Clinical Characteristics and the Prognostic Factors of Acute Peripheral Facial Palsy in Children
Abstract
1. Introduction
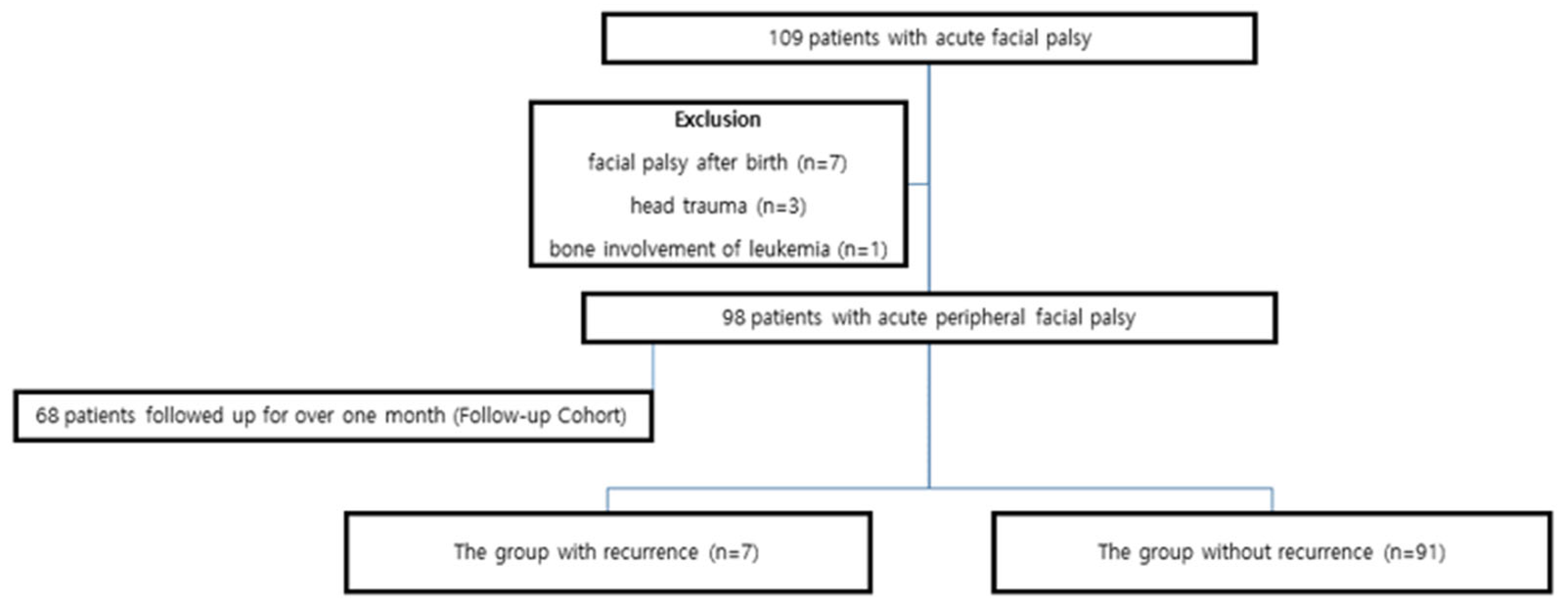
2. Materials and Methods
2.1. Patient Population
2.2. Methods
2.3. Statistical Analysis
3. Results
Analysis of the Follow-Up Cohort
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PFP | Peripheral Facial Palsy |
| H–B grade | The House-Brackmann grading system |
| MRI | Magnetic Resonance Imaging |
| SD | Standard Deviation |
| IQR | Interquartile Range |
| EMG | Electromyography |
| CI | Confidence Interval |
| OR | Odds Ratio |
| VIF | Variance Inflation Factor |
References
- Lunan, R.; Nagarajan, L. Bell’s palsy: A guideline proposal following a review of practice. J. Paediatr. Child Health. 2008, 44, 219–220. [Google Scholar] [CrossRef]
- Jenke, A.C.; Stoek, L.M.; Zilbauer, M.; Wirth, S.; Borusiak, P. Facial palsy: Etiology, outcome and management in children. Eur. J. Paediatr. Neurol. 2011, 15, 209–213. [Google Scholar] [CrossRef]
- Wright, H.; Waddington, C.; Geddes, J.; Newburger, J.W.; Burgner, D. Facial nerve palsy complicating Kawasaki disease. Pediatrics 2008, 122, e783-5. [Google Scholar] [CrossRef]
- Park, E.; Chang, Y.-S.; Kim, B.-J.; Chang, M.; Im, G.J.; Choi, J.; Jung, H.H.; Rah, Y.C. Improved Prediction of Hearing Loss after Temporal Bone Fracture by Applying a Detailed Classification for Otic Capsule-Violating Fracture: A Wide Scope Analysis with Large Case Series. Otol Neurotol. 2023, 44, 153–160. [Google Scholar] [CrossRef]
- Pitaro, J.; Waissbluth, S.; Daniel, S.J. Do children with Bell’s palsy benefit from steroid treatment? A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2012, 76, 921–926. [Google Scholar] [CrossRef]
- Salman, M.S.; MacGregor, D.L. Should children with Bell’s palsy be treated with corticosteroids? A systematic review. J. Child Neurol. 2001, 16, 565–568. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.R.; Guyatt, G.H.; Sud, S.; Dorion, J.; Hill, M.D.; Kolber, M.R.; Lea, J.; Reg, S.L.; Somogyi, B.K.; Westerberg, B.D.; et al. Management of Bell palsy: Clinical practice guideline. Can. Med Assoc. J. 2014, 186, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Eidlitz-Markus, T.; Gilai, A.; Mimouni, M.; Shuper, A. Recurrent facial nerve palsy in paediatric patients. Eur. J. Pediatr. 2001, 160, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.K.; Hollander, G.A.; McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. Biol. Sci. 2015, 282, 20143085. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef]
- Deoni, S.C.; Dean, D.C., 3rd; O’Muircheartaigh, J.; Dirks, H.; Jerskey, B.A. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage 2012, 63, 1038–1053. [Google Scholar] [CrossRef]
- Pavlou, E.; Gkampeta, A.; Arampatzi, M. Facial nerve palsy in childhood. Brain Dev. 2011, 33, 644–650. [Google Scholar] [CrossRef]
- Yoo, M.C.; Park, D.C.; Byun, J.Y.; Yeo, S.G. Clinical Prognostic Factors Associated with Good Outcomes in Pediatric Bell’s Palsy. J. Clin. Med. 2021, 10, 4368. [Google Scholar] [CrossRef] [PubMed]
- Karatoprak, E.; Yilmaz, S. Prognostic Factors Associated With Recovery in Children With Bell’s Palsy. J. Child Neurol. 2019, 34, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.W.; Lee, D.H.; Jun, B.C.; Chang, K.H.; Park, Y.S. Analysis of prognostic factors in Bell’s palsy and Ramsay Hunt syndrome. Auris Nasus Larynx 2007, 34, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.; Marsk, E.; Engström, M.; Hultcrantz, M.; Hadziosmanovic, N.; Jonsson, L. The effect of study design and analysis methods on recovery rates in Bell’s palsy. Laryngoscope 2009, 119, 2046–2050. [Google Scholar] [CrossRef]
- Peitersen, E. Bell’s palsy: The spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol. Suppl. 2002, 549, 4–30. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Swan, I.R.C.; Donnan, P.T.; Morrison, J.M.; Smith, B.H.; McKinstry, B.; Davenport, R.J.; Vale, L.D.; Clarkson, J.E.; Hammersley, V.; et al. Early treatment with prednisolone or acyclovir in Bell’s palsy. N. Engl. J. Med. 2007, 357, 1598–1607. [Google Scholar] [CrossRef]
- Yoo, M.C.; Soh, Y.; Chon, J.; Lee, J.H.; Jung, J.; Kim, S.S.; You, M.-W.; Byun, J.Y.; Kim, S.H.; Yeo, S.G. Evaluation of Factors Associated With Favorable Outcomes in Adults With Bell Palsy. JAMA Otolaryngol Head Neck Surg. 2020, 146, 256–263. [Google Scholar] [CrossRef]
- Drack, F.D.; Weissert, M. Outcome of peripheral facial palsy in children—A catamnestic study. Eur. J. Paediatr. Neurol. 2013, 17, 185–191. [Google Scholar] [CrossRef]
- Xiong, X.; Huang, L.; Herd, D.; Borland, M.; Davidson, A.; Hearps, S.; Mackay, M.; Lee, K.; Dalziel, S.; Dalziel, K.; et al. Cost-effectiveness of Prednisolone to Treat Bell Palsy in Children: An Economic Evaluation Alongside a Randomized Controlled Trial. Neurology 2023, 100, e2432–e2441. [Google Scholar] [CrossRef]
- Unüvar, E.; Oğuz, F.; Sidal, M.; Kiliç, A. Corticosteroid treatment of childhood Bell’s palsy. Pediatr. Neurol. 1999, 21, 814–816. [Google Scholar] [CrossRef]
- Yoo, H.W.; Yoon, L.; Kim, H.Y.; Kwak, M.J.; Park, K.H.; Bae, M.H.; Lee, Y.; Nam, S.O.; Kim, Y.M. Comparison of conservative therapy and steroid therapy for Bell’s palsy in children. Korean J. Pediatr. 2018, 61, 332–337. [Google Scholar] [CrossRef]
- Hato, N.; Matsumoto, S.; Kisaki, H.; Takahashi, H.; Wakisaka, H.; Honda, N.; Gyo, K.; Murakami, S.; Yanagihara, N. Efficacy of early treatment of Bell’s palsy with oral acyclovir and prednisolone. Otol Neurotol. 2003, 24, 948–951. [Google Scholar] [CrossRef] [PubMed]
- Guidi, M.; Giordano, F.; Peraio, S.; Conti, G.; Guerrini, R.; Trabalzini, F. Facial Nerve Tumors in Children: Two Clinical Cases and a Review of the Literature. J. Int. Adv. Otol. 2023, 19, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Vrinceanu, D.; Dumitru, M.; Popa-Cherecheanu, M.; Marinescu, A.N.; Patrascu, O.M.; Bobirca, F. Extracranial Facial Nerve Schwannoma-Histological Surprise or Therapeutic Planning? Medicina 2023, 59, 1167. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.S.; Gray, J.M.; Ramgopal, S.; Lipshaw, M.J. Risk of malignancy following emergency department Bell’s palsy diagnosis in children. Am. J. Emerg. Med. 2022, 53, 63–67. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Prengel, J.; Cohen, O.; Mäkitie, A.A.; Poorten, V.V.; Ronen, O.; Shaha, A.; Ferlito, A. Pathogenesis, diagnosis and therapy of facial synkinesis: A systematic review and clinical practice recommendations by the international head and neck scientific group. Front. Neurol. 2022, 13, 1019554. [Google Scholar] [CrossRef]
- Petrides, G.A.; Hayler, R.; Lee, J.W.; Jankelowitz, S.; Low, T.H. Electromyography in the prognostication of recovery in patients with acute peripheral facial nerve palsy: A systematic review. Clin. Otolaryngol. 2023, 48, 563–575. [Google Scholar] [CrossRef]
- Bylund, N.; Jensson, D.; Enghag, S.; Berg, T.; Marsk, E.; Hultcrantz, M.; Hadziosmanovic, N.; Rodriguez-Lorenzo, A.; Jonsson, L. Synkinesis in Bell’s palsy in a randomised controlled trial. Clin. Otolaryngol. 2017, 42, 673–680. [Google Scholar] [CrossRef]
| Group Without Recurrence (n = 91) | Group with Recurrence (n = 7) | Comparison | |
|---|---|---|---|
| Sex | |||
| Male | 44 | 4 | p = 0.48 |
| Female | 47 | 3 | chi-squared |
| Age (months) | 143.8 ± 52.5 | 79.1 ± 29.9 | p = 0.001 Mann–Whitney U test |
| Age (Months) | Steroid Initiation (Days) | Recovery Period (Days) | Side | Initial Severity (H–B Grade) | MRI | |
|---|---|---|---|---|---|---|
| Patient 1 | ||||||
| 1st | 107 | 1 | ||||
| 2nd | 151 | 2 | 28 | Lt | V | Facial nerve enhancement, Lt |
| 3rd | 239 | 3 | 6 | Rt | II | |
| Patient 2 | ||||||
| 1st | 99 | 8 | Rt | III | ||
| 2nd | 199 | 6 | 14 | Lt | III | |
| Patient 3 | ||||||
| 1st | 81 | 7 | 21 | Rt | IV | |
| 2nd | 155 | 0 | 5 | Rt | III | |
| Patient 4 | ||||||
| 1st | 105 | 7 | Lt | III | ||
| 2nd | 226 | 1 | 39 | Rt | IV | |
| Patient 5 | ||||||
| 1st | 72 | 1 | 6 | Lt | IV | |
| 2nd | 138 | 1 | 5 | Lt | III | |
| Patient 6 | ||||||
| 1st | 21 | 0 | Rt | |||
| 2nd | 48 | 0 | 8 | Rt | Facial nerve enhancement, Rt | |
| 3rd | 125 | 2 | 6 | Rt | III | Normal findings |
| Patient 7 | ||||||
| 1st | 69 | 14 | Normal findings | |||
| 2nd | 95 | 14 | Lt | |||
| 3rd | 110 | 0 | 6 | Lt | IV | |
| 4th | 176 | 1 | 11 | Lt | III | Normal findings |
| Patients Followed up for over One Month (n = 68) | |
|---|---|
| Sex | |
| Male | 34 (50%) |
| Female | 34 (50%) |
| Age (months) | |
| Mean (± SD) | 131.9 ± 53.5 |
| Range | 12–227 |
| Etiology | |
| Bell’s palsy | 60 (88.2%) |
| Otitis media | 3 (4.4%) |
| Ramsay-Hunt syndrome | 3 (4.4%) |
| Vaccination | 2 (2.9%) |
| Steroid initiation (days) | |
| Mean (± SD) | 2.8 ± 3.1 |
| Range | 0–16 |
| Treatment | |
| Steroid | 68 (100%) |
| Antiviral agents | 38 (55.9%) |
| Recovery period (days) | |
| Mean (± SD) | 39.5 ± 55.2 |
| Median (IQR) | 28 (21) |
| Range | 6–365 |
| Initial severity (H–B grade) | |
| II | 11 (16.2%) |
| III | 39 (57.4%) |
| IV | 18 (26.5%) |
| Final outcome (H–B grade) | |
| I | 64 (94.1%) |
| II | 4 (5.9%) |
| Variable | Categories | Odds Ratio (OR) | 95% CI | p-Value |
|---|---|---|---|---|
| Initial Severity | Complete palsy | 5.83 | 1.48–22.97 | 0.01 * |
| Incomplete palsy | Reference | |||
| Age | ≥6 years | 0.90 | 0.26–3.09 | 0.87 |
| <6 years | Reference | |||
| Sex | Male | 1.15 | 0.33–3.99 | 0.82 |
| Female | Reference | |||
| Steroid Initiation | ≥7 days | 2.50 | 0.40–15.70 | 0.33 |
| <7 days | Reference | |||
| Antiviral use | No | 0.87 | 0.27–2.77 | 0.82 |
| Yes | Reference |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-S.; You, S.J. Clinical Characteristics and the Prognostic Factors of Acute Peripheral Facial Palsy in Children. Medicina 2025, 61, 1790. https://doi.org/10.3390/medicina61101790
Chang Y-S, You SJ. Clinical Characteristics and the Prognostic Factors of Acute Peripheral Facial Palsy in Children. Medicina. 2025; 61(10):1790. https://doi.org/10.3390/medicina61101790
Chicago/Turabian StyleChang, Young-Soo, and Su Jeong You. 2025. "Clinical Characteristics and the Prognostic Factors of Acute Peripheral Facial Palsy in Children" Medicina 61, no. 10: 1790. https://doi.org/10.3390/medicina61101790
APA StyleChang, Y.-S., & You, S. J. (2025). Clinical Characteristics and the Prognostic Factors of Acute Peripheral Facial Palsy in Children. Medicina, 61(10), 1790. https://doi.org/10.3390/medicina61101790






