Fertility Preservation Strategies in Female Cancer Patients: Current Approaches and Future Directions
Abstract
1. Introduction
2. Review Methodology
3. Overview of Fertility Preservation Strategies in Cancer Patients
4. Critical Appraisal of Fertility Preservation Strategies
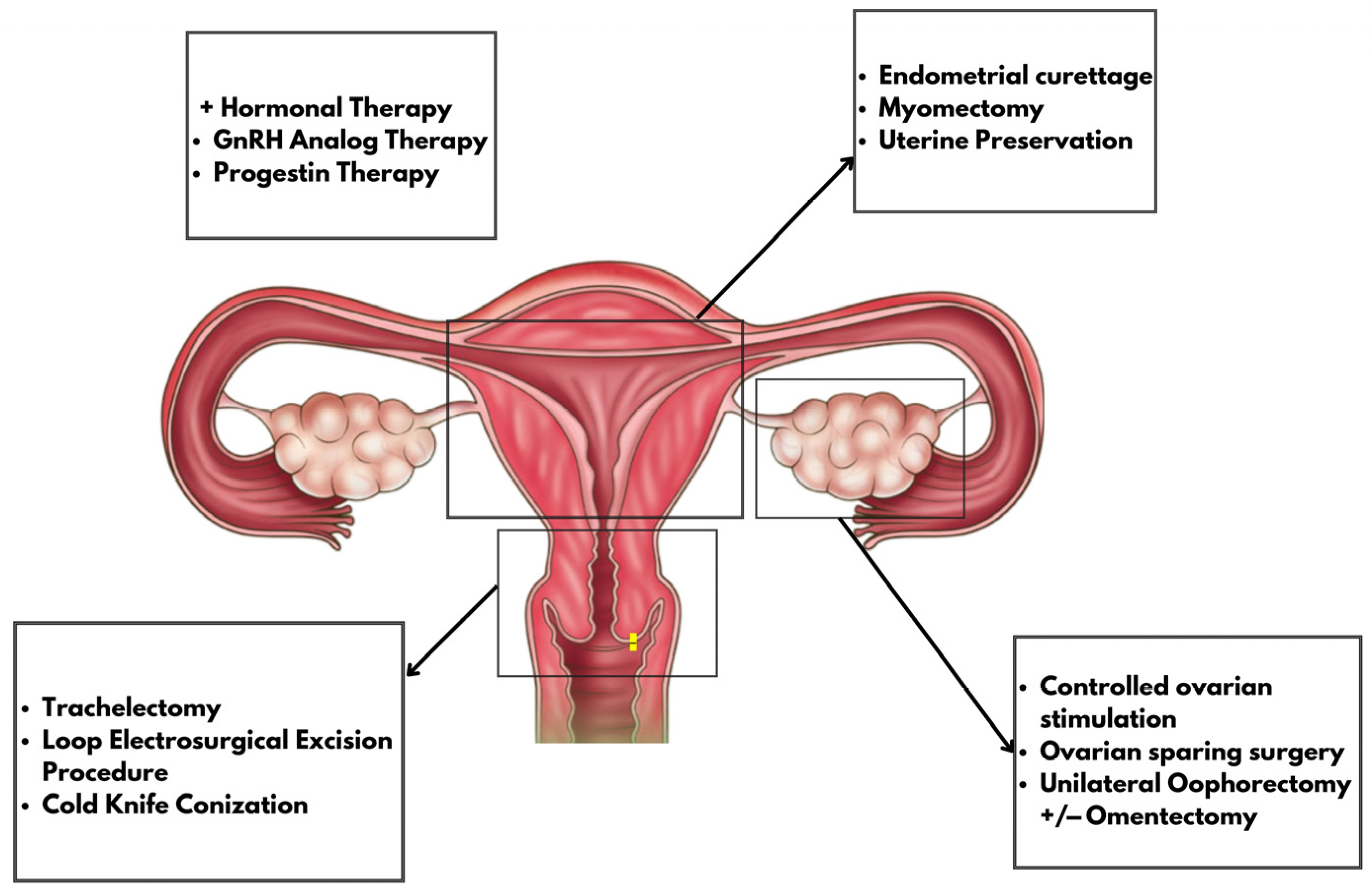
4.1. Gynecological Malignancies and FP
4.1.1. Hormonal Treatment
4.1.2. Controlled Ovarian Hyperstimulation
4.1.3. Ovarian Sparing Surgery
4.1.4. Uterine Procedures
Endometrial Curettage
Myomectomy
Uterine Preservation
4.1.5. Cervical Procedures
4.2. Non-Gynecological Malignancies and FP
4.2.1. Ovarian Stimulation and Neoadjuvant Treatment Delay
4.2.2. Use of GnRH Analogs During Chemotherapy
4.2.3. Oocyte and Embryo Cryopreservation
4.2.4. Ovarian Tissue Cryopreservation
4.3. Future Directions and Research Prospects
4.3.1. Development of the Artificial Ovary
4.3.2. Robotic Surgery Advantages
4.4. Ethical and Psychological Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- You, L.; Lv, Z.; Li, C.; Ye, W.; Zhou, Y.; Jin, J.; Han, Q. Worldwide cancer statistics of adolescents and young adults in 2019: A systematic analysis of the Global Burden of Disease Study 2019. ESMO Open 2021, 6, 100255. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.J.; Stratton, K.L.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Donaldson, S.S.; Ginsberg, J.P.; Kenney, L.B.; Levine, J.M.; Robison, L.L.; et al. Pregnancy after chemotherapy in male and female survivors of childhood cancer treated between 1970 and 1999: A report from the Childhood Cancer Survivor Study cohort. Lancet. Oncol. 2016, 17, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Singh, S.; Shukla, S.; Shrivastava, S. Oncofertility: Treatment Options from Bench to Bedside. Cancer Pathog. Ther. 2023, 1, 284–289. [Google Scholar] [CrossRef]
- Poorvu, P.D.; Frazier, A.L.; Feraco, A.M.; Manley, P.E.; Ginsburg, E.S.; Laufer, M.R.; LaCasce, A.S.; Diller, L.R.; Partridge, A.H. Cancer Treatment-Related Infertility: A Critical Review of the Evidence. JNCI Cancer Spectr. 2019, 3, pkz008. [Google Scholar] [CrossRef]
- Di Tucci, C.; Galati, G.; Mattei, G.; Chinè, A.; Fracassi, A.; Muzii, L. Fertility after Cancer: Risks and Successes. Cancers 2022, 14, 2500. [Google Scholar] [CrossRef]
- Xiong, J.; Zhang, Z.; Liu, Y.; Fan, G.; Wu, K.; Zhang, W. Prevalence and Outcomes of Unilateral Versus Bilateral Oophorectomy in Women with Ovarian Cancer: A Population-Based Study. Front. Oncol. 2022, 12, 866443. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, B.; Guan, J.; Shan, W.; Liao, J.; Shao, W.; Chen, X. Comparison of the effect of oral megestrol acetate with or without levonorgestrel-intrauterine system on fertility-preserving treatment in patients with early-stage endometrial cancer: A prospective, open-label, randomized controlled phase ii trial (ClinicalTrials.Gov NCT03241914). J. Gynecol. Oncol. 2023, 34, e32. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.K.; Lee, H.J.; Lee, J.R.; Jee, B.C.; Suh, C.S.; Kim, S.H. Efficacy of Random-Start Controlled Ovarian Stimulation in Cancer Patients. J. Korean Med. Sci. 2015, 30, 290. [Google Scholar] [CrossRef]
- Arthur, F.; Hennessey, I.; Pizer, B.; Losty, P.D. surgical management and outcomes of paediatric ovarian tumours—A 25-year UK single centre experience. Pediatr. Surg. Int. 2021, 37, 1355–1359. [Google Scholar] [CrossRef]
- Dellino, M.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Loiacono, R.; Minoia, C.; Daniele, A.; Cormio, G. Germinal ovarian tumors in reproductive age women: Fertility-sparing and outcome. Medicine 2020, 99, e22146. [Google Scholar] [CrossRef]
- Ayhan, A.; Tohma, Y.A.; Tunc, M. Fertility preservation in early-stage endometrial cancer and endometrial intraepithelial neoplasia: A single-center experience. Taiwan J. Obstet. Gynecol. 2020, 59, 415–419. [Google Scholar] [CrossRef]
- Şahin, H.; Karatas, F.; Coban, G.; Özen, Ö.; Erdem, Ö.; Onan, M.A.; Ayhan, A. uterine smooth muscle tumor of uncertain malignant potential: Fertility and clinical outcomes. J. Gynecol. Oncol. 2019, 30, e54. [Google Scholar] [CrossRef]
- Petiot, F.; Descargues, P.; Devouassoux-Shisheboran, M.; You, B.; Rousset-Jablonski, C.; Raffin, D.; Hajri, T.; Gertych, W.; Glehen, O.; Philip, C.-A.; et al. Retrospective analysis of uterine involvement in low grade serous ovarian carcinoma. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 294, 191–197. [Google Scholar] [CrossRef]
- Chen, J.; Wang, F.; Zhang, Y.; Yang, B.; Ai, J.; Wang, X.; Cheng, X.; Li, K. Oncological and Reproductive Outcomes of Fertility-Sparing Surgery in Women with Early-Stage Epithelial Ovarian Carcinoma: A Multicenter Retrospective Study. Curr. Med. Sci. 2020, 40, 745–752. [Google Scholar] [CrossRef]
- Gouy, S.; Saidani, M.; Maulard, A.; Faron, M.; Bach-Hamba, S.; Bentivegna, E.; Leary, A.; Pautier, P.; Devouassoux-Shisheboran, M.; Genestie, C.; et al. Is uterine preservation combined with bilateral salpingo-oophorectomy to promote subsequent fertility safe in infiltrative mucinous ovarian cancer? Gynecol. Oncol. Rep. 2017, 22, 52–54. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Feasibility of uterine preservation in the management of early-stage uterine adenosarcomas: A single institute experience. World J. Surg. Oncol. 2017, 15, 87. [Google Scholar] [CrossRef]
- Machida, H.; Iwata, T.; Okugawa, K.; Matsuo, K.; Saito, T.; Tanaka, K.; Morishige, K.; Kobayashi, H.; Yoshino, K.; Tokunaga, H.; et al. Fertility-sparing trachelectomy for early-stage cervical cancer: A proposal of an ideal candidate. Gynecol. Oncol. 2020, 156, 341–348. [Google Scholar] [CrossRef]
- Ekdahl, L.; Paraghamian, S.; Eoh, K.J.; Thumuluru, K.M.; Butler-Manuel, S.A.; Kim, Y.T.; Boggess, J.F.; Persson, J.; Falconer, H. Long term oncologic and reproductive outcomes after robot-assisted radical trachelectomy for early-stage cervical cancer. An international multicenter study. Gynecol. Oncol. 2022, 164, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Chambers, J.; Mcauley, F.; Kaplan, T.; Letourneau, J.; Hwang, J.; Kim, M.-O.; Melisko, M.E.; Rugo, H.S.; Esserman, L.J.; et al. Fertility preservation with ovarian stimulation and time to treatment in women with stage ii–iii breast cancer receiving neoadjuvant therapy. Breast Cancer Res. Treat. 2017, 165, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Volodarsky-Perel, A.; Tulandi, T.; Son, W.-Y.; Khojah, M.; Buckett, W. Impact of extent and biochemical parameters of lymphoma on fertility preservation outcome. Fertil. Steril. 2020, 113, 400–407.e1. [Google Scholar] [CrossRef] [PubMed]
- Goldrat, O.; Van Den Steen, G.; Gonzalez-Merino, E.; Dechène, J.; Gervy, C.; Delbaere, A.; Devreker, F.; De Maertelaer, V.; Demeestere, I. Letrozole-associated controlled ovarian hyperstimulation in breast cancer patients versus conventional controlled ovarian hyperstimulation in infertile patients: Assessment of oocyte quality related biomarkers. Reprod. Biol. Endocrinol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Kira, A.T.F.; Hentschke, M.R.; Vasconcelos, N.F.D.; Colombo, T.; Trindade, V.D.; Petracco, A.; Costa, B.E.P.D.; Badalotti, M. Patients undergoing elective and oncofertility preservation respond similarly to controlled ovarian stimulation for fertility preservation. JBRA 2022, 26, 407–411. [Google Scholar] [CrossRef]
- Creux, H.; Monnier, P.; Son, W.-Y.; Tulandi, T.; Buckett, W. Immature oocyte retrieval and in vitro oocyte maturation at different phases of the menstrual cycle in women with cancer who require urgent gonadotoxic treatment. Fertil. Steril. 2017, 107, 198–204. [Google Scholar] [CrossRef]
- Poirot, C.; Fortin, A.; Lacorte, J.M.; Akakpo, J.P.; Genestie, C.; Vernant, J.P.; Brice, P.; Morice, P.; Leblanc, T.; Gabarre, J.; et al. Impact of cancer chemotherapy before ovarian cortex cryopreservation on ovarian tissue transplantation. Hum. Reprod. 2019, 34, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Moffitt Cancer Center. Omentectomy, Ovarian Cancer Surgery. Available online: https://www.moffitt.org/cancers/ovarian-cancer/omentectomy/ (accessed on 20 August 2025).
- Salvo, G.; Ramirez, P.T.; Leitao, M.; Cibula, D.; Fotopoulou, C.; Kucukmetin, A.; Rendon, G.; Perrotta, M.; Ribeiro, R.; Vieira, M.; et al. International radical trachelectomy assessment: IRTA Study. Int. J. Gynecol. Cancer 2019, 29, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Cancer.gov. NCI Dictionary of Cancer Terms. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/trachelectomy (accessed on 20 August 2025).
- Cleveland Clinic. Loop Electrosurgical Excision Procedure (LEEP). Available online: https://my.clevelandclinic.org/health/treatments/4711-loop-electrosurgical-excision-procedure-leep (accessed on 20 August 2025).
- Garrido Colino, C.; González Urdiales, P.; Molinés Honrubia, A.; Ortega Acosta, M.J.; García Abos, M. Primary ovarian insufficiency in cancer survivors: Keys to optimal management. An. Pediatría (Engl. Ed.) 2023, 99, 385–392. [Google Scholar] [CrossRef]
- Rodriguez-Wallberg, K.A.; Nilsson, H.P.; Bergh, J.; Malmros, J.; Ljungman, P.; Foukakis, T.; Stragliotto, C.L.; Friman, E.I.; Linderholm, B.; Valachis, A.; et al. ProFertil study protocol for the investigation of gonadotropin-releasing hormone agonists (GnRHa) during chemotherapy aiming at fertility protection of young women and teenagers with cancer in Sweden—A phase iii randomised double-blinded placebo-controlled study. BMJ Open 2023, 13, e078023. [Google Scholar] [CrossRef]
- Arian, S.E.; Flyckt, R.L.; Herman, R.; Erfani, H.; Falcone, T. Fertility preservation in pediatric female cancer patients. Fertil. Steril. 2018, 109, 941. [Google Scholar] [CrossRef] [PubMed]
- Oqani, R.K.; So, S.; Lee, Y.; Ko, J.J.; Kang, E. Artificial Oocyte: Development and Potential Application. Cells 2022, 11, 1135. [Google Scholar] [CrossRef]
- Cho, E.; Kim, Y.Y.; Noh, K.; Ku, S. A new possibility in fertility preservation: The artificial ovary. J. Tissue Eng. Regen. Med. 2019, 13, 1294–1315. [Google Scholar] [CrossRef]
- Cianci, S.; Arcieri, M.; Vizzielli, G.; Martinelli, C.; Granese, R.; La Verde, M.; Fagotti, A.; Fanfani, F.; Scambia, G.; Ercoli, A. robotic pelvic exenteration for gynecologic malignancies, anatomic landmarks, and surgical Steps: A systematic review. Front. Surg. 2021, 8, 790152. [Google Scholar] [CrossRef] [PubMed]
- Dudus, L.; Minciuna, C.; Tudor, S.; Lacatus, M.; Stefan, B.; Vasilescu, C. Robotic or laparoscopic pelvic exenteration for gynecological malignancies: Feasible options to open surgery. J. Gynecol. Oncol. 2024, 35, e12. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, V.; van Poecke, N.; Waterman, L.; Lok, C.A.R.; Beerendonk, C.C.M.; Smets, E.M.A. Are we Only Doing Good? Long-Term Psychosocial Effects of Fertility Preservation (or Lack Thereof) on Survivors of Cancer During Adolescence and Young Adulthood. Cancer J. 2025, 31, e0774. [Google Scholar] [CrossRef] [PubMed]

| Paper | Method | Malignancy | Sample Size | Pregnancy Rate | Live Birth Rate | Study Duration | Outcomes |
|---|---|---|---|---|---|---|---|
| Xiong et al., 2022 [6] | Unilateral vs. bilateral Oophorectomy | Gynecological | 28480 patients | Not reported | Not reported | 17 years | Unilateral oophorectomy associated with better survival in stage IA patients < 50 years; bilateral approach yielded worse outcomes in stage II–III |
| Xu et al., 2023 [7] | Megestrol Acetate(MA) vs. MA + Levonorgestrel Intrauterine System(LNG-IUS) | 26 patients MA, 28 patients MA + LNG-IUS | Not significantly different between groups | Not reported | Median 31.6 months follow-up (range 3.1–94) | Complete response at 32 weeks: 57.1% (MA) vs. 61.5% (MA + LNG-IUS), with no significant difference between groups | |
| Kim et al., 2015 [8] | Random-start Controlled Ovarian Hyperstimulation | 22 cancer patients (6 in early follicular phase, 11 in late follicular phase, 5 in luteal phase) vs. 40 controls | Not reported | Not reported | Between 10 and 14 days follow-up | Random-start controlled ovarian hyperstimulation was feasible across cycle phases, though fertility outcomes were not reported | |
| Arthur et al., 2021 [9] | Surgical management of ovarian tumors (pediatric): salpingo-oopherectomy n = 21 (21%), ovary excision n = 33 (33%), ovary sparing tumourectomy n = 34 (34%), and cyto-reductive extirpation in 2 cases (2%) | 88 patients | Not reported | Not reported | 1990–2018 (18 years) | Recurrence rate 10%, with overall survival of 97% over 18 years | |
| Dellino et al., 2020 [10] | Fertility-sparing surgery for malignant ovarian germ cell tumors | 28 patients | 100% (5/5 patients with spontaneous pregnancies) | Not reported | 90 months follow-up | One recurrence observed; ovarian function preserved in all others, with 100% spontaneous pregnancy rate in those attempting conception | |
| Ayhan et al., 2020 [11] | Endometrial curettage in Atypical Hyperplasia(AH)/Endometrial Intraepithelial Neoplasia(EIN) and Endometrioid Endometrial Cancer(EEC) | 57 patients (27 AH/EIN-group A, 30 EEC-group B) | 50% in group A, 47% in group B | 37.5% in group A, 16.6% in group B; in group B, 1 patient experienced intrauterine exitus | January 2007–October 2018 | Recurrence rate higher in EEC (16.7%) vs. AH/EIN (7.4%) | |
| Şahin et al., 2019 [12] | Myomectomy/Hysterectomy in smooth uterine muscle of uncertain malignant potential(STUMP) | 57 patients | 7 pregnancies out of 10 attempts (in patients who underwent myomectomy) | Not reported | January 2006–December 2017 Median 57 (16–125) months follow-up | 7 recurrences of STUMP and 1 leiomyosarcoma transformation reported | |
| Petiot et al., 2024 [13] | Uterine preservation in Low Grade Serous Ovarian Carcinoma | 26 patients, 73% in stage FIGO III | Not reported | Not reported | January 2000–May 2022 | Uterine preservation was feasible in early-stage disease, but hysterectomy remained necessary in advanced stages | |
| Chen et al., 2020 [14] | Uterine preservation in epithelial ovarian carcinoma | 87 patients, 36 undergoing fertility-sparing surgery | 93.75% (15 out of 16 patients) | Not reported | January 2005–December 2014 | Fertility-sparing surgery has to be considered in early-stage epithelial ovarian carcinoma | |
| Gouy et al., 2017 [15] | Uterine preservation combined with bilateral salpingo-oophorectomyin infiltrative mucinous ovarian cancer | 6 patients | 33.33% (2 patients) | Not reported | 1976–2016 | 33.3% recurrence rate, questioning oncologic safety | |
| Lee et al., 2017 [16] | Uterine preservation in early-stage uterine adenosarcoma | 31 patients, 7 undergoing uterine preservation surgery | 1 patient-vaginal delivery at term (14.28%) | Not reported | 1998–2014 Median 32 months follow-up | Persistent disease observed in 2 patients, while 2 patients experienced disease recurrence | |
| Machida et al., 2020 [17] | Trachelectomy for early-stage cervical cancer | 401 patients | Not reported | Not reported | 2009–2013 | Five-year recurrence rates were 2.8% in low-risk tumors (≤2 cm) vs. 16.6% in high-risk tumors, supporting trachelectomy mainly in low-risk cases | |
| Ekdahl et al. 2022 [18] | Robot-assisted radical trachelectomy for early stage cervical cancer | 149 patients | 70 out of 88 conceived (80%) | 76 livebirths in 81 pregnancies | 2007–2019, Median follow-up 58 months | 6% recurrence rate; NB: short postoperative cervical length was associated with impaired fertility | |
| Chien et al., 2017 [19] | Ovarian stimulation(OS) and neoadjuvant treatment delay in stage II-II breast cancer | 82 patients were included (34 undergoing OS and 48 control) | 6 underwent embryo transfer, two pregnancies in one patient | 2 live births | April 2010–February 2017 Median follow-up 79 months | Recurrence and death rates were similar in both groups; no adverse effect on survival compared with controls | |
| Volodarsky-Perel et al., 2020 [20] | Ovarian stimulation in women with lymphoma | Non-Gynecological | 141 patients | Not reported | Not reported | 2009–2018 | Lower mature oocyte yield observed in advanced-stage lymphoma and in patients with poor biochemical markers |
| Goldrat et al., 2019 [21] | Mature oocyte cryopreservation following letrozole associated controlled ovarian hyperstimulation (Let-COH) in breast cancer patients | 47 (23 Let-OH, 24 control) | Not reported | Not reported | December 2012–February 2017 | Let-COH resulted in reduced estradiol and increased testosterone in the follicular fluid relative to conventional COH. The oocyte environment was suboptimal under hCG triggering, whereas GnRHa triggering enhanced oocyte quality | |
| Kira et al., 2022 [22] | Oocyte vitrification in breast cancer (elective vs. oncofertility preservation) | 40 cancer patients vs. 327 elective | Not reported | Not reported | 2009–2018 | Oocyte vitrification outcomes in breast cancer patients were comparable to elective preservation controls | |
| Creux et al., 2017 [23] | In vitro maturation(IVM) in cancer patients requiring urgent chemotherapy | 165 patients | Not reported | Not reported | January 2003–December 2015 | IVM was feasible at all cycle stages, offering a rapid fertility preservation option | |
| Poirot et al., 2019 [24] | Ovarian tissue preservation in various cancer patients (especially hematological malignancies) | 31 patients (22 with previous chemotherapy) | 36% | 23% | 2005–2015 | Prior chemotherapy did not influence ovarian function recovery or pregnancy incidence; the sole parameter associated with outcome variation was the quantity of ovarian tissue retrieved |
| Society | Cancer Type | Eligibility Criteria | Recommended Fertility-Sparing Treatment | Follow-Up Protocol |
|---|---|---|---|---|
| ESGO (2023) | Cervical cancer | Stage IA1-IB1, tumor < 2 cm, no LVSI, negative nodes | Conization (IA1), simple/radical trachelectomy ± SLNB (IB1) | MRI every 6 months; colposcopy, cytology |
| ESMO (2022) | Endometrial cancer | Grade 1, stage IA, no myometrial invasion (MRI), no LVSI | High-dose progestins ± LNG-IUS; hysteroscopic resection | Hysteroscopy + biopsy every 3–6 months |
| ASCO (2021) | Ovarian cancer | Stage IA–IC1, low-grade, unilateral tumors, no genetic risk | Unilateral salpingo-oophorectomy ± staging | Imaging and CA-125 every 3–6 months |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gică, N.; Vișoiu, I.; Mocanu, I.-C.; Năstac, A.; Sima, R.M.; Panaitescu, A.M.; Mehedințu, C. Fertility Preservation Strategies in Female Cancer Patients: Current Approaches and Future Directions. Medicina 2025, 61, 1794. https://doi.org/10.3390/medicina61101794
Gică N, Vișoiu I, Mocanu I-C, Năstac A, Sima RM, Panaitescu AM, Mehedințu C. Fertility Preservation Strategies in Female Cancer Patients: Current Approaches and Future Directions. Medicina. 2025; 61(10):1794. https://doi.org/10.3390/medicina61101794
Chicago/Turabian StyleGică, Nicolae, Ioana Vișoiu, Ioana-Catalina Mocanu, Ancuța Năstac, Romina Marina Sima, Anca Maria Panaitescu, and Claudia Mehedințu. 2025. "Fertility Preservation Strategies in Female Cancer Patients: Current Approaches and Future Directions" Medicina 61, no. 10: 1794. https://doi.org/10.3390/medicina61101794
APA StyleGică, N., Vișoiu, I., Mocanu, I.-C., Năstac, A., Sima, R. M., Panaitescu, A. M., & Mehedințu, C. (2025). Fertility Preservation Strategies in Female Cancer Patients: Current Approaches and Future Directions. Medicina, 61(10), 1794. https://doi.org/10.3390/medicina61101794











