Investigation of the Effect of Enamel Matrix Protein, Platelet-Rich Fibrin, and Bone Graft on New Bone Formation in Guided Tissue Regeneration in Rat Calvarium
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
- Control Group (n = 13): Titanium domes were placed over the decorticated cavities, leaving their interior empty.
- Emdogain Group (EMD, n = 13): Titanium domes filled with Emdogain were placed over the decorticated cavities.
- Emdogain and Bone Graft Group (EMD + BG, n = 13): Titanium domes filled with a mixture of bone xenograft and Emdogain were placed over the decorticated cavities.
- PRF Group (PRF, n = 13): Titanium domes filled with PRF were placed over the decorticated cavities.
- PRF and Bone Graft Group (PRF + BG, n = 13): Titanium domes filled with a mixture of bone xenograft and PRF derived from the rats’ blood were placed over the decorticated cavities.
- Bone Graft Group (BG, n = 13): Titanium domes filled with bone xenograft were placed over the decorticated cavities.
- Emdogain, PRF, and Bone Graft Group (EMD + PRF + BG, n = 13): Titanium domes filled with a mixture of Emdogain, PRF, and bone xenograft were placed over the decorticated cavities.
2.2. Surgical Method
2.3. PRF Preparation
2.4. Calvarial Bone Collection and Preparation
- (a)
- Amount of newly formed bone: New bone formation was histologically evaluated by identifying and scoring the presence of layered bone tissue. Unlike mature bone, lamellar bone tissue exhibits trabeculation and bone marrow. Bone formation was scored as follows (Figure 8, Figure 9, Figure 10, Figure 11 and Figure 12) [18]:
- (b)
- (c)
- (d)
- (e)
2.5. Immunohistochemical Examination
2.6. Statistical Analysis
3. Results
3.1. New Bone Formation:
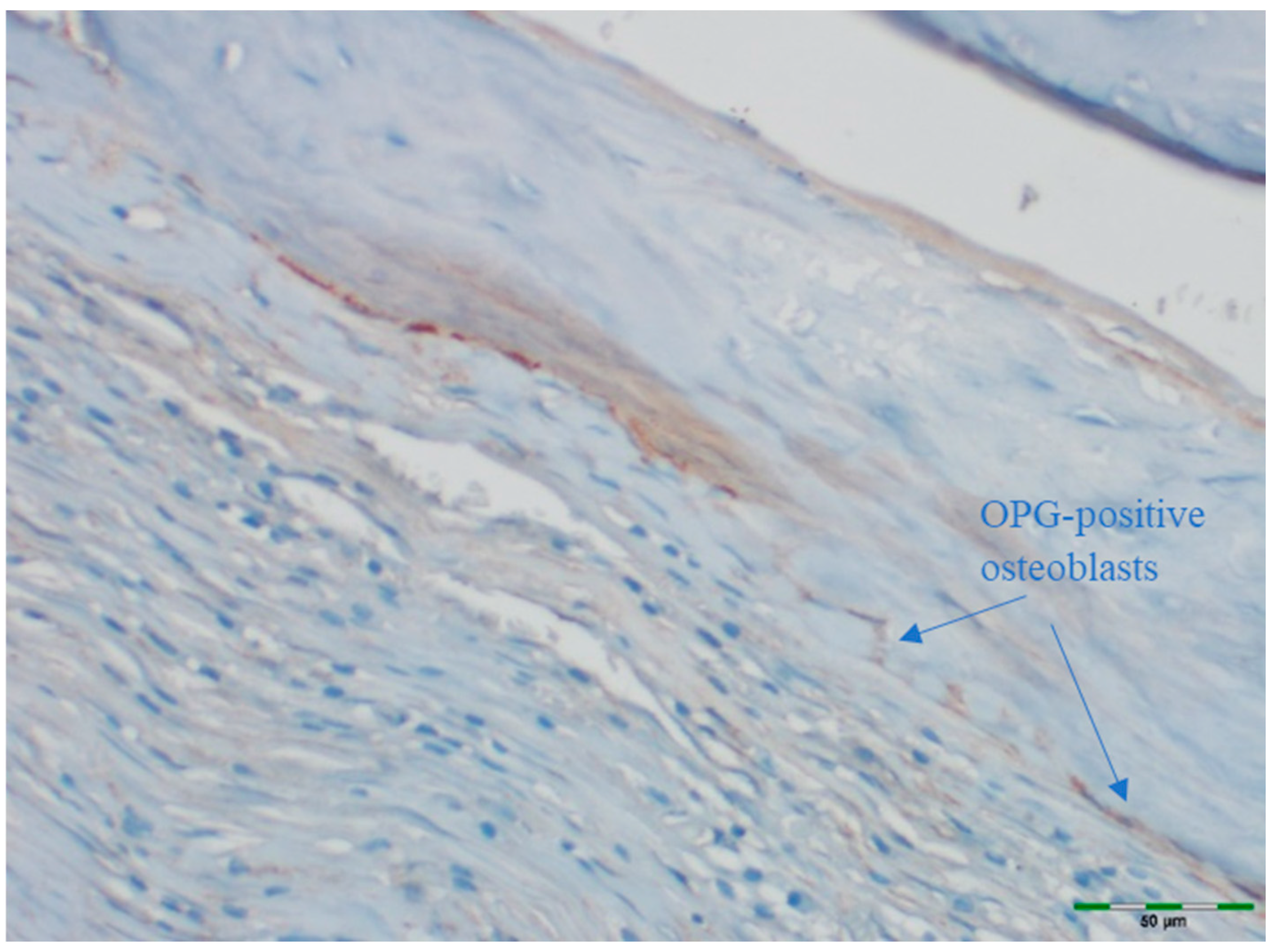
3.2. Osteoblast
3.3. Osteoclasts
3.4. Fibrosis
3.5. Angiogenesis
3.6. OPG Levels
3.7. RANKL Expression
3.8. OPG/RANKL Ratio
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| GTR | Guided Tissue Regeneration |
| BMPs | Bone Morphogenetic Proteins |
| EMD | Enamel Matrix Derivative |
| BG | Bone Graft |
| PRF | Platelet-Rich Fibrin |
| ENAM | Enamelin |
| AMBN | Ameloblastin |
| AMTN | Amelotin |
| ODAM | Odontogenic Ameloblast-Associated Protein |
| TGF-β | Transforming Growth Factor-beta |
| PRP | Platelet-Rich Plasma |
| OPG | Osteoprotegerin |
| RANKL | Receptor Activator of Nuclear Factor Kappa-B Ligand |
| H&E | Hematoxylin and Eosin |
| TNF | Tumor Necrosis Factor |
| DAB | 3,3′-Diaminobenzidine |
| BGLAP | Bone Gamma-Carboxyglutamic Acid-Containing Protein |
| OCN | Osteocalcin |
References
- Kunrath, M.F.; Magrin, G.L.; Zorzo, C.S.; Rigotto, I.; Aludden, H.; Dahlin, C. Membranes for Periodontal and Bone Regeneration: Everything You Need to Know. J. Periodontal Res. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Lindhe, J. (Eds.) Clinical Periodontology and Implant Denstry, 6th ed.; Ankara Nobel Tıp Kitapevleri: Ankara, Türkiye, 2017. [Google Scholar]
- Stavropoulos, A.; Bertl, K.; Sculean, A.; Kantarci, A. Regenerative Periodontal Therapy in Intrabony Defects and Long-Term Tooth Prognosis. Dent. Clin. N. Am. 2022, 66, 103–109. [Google Scholar] [CrossRef]
- Deng, Y.; Liang, Y.; Liu, X. Biomaterials for Periodontal Regeneration. Dent. Clin. N. Am. 2022, 66, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 87–105. [Google Scholar] [CrossRef]
- Margolis, H.C.; Beniash, E.; Fowler, C.E. Role of macromolecular assembly of enamel matrix proteins in enamel formation. J. Dent. Res. 2006, 85, 775–793. [Google Scholar] [CrossRef]
- El-Sayed, K.M.F.; Dörfer, C.; Ungefroren, H.; Kassem, N.; Wiltfang, J.; Paris, S. Effect of Emdogain enamel matrix derivative and BMP-2 on the gene expression and mineralized nodule formation of alveolar bone proper-derived stem/progenitor cells. J. Craniomaxillofac. Surg. 2014, 42, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Ohyama, M.; Maeno, M.; Ito, K.; Otsuka, K. Attachment of human periodontal ligament cells to enamel matrix-derived protein is mediated via interaction between BSP-like molecules and integrin alpha(v)beta3. J. Periodontol. 2001, 72, 1520–1526. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Papanikolaou, N.; Coulthard, P.; Worthington, H.V. Enamel matrix derivative (Emdogain®) for periodontal tissue regeneration in intrabony defects. Cochrane Database Syst. Rev. 2009, 2009, CD003875. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Damsaz, M.; Castagnoli, C.Z.; Eshghpour, M.; Alamdari, D.H.; Alamdari, A.H.; Noujeim, Z.E.F.; Haidar, Z.S. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front. Surg. 2020, 7, 537138. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Sunitha Raja, V.; Munirathnam Naidu, E. Platelet-rich fibrin: Evolution of a second-generation platelet concentrate. Indian J. Dent. Res. 2008, 19, 42–46. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behaviour Research Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.P.; Narmada, I.B.; Ernawati, D.S.; Dinaryanti, A.; Hendrianto, E.; Ihsan, I.S.; Riawan, W.; Rantam, F.A. Osteogenic potential of gingival stromal progenitor cells cultured in platelet rich fibrin is predicted by core-binding factor subunit-α1/Sox9 expression ratio (in vitro). F1000Research 2018, 7, 1134. [Google Scholar] [CrossRef]
- Artas, G.; Gul, M.; Acikan, I.; Kirtay, M.; Bozoglan, A.; Simsek, S.; Yaman, F.; Dundar, S. A comparison of different bone graft materials in peri-implant guided bone regeneration. Braz. Oral Res. 2018, 32, e59. [Google Scholar] [CrossRef]
- Yaman, F.; Acikan, I.; Dundar, S.; Simsek, S.; Gul, M.; Ozercan, I.H.; Komorowski, J.; Sahin, K. Dietary arginine silicate inositol complex increased bone healing: Histologic and histomorphometric study. Drug Des. Dev. Ther. 2016, 10, 2081–2086. [Google Scholar] [CrossRef]
- Gunay, A.; Arpag, O.F.; Atilgan, S.; Yaman, F.; Atalay, Y.; Acikan, I. Effects of caffeic acid phenethyl ester on palatal mucosal defects and tooth extraction sockets. Drug Des. Dev. Ther. 2014, 8, 2069–2074. [Google Scholar] [CrossRef] [PubMed]
- Hapa, O.; Cakici, H.; Kukner, A.; Aygun, H.; Sarkalan, N.; Baysal, G. Effect of platelet-rich plasma on tendon-to-bone healing after rotator cuff repair in rats: An in vivo experimental study. Acta Orthop. Traumatol. Turc. 2012, 46, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of bone defects by guided tissue regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Kao, D.W.; Fiorellini, J.P. Regenerative periodontal therapy. Front. Oral Biol. 2012, 15, 149–159. [Google Scholar] [CrossRef]
- Lundgren, A.K.; Lundgren, D.; Hämmerle, C.H.; Nyman, S.; Sennerby, L. Influence of decortication of the donor bone on guided bone augmentation. An experimental study in the rabbit skull bone. Clin. Oral Implant. Res. 2000, 11, 99–106. [Google Scholar] [CrossRef]
- Bodde, E.W.; Spauwen, P.H.; Mikos, A.G.; Jansen, J.A. Closing capacity of segmental radius defects in rabbits. J. Biomed. Mater. Res. Part A 2008, 85, 206–217. [Google Scholar] [CrossRef]
- Toker, H.; Ozdemir, H.; Ozer, H.; Eren, K. Alendronate enhances osseous healing in a rat calvarial defect model. Arch. Oral Biol. 2012, 57, 1545–1550. [Google Scholar] [CrossRef]
- Saçak, B.; Certel, F.; Akdeniz, Z.D.; Karademir, B.; Ercan, F.; Özkan, N.; Akpinar, I.N.; Çelebiler, Ö. Repair of critical size defects using bioactive glass seeded with adipose-derived mesenchymal stem cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Franke Stenport, V.; Johansson, C.B. Enamel matrix derivative and titanium implants. J. Clin. Periodontol. 2003, 30, 359–363. [Google Scholar] [CrossRef]
- Kawana, F.; Sawae, Y.; Sahara, T.; Tanaka, S.; Debari, K.; Shimizu, M.; Sasaki, T. Porcine enamel matrix derivative enhances trabecular bone regeneration during wound healing of injured rat femur. Anat. Rec. 2001, 264, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, S.; Itoh, D.; Kuroda, S.; Kondo, H.; Umezawa, A.; Ohya, K.; Ohyama, T.; Kasugai, S. The effects of enamel matrix derivative (EMD) on osteoblastic cells in culture and bone regeneration in a rat skull defect. J. Periodontal Res. 2003, 38, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Casati, M.Z.; Sallum, E.A.; Nociti, F.H., Jr.; Caffesse, R.G.; Sallum, A.W. Enamel matrix derivative and bone healing after guided bone regeneration in dehiscence-type defects around implants. A histomorphometric study in dogs. J. Periodontol. 2002, 73, 789–796. [Google Scholar] [CrossRef]
- Donos, N.; Bosshardt, D.; Lang, N.; Graziani, F.; Tonetti, M.; Karring, T.; Kostopoulos, L. Bone formation by enamel matrix proteins and xenografts: An experimental study in the rat ramus. Clin. Oral Implant. Res. 2005, 16, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Jeon, S.H.; Park, J.-Y.; Chung, J.-H.; Choung, Y.-H.; Choung, H.-W.; Kim, E.-S.; Choung, P.-H. Platelet-rich fibrin is a Bioscaffold and reservoir of growth factors for tissue regeneration. Tissue Eng. Part A 2011, 17, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pan, S.; Dangaria, S.J.; Gopinathan, G.; Kolokythas, A.; Chu, S.; Geng, Y.; Zhou, Y.; Luan, X. Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. BioMed Res. Int. 2013, 2013, 638043. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Silva, A.D.; Ferreira, S.; Avelino, C.C.; Garcia, I.R., Jr.; Mariano, R.C. Influence of the association between platelet-rich fibrin and bovine bone on bone regeneration. A histomorphometric study in the calvaria of rats. Int. J. Oral Maxillofac. Surg. 2015, 44, 649–655. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Qu, Z.; Andrukhov, O.; Laky, M.; Ulm, C.; Matejka, M.; Dard, M.; Rausch-Fan, X. Effect of enamel matrix derivative on proliferation and differentiation of osteoblast cells grown on the titanium implant surface. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 111, 517–522. [Google Scholar] [CrossRef]
- Sumida, R.; Maeda, T.; Kawahara, I.; Yusa, J.; Kato, Y. Platelet-rich fibrin increases the osteoprotegerin/receptor activator of nuclear factor-κB ligand ratio in osteoblasts. Exp. Ther. Med. 2019, 18, 358–365. [Google Scholar] [CrossRef]
- Gupta, S.J.; Jhingran, R.; Gupta, V.; Bains, V.K.; Madan, R.; Rizvi, I. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: A clinical and cone beam computed tomography study. J. Int. Acad. Periodontol. 2014, 16, 86–96. [Google Scholar]
- Aydemir Turkal, H.; Demirer, S.; Dolgun, A.; Keceli, H.G. Evaluation of the adjunctive effect of platelet--rich fibrin to enamel matrix derivative in the treatment of intrabony defects. Six--month results of a randomized, split--mouth, controlled clinical study. J. Clin. Periodontol. 2016, 43, 955–964. [Google Scholar] [CrossRef] [PubMed]


















| Step | Procedure | Description | Duration | Temperature |
|---|---|---|---|---|
| 1 | Fixation | Samples fixed in 10% formalin solution | 72 h | Room temp |
| 2 | Decalcification | Immersion in 10% formic acid; solution changed every 2 days | 1 week | Room temp |
| 3 | Washing | Running tap water to remove residual acid | 12 h | Room temp |
| 4 | Dehydration | Immersion in graded ethanol series (70%, 80%, 95%, 100%) | ~2 h total | Room temp |
| 5 | Clearing | Immersion in xylene | 30 min | Room temp |
| 6 | Paraffin embedding | Infiltration with melted paraffin wax | Overnight | 60 °C |
| 7 | Sectioning | Paraffin blocks cut into 5 µm sections | – | Room temp |
| 8 | Deparaffinization | Sections incubated at 65 °C, then immersed in xylene and alcohol | 1 h + washes | 65 °C and RT |
| 9 | Rehydration | Stepwise rehydration with graded ethanol to distilled water | ~20 min | Room temp |
| 10 | H&E staining | Hematoxylin and eosin staining for histology | Standard Protocol | Room temp |
| 11 | Antigen retrieval | Heat-induced epitope retrieval for IHC | 20 min | 95–98 °C |
| 12 | Blocking | Non-specific binding blocked with serum | 30 min | Room temp |
| 13 | Immunohistochemistry | OPG and RANKL primary antibody incubation, followed by secondary antibody and DAB visualization | Overnight (primary), 1 h (secondary) | 4 °C (primary), RT (secondary) |
| New Bone Formation | Control | EMD | EMD + BG | PRF | PRF + BG | BG | EMD + PRF + BG | |
|---|---|---|---|---|---|---|---|---|
| (n = 13) | (n = 13) | (n = 13) | (n = 13) | (n = 13) | (n = 13) | (n = 13) | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | p | |
| Low Level | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0.000 * |
| (38.5%) | (0%) | (0%) | (0%) | (0%) | (0%) | (0%) | ||
| Moderate Level | 8 | 11 | 8 | 13 | 9 | 10 | 6 | |
| (61.5%) | (84.6%) | (61.5%) | (100%) | (69.2%) | (76.9%) | (46.2%) | ||
| High Level | 0 | 2 | 5 | 0 | 4 | 3 | 7 | |
| (0%) | (15.4%) | (30.8%) | (0%) | (30.8%) | (23.1%) | (53.8%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dönmezer, T.; Talo Yildirim, T.; Dündar, S.; Bozoğlan, A.; Özercan, İ.H. Investigation of the Effect of Enamel Matrix Protein, Platelet-Rich Fibrin, and Bone Graft on New Bone Formation in Guided Tissue Regeneration in Rat Calvarium. Medicina 2025, 61, 1795. https://doi.org/10.3390/medicina61101795
Dönmezer T, Talo Yildirim T, Dündar S, Bozoğlan A, Özercan İH. Investigation of the Effect of Enamel Matrix Protein, Platelet-Rich Fibrin, and Bone Graft on New Bone Formation in Guided Tissue Regeneration in Rat Calvarium. Medicina. 2025; 61(10):1795. https://doi.org/10.3390/medicina61101795
Chicago/Turabian StyleDönmezer, Tuğçe, Tuba Talo Yildirim, Serkan Dündar, Alihan Bozoğlan, and İbrahim Hanifi Özercan. 2025. "Investigation of the Effect of Enamel Matrix Protein, Platelet-Rich Fibrin, and Bone Graft on New Bone Formation in Guided Tissue Regeneration in Rat Calvarium" Medicina 61, no. 10: 1795. https://doi.org/10.3390/medicina61101795
APA StyleDönmezer, T., Talo Yildirim, T., Dündar, S., Bozoğlan, A., & Özercan, İ. H. (2025). Investigation of the Effect of Enamel Matrix Protein, Platelet-Rich Fibrin, and Bone Graft on New Bone Formation in Guided Tissue Regeneration in Rat Calvarium. Medicina, 61(10), 1795. https://doi.org/10.3390/medicina61101795






