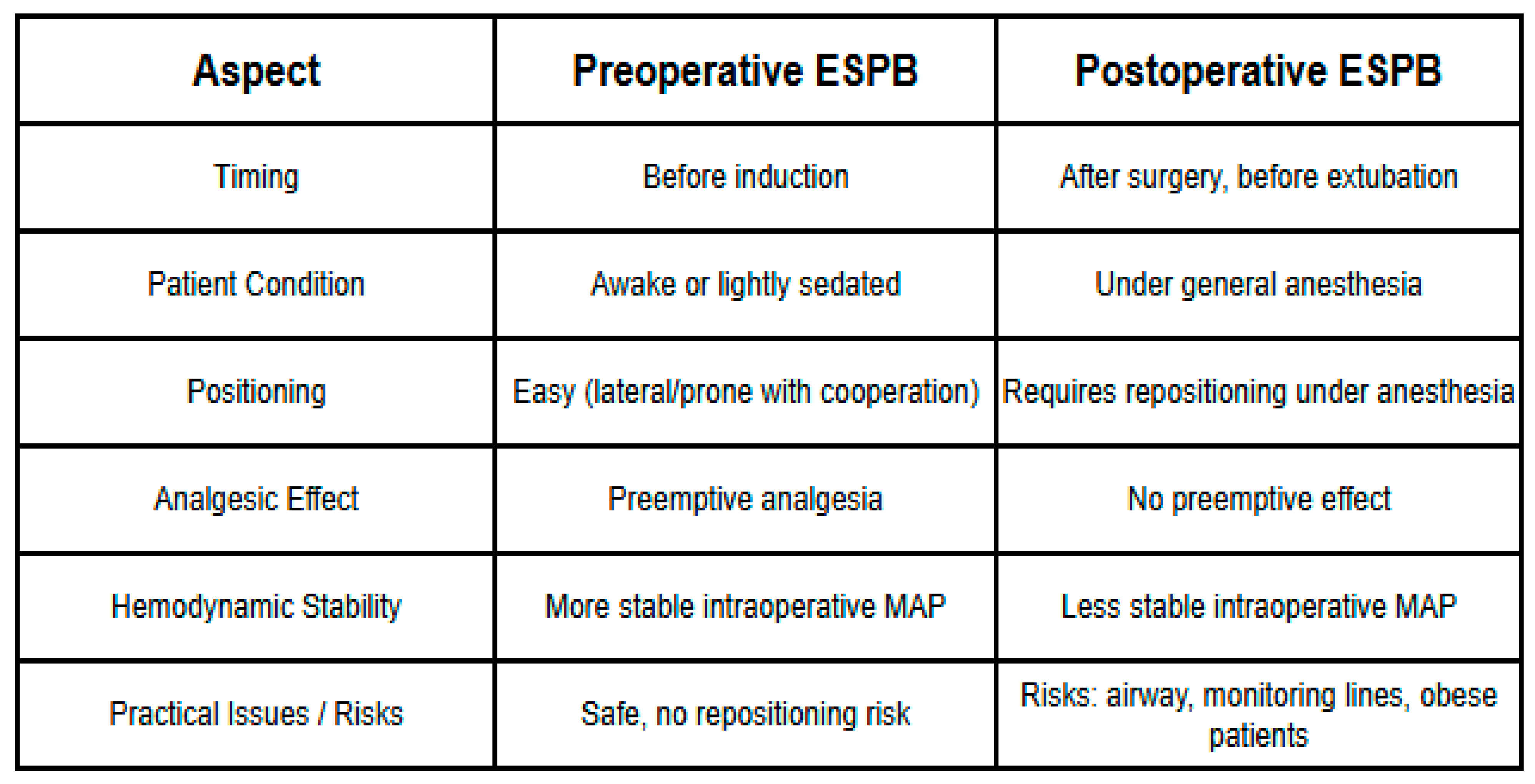
2.5. Anesthetic Management and ESPB Protocol
2.6. Preoperative ESPB Protocol (Group 1)
2.7. Postoperative ESPB Protocol (Group 2)
2.8. Outcome Measures
- NRS scores at other time points: postoperative 0 (PACU), 4, 6, 12, and 24 h.
- Hemodynamic parameters (heart rate, systolic and diastolic blood pressure, and mean arterial pressure) at specified intraoperative and postoperative time points.
- Cumulative rescue analgesic use within 24 h (tramadol 1 mg/kg IV, administered as needed).
- Patient satisfaction at 24 h (measured on a 5-point Likert scale).
- Adverse events such as pneumothorax, local anesthetic systemic toxicity (LAST), nausea, and vomiting.
2.9. Statistical Analysis
3. Results
3.1. Baseline Demographic and Clinical Characteristics of the Study Population
3.2. Effect of ESPB Timing on Postoperative Pain Intensity: NRS Score Trajectory
3.3. Effect of ESPB Timing on Intraoperative and Postoperative Hemodynamic Profiles
3.4. Effect of ESPB Timing on the Incidence and Dose of Rescue Tramadol Use
3.5. Impact of ESPB Administration Timing on 24-Hour Patient Satisfaction
3.6. Adverse Events
4. Discussion
Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESPB | Erector Spinae Plane Block |
| LC | Laparoscopic Cholecystectomy |
| ERAS | Enhanced Recovery After Surgery |
| NRS | Numeric Rating Scale |
| PACU | Post-Anesthesia Care Unit |
| MAP | Mean Arterial Pressure |
| HR | Heart Rate |
| SBP | Systolic Blood Pressure |
| DBP | Diastolic Blood Pressure |
| ASA | American Society of Anesthesiologists |
| EtCO2 | End-Tidal Carbon Dioxide |
| SD | Standard Deviation |
| LAST | Local Anesthetic Systemic Toxicity |
References
- Kang, S.H.; Park, M. Comparison of early postoperative recovery between laryngeal mask airway and endotracheal tube in laparoscopic cholecystectomy: A randomized trial. Medicine 2019, 98, e16022. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Liu, J.; Qin, S.; Geng, X.; Jing, L.; Fang, S. Safety and effectiveness of multimodal opioid-free anaesthesia for pain and recovery after laparoscopic surgery: A systematic review and meta-analysis. BMJ Open 2025, 15, e085988. [Google Scholar] [CrossRef]
- Gonçalves, A.M.; Abelenda, D.M.d.S.; Cruz, I.S.; Feitoza, M.J.T.; Azevedo Tda, C.S.; Perrucci, J.A.; Miranda, M.C.G.G.D.; Silva, K.C.M.; de Souza, G.T.Y.; Fonseca, K.S.; et al. Technical and quantitative analysis of pain management in major surgeries using regional anesthesia with opioid-sparing drugs. ARACÊ 2024, 6, 18732–18744. [Google Scholar] [CrossRef]
- Young-Fadok, T.M.; Craner, R.C. Regional Anesthesia Techniques for Abdominal Operations. Enhanced Recovery After Surgery; Springer International Publishing: Cham, Switzerland, 2020; pp. 149–162. [Google Scholar] [CrossRef]
- Forero, M.; Adhikary, S.D.; Lopez, H.; Tsui, C.; Chin, K.J. The Erector Spinae Plane Block. Reg. Anesth. Pain Med. 2016, 41, 621–627. [Google Scholar] [CrossRef]
- Chin, K.J.; Malhas, L.; Perlas, A. The Erector Spinae Plane Block Provides Visceral Abdominal Analgesia in Bariatric Surgery: A Report of 3 Cases. Reg. Anesth. Pain Med. 2017, 42, 372–376. [Google Scholar] [CrossRef]
- Tulgar, S.; Selvi, O.; Senturk, O.; Serifsoy, T.E.; Thomas, D.T. Ultrasound-guided Erector Spinae Plane Block: Indications, Complications, and Effects on Acute and Chronic Pain Based on a Single-center Experience. Cureus 2019, 11, e3815. [Google Scholar] [CrossRef]
- Tulgar, S.; Kapakli, M.S.; Senturk, O.; Selvi, O.; Serifsoy, T.E.; Ozer, Z. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: A prospective, randomized, controlled clinical trial. J. Clin. Anesth. 2018, 49, 101–106. [Google Scholar] [CrossRef]
- Altiparmak, B.; Toker, M.K.; Uysal, A.İ.; Kuşçu, Y.; Demirbilek, S.G. Efficacy of ultrasound-guided erector spinae plane block for analgesia after laparoscopic cholecystectomy: A randomized controlled trial. Braz. J. Anesthesiol. 2019, 69, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, N.; Krishnan, S.; Dua, A.; Cascella, M. Erector Spinae Plane Block; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545305/ (accessed on 27 September 2025). [PubMed]
- Liao, C.-A.; Chen, Y.-J.; Shen, S.-J.; Wang, Q.-A.; Chen, S.-A.; Liao, C.-H.; Lin, J.-R.; Lee, C.-W.; Tsai, H.-I. Erector spinae plane block (ESPB) enhances hemodynamic stability decreasing analgesic requirements in surgical stabilization of rib fractures (SSRFs). World J. Emerg. Surg. 2024, 19, 36. [Google Scholar] [CrossRef]
- Oezel, L.; Hughes, A.P.; Onyekwere, I.; Wang, Z.; Arzani, A.; Okano, I.; Zhu, J.; Sama, A.A.; Cammisa, F.P.; Girardi, F.; et al. Procedure-Specific Complications Associated with Ultrasound-Guided Erector Spinae Plane Block for Lumbar Spine Surgery: A Retrospective Analysis of 342 Consecutive Cases. J. Pain Res. 2022, 15, 655–661. [Google Scholar] [CrossRef]
- Yokoyama, M.; Ueda, W.; Hirakawa, M. Haemodynamic effects of the lateral decubitus position and the kidney rest lateral decubitus position during anaesthesia. Br. J. Anaesth. 2000, 84, 753–757. [Google Scholar] [CrossRef]
- Chauhan, S.; Gupta, A.; Harjai, M.; Giri, M.K. Evaluation of efficacy of ultrasound guided erector spinae plane block (ESPB) for post-operative analgesia in patients undergoing laparoscopic cholecystectomy. Turk. J. Surg. 2025, 41, 180–185. [Google Scholar] [CrossRef]
- Tunay, D.L.; Ilginel, M.T.; Karacaer, F.; Biricik, E.; Tabakan, I.; Ozmete, O. Bilateral Ultrasound-Guided Erector Spinae Plane Block for Perioperative Analgesia in Breast Reduction Surgery: A Prospective Randomized and Controlled Trial. Aesthetic Plast. Surg. 2023, 47, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.K.; Shrestha, B.; Yadav, K.K.; Dahal, S.; Yadav, P.; Yadav, P.; Deo, S. Ultrasound-guided bilateral Erector Spinae Plane Block (ESPB) for postoperative analgesia in laparoscopic cholecystectomy: A randomized controlled trial. Ann. Med. Surg. 2025, 87, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Tsai, H.-I.; Kao, F.-C.; Tsai, T.-T.; Niu, C.-C.; Chen, L.-H.; Lai, P.-L.; Chiu, P.-Y. Effects of erector spinae plane block on perioperative hemodynamic stability, blood loss, and postoperative pain in transforaminal lumbar interbody fusion. Res. Sq. 2025. preprint. [Google Scholar] [CrossRef]
- Adhikary, S.D.; Liu, W.M.; Fuller, E.; Cruz-Eng, H.; Chin, K.J. The effect of erector spinae plane block on respiratory and analgesic outcomes in multiple rib fractures: A retrospective cohort study. Anaesthesia 2019, 74, 585–593. [Google Scholar] [CrossRef]
- Malawat, A.; Verma, K.; Jethava, D.; Jethava, D.D. Erector spinae plane block for complete surgical anaesthesia and postoperative analgesia for breast surgeries: A prospective feasibility study of 30 cases. Indian J. Anaesth. 2020, 64, 118–124. [Google Scholar] [CrossRef]
- Li, X.; Eichinger, J.K.; Hartshorn, T.; Zhou, H.; Matzkin, E.G.; Warner, J.P. A comparison of the lateral decubitus and beach-chair positions for shoulder surgery: Advantages and complications. J. Am. Acad. Orthop. Surg. 2015, 23, 18–28. [Google Scholar] [CrossRef]
- Quan, Z.-F.; He, H.-L.; Tian, M.; Chi, P.; Li, X. Influence of lateral decubitus positioning after combined use of hyperbaric and hypobaric ropivacaine on hemodynamic characteristics in spinal anesthesia for caesarean section. Int. J. Clin. Exp. Med. 2014, 7, 5669–5674. [Google Scholar]
- Bansal, T.; Singhal, S. Sacral erector spinae block: A new era for postoperative analgesia—A narrative review. J. Anaesthesiol. Clin. Pharmacol. 2025, 41, 404–409. [Google Scholar] [CrossRef]
- Zewdu, D.; Tantu, T.; Eanga, S.; Tilahun, T. Analgesic efficacy of erector spinae plane block versus transversus abdominis plane block for laparoscopic cholecystectomy: A systematic review and meta-analysis of randomized controlled trial. Front. Med. 2024, 11, 1399253. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.S.; Fayez Yousef Metias, M.; Mohamed, M.S.; Abd Elmohsen Bedewy, A.; IIsmail, T. Evaluation of Ultrasound-Guided Erector Spinae Plane Block Versus Oblique Subcostal Transversus Abdominis Plane Block in Laparoscopic Cholecystectomy: A Comparative Study. Anesth. Pain Med. 2025, 15, e157680. [Google Scholar] [CrossRef] [PubMed]

| Group 1 (n:40) | Group 2 (n:40) | p | SMD | ||
|---|---|---|---|---|---|
| Age (years) | 45.4 ± 14.2 | 48.0 ± 13.6 | 0.413 | 0.18 | |
| BMI (kg/m2) | 26.1 ± 3.92 | 27.6 ± 3.00 | 0.054 | 0.42 | |
| n(%) | n(%) | p | |||
| Sex | Female | 29 (72.5%) | 28 (70%) | 0.805 | 0.06 |
| Male | 11 (27.5%) | 12 (30%) | |||
| Comorbidity | Present | 23 (57.5%) | 31 (77.5%) | 0.171 | 0.41 |
| Absent | 17 (42.5%) | 9 (22.5%) | |||
| Allergic Rhinitis | 1 (2.5%) | 0 (0.0%) | 1.000 | 0.07 | |
| Asthma + Diabetes Mellitus | 0 (0.0%) | 2 (5.0%) | 0.494 | 0.22 | |
| Asthma + Hypertension | 0 (0.0%) | 1 (2.5%) | 1.000 | 0.16 | |
| Diabetes Mellitus | 1 (2.5%) | 4 (10.0%) | 0.363 | 0.29 | |
| Diabetes Mellitus + Hypertension | 4 (10.0%) | 2 (5.0%) | 0.673 | 0.18 | |
| Hypertension | 4 (10.0%) | 5 (12.5%) | 1.000 | 0.09 | |
| Asthma | 1 (2.5%) | 3 (7.5%) | 0.615 | 0.22 | |
| Coronary Artery Disease | 1 (2.5%) | 1 (2.5%) | 1.000 | 0.00 | |
| Facial Paralysis | 1 (2.5%) | 1 (%) | 1.000 | 0.00 | |
| Postoperative Time | Group 1 (Mean ± SD) | Group 2 (Mean ± SD) | p Value |
|---|---|---|---|
| 0 min | 3.30 ± 2.63 | 4.25 ± 2.54 | 0.105 |
| 2 h | 3.33 ± 2.08 | 3.40 ± 1.91 | 0.867 |
| 4 h | 2.75 ± 1.43 | 3.13 ± 1.49 | 0.254 |
| 6 h | 3.25 ± 1.69 | 3.38 ± 1.94 | 0.760 |
| 12 h | 3.02 ± 1.56 | 3.58 ± 1.91 | 0.162 |
| 24 h | 3.14 ± 0.07 | 3.48 ± 1.91 | 0.658 |
| Time Point | Group 1 (n = 40) (Mean ± SD) | Group 2 (n = 40) (Mean ± SD) | p | |
|---|---|---|---|---|
| Baseline (0 min) | HR | 81.3 ± 12.9 | 84.0 ± 15.4 | 0.395 |
| SBP | 109.0 ± 21.5 | 114.0 ± 21.6 | 0.266 | |
| DBP | 69.1 ± 14.4 | 71.0 ± 16.2 | 0.586 | |
| MAP | 82.4 ± 16.2 | 84.9 ± 16.7 | 0.488 | |
| 15 min | HR | 80.4 ± 12.6 | 82.7 ± 15.5 | 0.468 |
| SBP | 107.0 ± 24.4 | 114.0 ± 28.6 | 0.284 | |
| DBP | 70.3 ± 16.0 | 73.0 ± 18.4 | 0.473 | |
| MAP | 82.5 ± 18.3 | 86.5 ± 21.3 | 0.372 | |
| 30 min | HR | 77.9 ± 10.4 | 78.9 ± 12.0 | 0.692 |
| SBP | 107.0 ± 19.0 | 117.0 ± 18.8 | 0.016 * | |
| DBP | 67.9 ± 12.1 | 73.5 ± 11.4 | 0.036 * | |
| MAP | 80.8 ± 13.8 | 88.0 ± 12.7 | 0.018 * | |
| 60 min | HR | 77.3 ± 11.7 | 77.1 ± 10.5 | 0.956 |
| SBP | 102.0 ± 13.7 | 119.0 ± 19.6 | 0.009 * | |
| DBP | 66.6 ± 9.39 | 71.5 ± 10.0 | 0.153 | |
| MAP | 78.0 ± 10.3 | 87.3 ± 12.0 | 0.023 * |
| Time Point | Group 1 (n = 40) (Mean ± SD) | Group 2 (n = 40) (Mean ± SD) | p | |
|---|---|---|---|---|
| 0 min | HR | 87.7 ± 12.1 | 84.6 ± 12.3 | 0.258 |
| SBP | 122.0 ± 15.7 | 134.0 ± 23.3 | 0.005 * | |
| DBP | 76.1 ± 12.2 | 81.5 ± 12.4 | 0.056 | |
| MAP | 91.2 ± 12.1 | 99.1 ± 14.5 | 0.011 * | |
| 2 h | HR | 82.8 ± 11.0 | 80.5 ± 11.5 | 0.376 |
| SBP | 118.0 ± 13.5 | 121.0 ± 17.6 | 0.391 | |
| DBP | 75.0 ± 9.56 | 72.7 ± 10.1 | 0.300 | |
| MAP | 89.3 ± 10.2 | 88.6 ± 11.7 | 0.788 | |
| 4 h | HR | 81.3 ± 11.2 | 80.6 ± 8.30 | 0.735 |
| SBP | 115.0 ± 13.9 | 113.0 ± 14.0 | 0.678 | |
| DBP | 71.1 ± 8.21 | 71.4 ± 8.33 | 0.882 | |
| MAP | 85.4 ± 9.75 | 85.5 ± 9.71 | 0.976 | |
| 6 h | HR | 82.4 ± 11.6 | 80.8 ± 7.49 | 0.472 |
| SBP | 113.0 ± 14.4 | 114.0 ± 12.8 | 0.629 | |
| DBP | 70.7 ± 8.74 | 70.7 ± 8.73 | 0.980 | |
| MAP | 84.7 ± 9.90 | 85.0 ± 9.09 | 0.891 | |
| 12 h | HR | 81.1 ± 10.7 | 78.8 ± 13.8 | 0.413 |
| SBP | 112.0 ± 14.9 | 115.0 ± 11.7 | 0.305 | |
| DBP | 69.9 ± 9.23 | 70.5 ± 8.01 | 0.767 | |
| MAP | 83.8 ± 10.5 | 85.2 ± 8.20 | 0.502 | |
| 24 h | HR | 80.9 ± 10.2 | 81.0 ± 8.54 | 0.953 |
| SBP | 110.0 ± 13.1 | 113.0 ± 14.3 | 0.441 | |
| DBP | 68.0 ± 8.64 | 69.0 ± 9.11 | 0.616 | |
| MAP | 82.0 ± 9.35 | 83.7 ± 10.2 | 0.438 |
| Postoperative Time | Supplemental Analgesia | Group 1 | Group 2 | p Value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| 0 min | Present | 9 | 22.5% | 13 | 32.5% | 0.317 |
| Absent | 31 | 77.5% | 27 | 67.5% | ||
| 2 h | Present | 12 | 30% | 15 | 37.5% | 0.478 |
| Absent | 28 | 70% | 25 | 62.5% | ||
| 4 h | Present | 7 | 17.5% | 9 | 22.5% | 0.576 |
| Absent | 33 | 77.5% | 31 | 82.5% | ||
| 6 h | Present | 11 | 27.5% | 12 | 30% | 0.805 |
| Absent | 29 | 62.5% | 28 | 70% | ||
| 12 h | Present | 11 | 27.5% | 13 | 32.5% | 0.626 |
| Absent | 29 | 72.5% | 27 | 67.5% | ||
| 24 h | Present | 7 | 17.5% | 8 | 20% | 0.775 |
| Absent | 33 | 82.5% | 32 | 80% | ||
| 24 h Tramadol Requirement | ||||||
| Tramadol (mg) | 30.0 ± 45.9 | 22.5 ± 41.8 | 0.062 | |||
| Likert Scale (1–5) | Group 1 | Group 2 | |||
|---|---|---|---|---|---|
| Patient Satisfaction Score (1 = Very Dissatisfied; 5 = Very Satisfied) | n | % | n | % | |
| Very Dissatisfied (1) | 0 | 0.0% | 1 | 2.5% | |
| Dissatisfied (2) | 0 | 0.0% | 3 | 7.5% | |
| Neutral (3) | 10 | 25.0% | 10 | 25.0% | |
| Satisfied (4) | 25 | 62.5% | 20 | 50.0% | |
| Very Satisfied (5) | 5 | 12.5% | 6 | 15% | |
| p-value | 0.191 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acar, M.S.; Pehlivan, V.F.; Pehlivan, B.; Duran, E. Timing Matters: A Randomized Controlled Trial Comparing Preoperative and Postoperative Erector Spinae Plane Block for Analgesia in Laparoscopic Cholecystectomy. Medicina 2025, 61, 1806. https://doi.org/10.3390/medicina61101806
Acar MS, Pehlivan VF, Pehlivan B, Duran E. Timing Matters: A Randomized Controlled Trial Comparing Preoperative and Postoperative Erector Spinae Plane Block for Analgesia in Laparoscopic Cholecystectomy. Medicina. 2025; 61(10):1806. https://doi.org/10.3390/medicina61101806
Chicago/Turabian StyleAcar, Mehmet Sait, Veli Fahri Pehlivan, Basak Pehlivan, and Erdogan Duran. 2025. "Timing Matters: A Randomized Controlled Trial Comparing Preoperative and Postoperative Erector Spinae Plane Block for Analgesia in Laparoscopic Cholecystectomy" Medicina 61, no. 10: 1806. https://doi.org/10.3390/medicina61101806
APA StyleAcar, M. S., Pehlivan, V. F., Pehlivan, B., & Duran, E. (2025). Timing Matters: A Randomized Controlled Trial Comparing Preoperative and Postoperative Erector Spinae Plane Block for Analgesia in Laparoscopic Cholecystectomy. Medicina, 61(10), 1806. https://doi.org/10.3390/medicina61101806






