Characteristics of Infective Endocarditis in Intravenous Drug Users vs. Non-Users: A Retrospective Study Conducted in Bucharest, Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Inclusion Criteria
2.2. Exclusion Criteria
2.3. Admission and Diagnostic Procedures
2.4. Data Retrieval
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Valvular Involvement
3.3. Clinical Presentation
3.4. Microbiological Findings
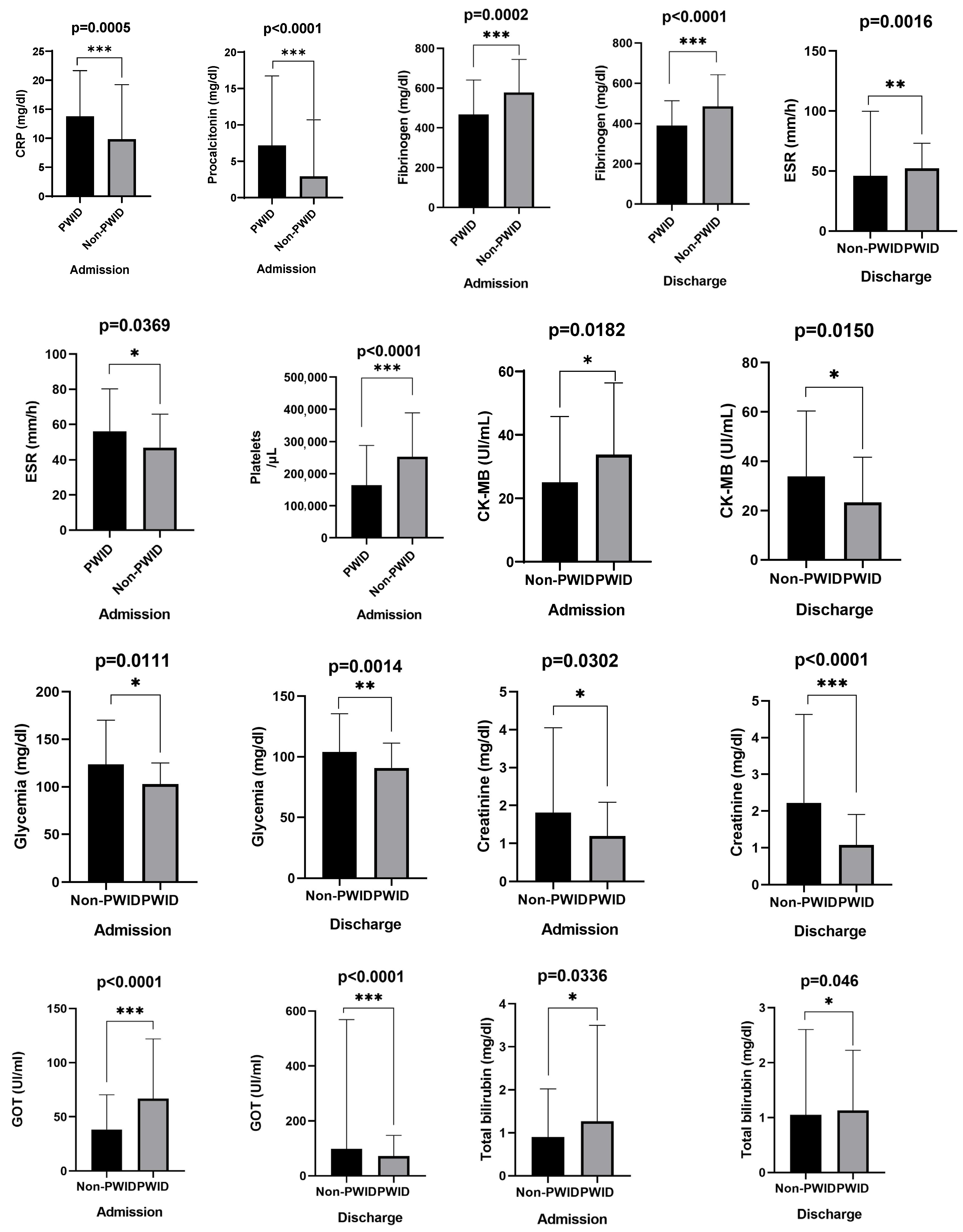
3.5. Laboratory Findings
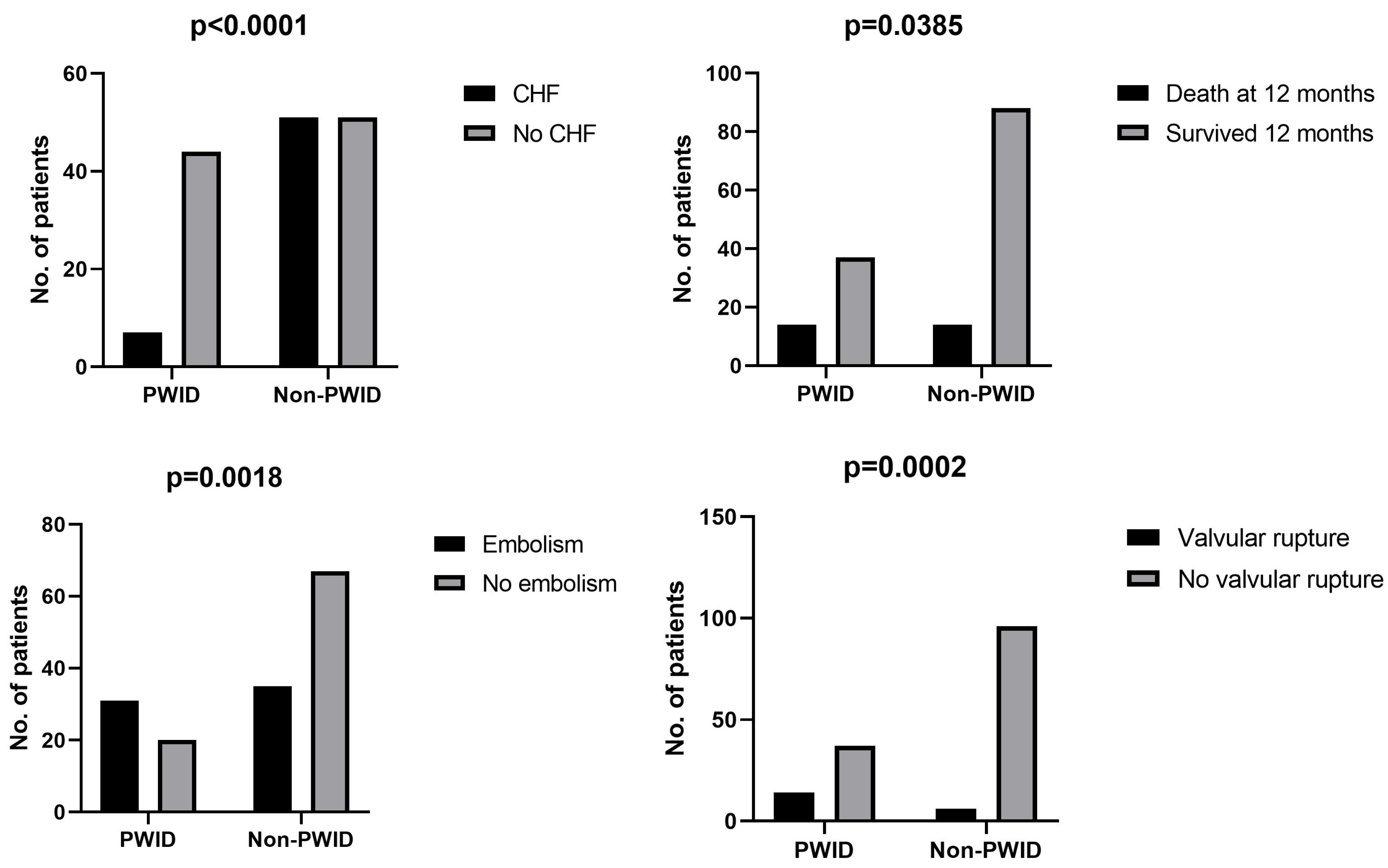
3.6. Complications and Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IE | Infective Endocarditis |
| PWID | People Who Inject Drugs |
| HIV | Human Immunodeficiency Virus |
| HBV | Hepatitis B Virus |
| HCV | Hepatitis C Virus |
| CRP | C-Reactive Protein |
| ESR | Erythrocyte Sedimentation Rate |
| TTE | Transthoracic Echocardiography |
| TEE | Transesophageal Echocardiography |
| PET-CT | Positron Emission Tomography–Computed Tomography |
| CHF | Congestive Heart Failure |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| MSSA | Methicillin-Sensitive Staphylococcus aureus |
| AST (GOT) | Aspartate Aminotransferase |
| ALT (GPT) | Alanine Aminotransferase |
| LDH | Lactate Dehydrogenase |
| ICU | Intensive Care Unit |
| BMI | Body Mass Index |
| PI | Prothrombin Index |
| ESCAPE | European Syringe Collection and Analysis Project |
References
- Liesenborghs, L.; Meyers, S.; Vanassche, T.; Verhamme, P. Coagulation: At the heart of infective endocarditis. J. Thromb. Haemost. 2020, 18, 995–1008. [Google Scholar] [CrossRef]
- Shmueli, H.; Thomas, F.; Flint, N.; Setia, G.; Janjic, A.; Siegel, R.J. Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment. J. Am. Heart Assoc. 2020, 9, 17293. [Google Scholar] [CrossRef]
- Bearpark, L.; Sartipy, U.; Franco-Cereceda, A.; Glaser, N. Surgery for Endocarditis in Intravenous Drug Users. Ann. Thorac. Surg. 2021, 112, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Verhamme, K.M.C.; Bohnen, A.M. Are we facing an opioid crisis in Europe? Lancet Public Health 2019, 4, e483–e484. [Google Scholar] [CrossRef]
- Loghin, I.I.; Surdu, A.E.; Rusu, Ș.A.; Cecan, I.; Dorobăț, V.D.; Mihăescu, A.A.; Dorobăţ, C.M. Etiological Aspects of Infectious Endocarditis in a Tertiary Hospital in Northeastern Romania. Medicina 2025, 61, 95. [Google Scholar] [CrossRef]
- Yucel, E.; Bearnot, B.; Paras, M.L.; Zern, E.K.; Dudzinski, D.M.; Soong, C.-P.; Jassar, A.S.; Rosenfield, K.; Lira, J.; Lambert, E.; et al. Diagnosis and Management of Infective Endocarditis in People Who Inject Drugs: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 2037–2057. [Google Scholar] [CrossRef]
- Hull, S.C.; Jadbabaie, F.; Weimer, M.B.; Golden, M.; Vallabhajosyula, P.; Rosenfeld, L.E. Revisiting Ethical Considerations in Recurrent Injection Drug Use-Related Infective Endocarditis. Ann. Thorac. Surg. Short Rep. 2023, 1, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Schranz, A.; Barocas, J.A. Infective endocarditis in persons who use drugs: Epidemiology, current management, and emerging treatments. Infect. Dis. Clin. N. Am. 2020, 34, 479. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.A.; Spence, C.; Shojaei, E.; Thandrasisla, P.; Gupta, A.; Choi, Y.-H.; Skinner, S.; Silverman, M. Infective Endocarditis Among Women Who Inject Drugs. JAMA Netw. Open 2024, 7, e2437861. [Google Scholar] [CrossRef]
- Rigau, P.V.; Moral, S.; Bosch, D.; Morales, M.; Frigola, J.M.; Albert, X.; Robles, R.; Ballesteros, E.; Roqué, M.; Aboal, J.; et al. Clinical Prognosis of Right-Sided Infective Endocarditis not Associated with Cardiac Devices or Intravenous Drug use: A Cohort Study and Meta-Analysis. Sci. Rep. 2020, 10, 7179. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Apostolakis, E.; Marangos, M.; Pasvol, G. Native valve right sided infective endocarditis. Eur. J. Intern. Med. 2013, 24, 510–519. [Google Scholar] [CrossRef]
- Habib, G.; Erba, P.A.; Iung, B.; Donal, E.; Cosyns, B.; Laroche, C.; Popescu, B.A.; Prendergast, B.; Tornos, P.; Sadeghpour, A.; et al. Clinical presentation, aetiology and outcome of infective endocarditis. Results of the ESC-EORP EURO-ENDO (European infective endocarditis) registry: A prospective cohort study. Eur. Heart J. 2019, 40, 3222–3232. [Google Scholar] [CrossRef]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3123. [Google Scholar]
- Thakarar, K.; Rokas, K.E.; Lucas, F.L.; Powers, S.; Andrews, E.; DeMatteo, C.; Mooney, D.; Sorg, M.H.; Valenti, A.; Cohen, M. Mortality, morbidity, and cardiac surgery in Injection Drug Use (IDU)-associated versus non-IDU infective endocarditis: The need to expand substance use disorder treatment and harm reduction services. PLoS ONE 2019, 14, e0225460. [Google Scholar] [CrossRef]
- Corcorran, M.A.; Stewart, J.; Lan, K.; Gupta, A.; Glick, S.N.; Seshadri, C.; Koomalsingh, K.J.; Gibbons, E.F.; Harrington, R.D.; Dhanireddy, S.; et al. Correlates of 90-Day Mortality Among People Who Do and Do Not Inject Drugs With Infective Endocarditis in Seattle, Washington. Open Forum Infect. Dis. 2022, 9, ofac150. [Google Scholar] [CrossRef]
- Straw, S.; Baig, M.W.; Gillott, R.; Wu, J.; Witte, K.K.; O’rEgan, D.J.; Sandoe, J.A.T. Long-term Outcomes Are Poor in Intravenous Drug Users Following Infective Endocarditis, Even After Surgery. Clin. Infect. Dis. 2020, 71, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Barnes, E.W.; Peacock, J.E. Repeat Infective Endocarditis in Persons Who Inject Drugs: ‘Take Another Little Piece of my Heart’. Open Forum Infect. Dis. 2018, 5, ofy304. [Google Scholar] [CrossRef]
- Halavaara, M.; Anttila, V.J.; Järvinen, A. Infective Endocarditis in People Who Inject Drugs—A 5-Year Follow-up: “I’ve Seen the Needle and the Damage Done”. Open Forum Infect. Dis. 2025, 12, ofaf057. [Google Scholar] [CrossRef] [PubMed]
- Pericàs, J.M.; Llopis, J.; Athan, E.; Hernández-Meneses, M.; Hannan, M.M.; Murdoch, D.R.; Kanafani, Z.; Freiberger, T.; Strahilevitz, J.; Fernández-Hidalgo, N.; et al. Prospective Cohort Study of Infective Endocarditis in People Who Inject Drugs. J. Am. Coll. Cardiol. 2021, 77, 544–555. [Google Scholar] [CrossRef]
- Cecchi, E.; Corcione, S.; Lupia, T.; De Benedetto, I.; Shbaklo, N.; Chirillo, F.; Moreo, A.; Rinaldi, M.; Faggiano, P.; Cecconi, M.; et al. Infective Endocarditis in People Who Inject Drugs: Report from the Italian Registry of Infective Endocarditis. J. Clin. Med. 2022, 11, 4082. [Google Scholar] [CrossRef] [PubMed]
- Rodger, L.; Glockler-Lauf, S.D.; Shojaei, E.; Sherazi, A.; Hallam, B.; Koivu, S.; Gupta, K.; Hosseini-Moghaddam, S.M.; Silverman, M. Clinical Characteristics and Factors Associated With Mortality in First-Episode Infective Endocarditis Among Persons Who Inject Drugs. JAMA Netw. Open 2018, 1, e185220. [Google Scholar] [CrossRef] [PubMed]
- Goodman-Meza, D.; Weiss, R.E.; Gamboa, S.; Gallegos, A.; Bui, A.A.T.; Goetz, M.B.; Shoptaw, S.; Landovitz, R.J. Long term surgical outcomes for infective endocarditis in people who inject drugs: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 918. [Google Scholar] [CrossRef] [PubMed]
| Non-PWID (n = 102) | PWID (n = 51) | p-Value | |
|---|---|---|---|
| Female sex, n (%) | 38 (37.3) | 7 (13.7) | 0.003 |
| Male sex, n (%) | 64 (62.7) | 44 (86.3) | |
| Age, years (mean ± SD; median [IQR]) | 64.29 ± 13.12; 67 [56–73.25] | 34.04 ± 6.57; 34 [29–40] | <0.001 |
| BMI (kg/m2) (mean ± SD; median [IQR]) | 26.68 ± 5.91; 25.8 [22.5–31] | 19.19 ± 2.36; 18.6 [17.8–20] | <0.001 |
| Stable housing, n (%) | 100 (98.0) | 31 (60.8) | <0.001 |
| Smoking, n (%) | 39 (38.2) | 51 (100.0) | <0.001 |
| Alcohol consumption, n (%) | 27 (26.5) | 44 (86.3) | <0.001 |
| Non-infectious comorbidities, n (%) | 101 (99.0) | 11 (21.6) | <0.001 |
| Infectious comorbidities, n (%) | 66 (64.7) | 37 (72.5) | 0.330 |
| Non-PWID (n = 102) | PWID (n = 51) | p-Value | |
|---|---|---|---|
| Prosthetic valve IE, n (%) | 25 (24.5) | 1 (2.0) | 0.002 |
| Biological valve, n (%) | 12 (48.0) | 1 (100.0) | 0.061 |
| Mechanical valve, n (%) | 12 (48.0) | 0 (0.0) | 0.009 |
| Pacemaker-related IE, n (%) | 1 (4.0) | 0 (0.0) | 1.000 |
| Pre-existing valve disease, n (%) | 61 (60.4) | 2 (3.9) | <0.001 |
| 40 (65.6) | 1 (50.0) | 0.649 |
| 9 (8.8) | 0 (0.0) | 0.030 |
| 46 (45.1) | 0 (0.0) | <0.001 |
| 14 (13.7) | 0 (0.0) | 0.005 |
| 37 (36.3) | 1 (2.0) | <0.001 |
| 7 (6.9) | 0 (0.0) | 0.096 |
| 2 (2.0) | 0 (0.0) | 1.000 |
| Vegetation identified, n (%) | 91 (89.2) | 47 (92.2) | 0.564 |
| TTE performed, n (%) | 64 (62.7) | 47 (92.2) | <0.001 |
| TEE performed, n (%) | 44 (43.1) | 3 (5.9) | <0.001 |
| PET-CT performed, n (%) | 1 (1.0) | 0 (0.0) | 1.000 |
| IE affecting tricuspid valve, n (%) | 8 (7.8) | 37 (72.5) | <0.001 |
| IE affecting mitral valve, n (%) | 53 (52.0) | 11 (21.6) | <0.001 |
| IE affecting aortic valve, n (%) | 48 (47.1) | 7 (13.7) | <0.001 |
| IE affecting pulmonary valve, n (%) | 0 (0.0) | 1 (2.0) | 0.336 |
| IE affecting interventricular septum, n (%) | 0 (0.0) | 1 (2.0) | 0.336 |
| Vegetation size, longitudinal (cm, mean ± SD; median [IQR]) | 1.06 ± 1.22; 0.8 [0.5–1.25] | 1.76 ± 0.84; 1.5 [1.2–2.1] | <0.001 |
| Vegetation size, transverse (cm, mean ± SD; median [IQR]) | 0.72 ± 0.46; 0.5 [0.5–0.96] | 1.20 ± 0.58; 1.0 [0.8–1.7] | <0.001 |
| More than one valve affected, n (%) | 4 (3.9) | 4 (7.8) | 0.442 |
| Major Duke criteria (mean ± SD; median [IQR]) | 1.68 ± 0.47; 2 [1–2] | 1.68 ± 0.47; 2 [1–2] | 0.894 |
| Minor Duke criteria (mean ± SD; median [IQR]) | 2.3 ± 0.8; 2 [2–3] | 3.32 ± 0.62; 3 [3–4] | <0.001 |
| Non-PWID (n = 102) | PWID (n = 51) | p-Value | |
|---|---|---|---|
| Subfebrility, n (%) | 7 (6.9) | 0 (0.0) | 0.096 |
| Fever, n (%) | 72 (70.6) | 49 (96.1) | <0.001 |
| Chills, n (%) | 40 (39.2) | 31 (60.8) | 0.012 |
| Maximum temperature (°C), mean ± SD, median [IQR] | 38.14 ± 1.08, 38.3 [37.8–38.7] | 38.78 ± 0.71, 38.7 [38.2–39.2] | <0.001 |
| Intense sweating, n (%) | 18 (17.6) | 16 (31.4) | 0.054 |
| Dyspnea, n (%) | 25 (24.5) | 19 (37.3) | 0.101 |
| Palpitations, n (%) | 11 (10.8) | 2 (3.9) | 0.221 |
| Cough, n (%) | 17 (16.7) | 37 (72.5) | <0.001 |
| Edema, n (%) | 20 (19.6) | 13 (25.5) | 0.404 |
| Precordial pain, n (%) | 9 (8.8) | 4 (7.8) | 1.000 |
| Asthenia, n (%) | 96 (94.1) | 47 (92.2) | 0.732 |
| Myalgias, n (%) | 9 (8.8) | 15 (29.4) | 0.001 |
| Arthralgias, n (%) | 8 (7.8) | 7 (13.7) | 0.249 |
| Fatigability, n (%) | 96 (94.1) | 49 (96.1) | 0.719 |
| Inappetence, n (%) | 95 (93.1) | 45 (88.2) | 0.360 |
| Chest pain, n (%) | 6 (5.9) | 6 (11.8) | 0.216 |
| Syncope, n (%) | 4 (3.9) | 7 (13.7) | 0.043 |
| Systolic BP ≤ 90 mmHg, n (%) | 7 (6.9) | 8 (15.7) | 0.084 |
| Heart rate (bpm), mean ± SD, median [IQR] | 91.02 ± 17.26, 88.5 [80–101.2] | 101.18 ± 17.57, 100 [90–113] | <0.001 |
| Respiratory rate, mean ± SD, median [IQR] | 19.52 ± 3.79, 19 [17–25] | 22.84 ± 6.91, 20 [18–25] | 0.005 |
| SpO2 (%), mean ± SD, median [IQR] | 95.81 ± 3.4, 97 [95–98] | 94.92 ± 4.4, 97 [94–98] | 0.263 |
| Pathological lung sounds, n (%) | 28 (27.5) | 25 (49.0) | 0.008 |
| Crepitants, n (%) | 23 (82.1) | 8 (32.0) | 0.0003 |
| Bronchial rales, n (%) | 5 (17.8) | 17 (68.0) | — |
| Systolic murmur, n (%) | 63 (61.8) | 33 (64.7) | 0.723 |
| Diastolic murmur, n (%) | 4 (3.9) | 2 (3.9) | 1.000 |
| Palpable lymph nodes, n (%) | 3 (2.9) | 32 (62.7) | <0.001 |
| Abdominal tenderness, n (%) | 8 (7.9) | 19 (37.3) | <0.001 |
| Hepatomegaly, n (%) | 12 (11.8) | 37 (72.5) | <0.001 |
| Diarrhea, n (%) | 8 (7.8) | 7 (13.7) | 0.249 |
| Focal neurological signs, n (%) | 24 (23.5) | 1 (2.0) | 0.001 |
| Meningitis signs, n (%) | 8 (7.8) | 4 (7.8) | 1.000 |
| Alveolar lung disease on X-ray, n (%) | 25 (24.5) | 36 (70.6) | <0.001 |
| Pleural fluid on X-ray, n (%) | 26 (25.5) | 15 (29.4) | 0.606 |
| Non-PWID (n = 102) | PWID (n = 51) | p-Value | |
|---|---|---|---|
| Identified microorganism, n (%) | 76 (74.5) | 40 (78.4) | 0.593 |
| Staphylococcus spp., n (%) | 28 (27.5) | 35 (68.6) | <0.001 |
| Methicillin-resistant S. aureus (MRSA), n (%) | 9 (8.8) | 14 (27.5) | 0.002 |
| Methicillin-sensitive S. aureus (MSSA), n (%) | 11 (10.9) | 21 (41.2) | <0.001 |
| Streptococcus spp. (pyogenes, agalactiae, dysgalactiae, gallolyticus, anginosus, constellatus, gordonii, mitis, oralis, salivarius, sanguinis, viridans), n (%) | 23 (22.5) | 6 (11.8) | 0.109 |
| Coxiella burnetii, n (%) | 6 (5.9) | 0 (0.0) | 0.179 |
| Klebsiella spp., n (%) | 3 (3.0) | 0 (0.0) | 0.551 |
| Enterococcus spp., n (%) | 11 (10.8) | 1 (2.0) | 0.062 |
| Coexisting fungal infection, n (%) | 5 (4.9) | 0 (0.0) | 0.170 |
| Polymicrobial acute infection, n (%) | 11 (10.8) | 2 (3.9) | 0.221 |
| Non-PWID (n = 102) | PWID (n = 51) | p-Value | |
|---|---|---|---|
| Leukocytes (×103/µL) | |||
| Admission, mean ± SD, median [IQR] | 11.61 ± 4.99, 10.9 [7.7–14.3] | 12.91 ± 6.26, 12.3 [9.2–16.6] | 0.199 |
| Discharge, mean ± SD, median [IQR] | 8.89 ± 4.19, 8.1 [5.9–10.7] | 12.59 ± 10.97, 9.75 [5.8–15.3] | 0.092 |
| C-Reactive Protein (mg/dL) | |||
| Admission | 9.81 ± 9.43, 6.88 [2.6–13.5] | 13.75 ± 7.9, 13.9 [8.4–18.5] | <0.001 |
| Discharge | 4.05 ± 5.7, 1.7 [0.5–5.4] | 5.19 ± 5.8, 2.83 [0.6–9.5] | 0.286 |
| Procalcitonin (mg/dL) | |||
| Admission | 2.90 ± 7.79, 0.29 [0.11–1.45] | 7.18 ± 9.54, 2.65 [0.67–10] | <0.001 |
| Discharge | 0.72 ± 2.93, 0.11 [0.04–0.5] | 2.80 ± 6.95, 0.11 [0.05–1.27] | 0.102 |
| Fibrinogen (mg/dL) | |||
| Admission | 578.5 ± 165.6, 562 [479–685] | 467.1 ± 173.8, 451 [338–589] | <0.001 |
| Discharge | 485.8 ± 156.6, 487.5 [390–559] | 390.0 ± 123.6, 391.5 [300–466] | <0.001 |
| ESR (mm/h) | |||
| Admission | 47.0 ± 18.9, 44.5 [33–55] | 56.2 ± 24.0, 55 [38–71] | 0.036 |
| Discharge | 46.1 ± 53.7, 38 [26–49] | 52.3 ± 20.9, 50 [33.8–63.5] | 0.002 |
| Platelets (×103/µL) | |||
| Admission | 252.8 ± 135.7, 241 [173–325] | 164.0 ± 123.9, 120.3 [57–259] | <0.001 |
| Discharge | 229.5 ± 115.3, 218.5 [157–275] | 232.6 ± 132.7, 212 [124.5–355] | 0.900 |
| Hemoglobin (g/dL) | |||
| Admission | 10.53 ± 1.77, 10.4 [9.1–11.8] | 9.97 ± 1.95, 9.8 [9.0–10.7] | 0.081 |
| Discharge | 10.42 ± 1.79, 10.1 [8.8–11.8] | 10.16 ± 1.84, 9.9 [8.9–11.1] | 0.547 |
| Prothrombin Index (%) | |||
| Admission | 71.3 ± 20.0, 74 [59.8–85] | 72.5 ± 14.0, 75 [64–81] | 0.895 |
| Discharge | 72.1 ± 23.6, 77 [56–91] | 81.7 ± 17.0, 86.5 [73.8–93.5] | 0.035 |
| D-dimers (mg/L) | |||
| Admission | 3.42 ± 3.35, 2.36 [1.4–4.4] | 3.60 ± 2.55, 3.5 [1.3–5.5] | 0.337 |
| Discharge | 2.88 ± 3.24, 2.06 [1.2–3.1] | 2.81 ± 2.03, 2.42 [1.3–3.5] | 0.440 |
| Troponin I ultrasensitive (ng/mL) | |||
| Admission | 162.2 ± 375.9, 30 [11–56.9] | 155.0 ± 293.7, 55 [21.5–155] | 0.057 |
| Discharge | 268.5 ± 977.8, 28.95 [11–44.5] | 139.3 ± 488.7, 32.5 [16–93.3] | 0.393 |
| CK-MB (U/L) | |||
| Admission | 25.1 ± 20.7, 19.3 [10.2–33] | 33.8 ± 22.6, 26.3 [16.3–50.6] | 0.019 |
| Discharge | 23.3 ± 18.3, 19 [12.6–23] | 33.9 ± 26.5, 27 [16.2–43] | 0.015 |
| Potassium (mEq/L) | |||
| Admission | 4.03 ± 0.7, 4.1 [3.5–4.5] | 3.8 ± 0.7, 3.7 [3.3–4.1] | 0.015 |
| Discharge | 4.24 ± 0.8, 4.2 [3.8–4.7] | 4.11 ± 0.7, 4.05 [3.8–4.6] | 0.150 |
| Sodium (mEq/L) | |||
| Admission | 136.9 ± 4.7, 137 [134.5–140.3] | 133.3 ± 6.0, 134 [129–138] | <0.001 |
| Discharge | 137.6 ± 4.8, 138 [135–140.3] | 133.3 ± 6.0, 134 [134–139] | 0.255 |
| Glycemia (mg/dL) | |||
| Admission | 123.6 ± 46.5, 112 [91–135] | 102.9 ± 22.2, 100 [88–117] | 0.011 |
| Discharge | 104.0 ± 31.5, 97.4 [84–117] | 90.8 ± 20.6, 87.5 [76–98.3] | 0.001 |
| Creatinine (mg/dL) | |||
| Admission | 1.81 ± 2.24, 1.1 [0.83–1.47] | 1.19 ± 0.89, 1.0 [0.7–1.17] | 0.030 |
| Discharge | 2.22 ± 2.41, 1.19 [0.89–2.84] | 1.08 ± 0.82, 0.86 [0.7–1.12] | <0.001 |
| GPT/ALT (U/L) | |||
| Admission | 42.1 ± 35.2, 32 [20.5–50] | 46.2 ± 40.4, 30 [21–53] | 0.761 |
| Discharge | 70.1 ± 187.5, 26.5 [19–60.2] | 49.4 ± 38.0, 37 [21–65] | 0.144 |
| GOT/AST (U/L) | |||
| Admission | 38.2 ± 32.2, 27.5 [19–47] | 66.8 ± 55.1, 56 [29–76] | <0.001 |
| Discharge | 98.1 ± 470.5, 29 [21–43.3] | 72.4 ± 75.0, 51.5 [33.9–86.3] | <0.001 |
| LDH (U/L) | |||
| Admission | 287.3 ± 112.8, 268.5 [198.8–367] | 373.0 ± 232.1, 306 [232–455] | 0.045 |
| Discharge | 284.7 ± 102.2, 281 [211–339] | 357.6 ± 324.3, 288 [211–388] | 0.466 |
| Total bilirubin (mg/dL) | |||
| Admission | 0.90 ± 1.12, 0.57 [0.36–1.04] | 1.26 ± 2.23, 0.86 [0.54–1.10] | 0.034 |
| Discharge | 1.05 ± 1.55, 0.7 [0.5–0.9] | 1.13 ± 1.09, 0.9 [0.8–1.1] | 0.005 |
| Non-PWID (n = 102) | PWID (n = 51) | p-Value | |
|---|---|---|---|
| In-hospital course | |||
| Congestive heart failure—n (%) | 51 (50%) | 7 (13.7%) | <0.001 |
| ICU admission—n (%) | 16 (15.7%) | 13 (25.5%) | 0.144 |
| Transfer to emergency hospitals—n (%) | 4 (3.9%) | 3 (5.9%) | 0.584 |
| Cardiovascular surgery scheduled—n (%) | 16 (15.7%) | 2 (3.9%) | 0.033 |
| Hospital outcomes | |||
| Fatal cases—n (%) | 12 (11.8%) | 9 (17.6%) | 0.318 |
| Hospital discharge on demand—n (%) | 7 (6.9%) | 18 (35.3%) | <0.001 |
| Improved clinical status at discharge—n (%) | 76 (74.5%) | 36 (70.6%) | 0.605 |
| Hospitalization days, mean ± SD, median [IQR] | 30.7 ± 16.2, 30.5 [18–41.3] | 30.4 ± 22.5, 30 [9–44] | 0.568 |
| Mortality | |||
| At 10 weeks—n (%) | 13 (12.7%) | 10 (19.6%) | 0.262 |
| At 12 months—n (%) | 14 (13.7%) | 14 (27.5%) | 0.038 |
| Complications | |||
| Embolic complications—n (%) | 35 (34.3%) | 31 (60.8%) | 0.002 |
| Pulmonary embolism—n (%) | 6 (17.1%) | 26 (83.8%) | <0.0001 |
| Abdominal organ embolism—n (%) | 11 (31.4%) | 4 (12.9%) | 0.086 |
| Valvular rupture—n (%) | 6 (5.9%) | 14 (27.5%) | <0.001 |
| IE recurrence after 6 months—n (%) | 0 (0%) | 2 (3.9%) | 0.109 |
| Domain | Non-PWID (n = 102) | PWID (n = 51) | p-Value |
|---|---|---|---|
| Demographics | Older age (mean 64 y), balanced sex (63% male), stable housing in 98% | Younger age (mean 34 y), predominantly male (86%), unstable housing in 39% | <0.001 |
| Comorbidities | Frequent non-infectious comorbidities (99%) | Rare non-infectious comorbidities (22%) | <0.001 |
| Infections | HIV absent; HCV rare (2%) | HIV in 65%; HCV in 98% | <0.001 |
| Valve involvement | Mostly left-sided IE (mitral 52%, aortic 47%), prosthetic valve IE 24% | Mostly right-sided IE (tricuspid 73%), larger vegetations | <0.001 |
| Microbiology | Streptococcus spp. 23%; culture-negative (6 cases with Coxiella burnetii) | Staphylococcus aureus 68% (MRSA 28%) | <0.001 |
| Clinical presentation | More heart failure (50%); less fever and systemic inflammation | More fever (96%), cough (73%), higher CRP/procalcitonin/ESR | <0.001 |
| Complications | More heart failure; more surgery (16%) | More embolic events (61%), pulmonary embolism (84%), valve rupture (28%) | <0.001 |
| Outcomes | In-hospital mortality 12%; 12-month mortality 14% | In-hospital mortality 18%; 12-month mortality 28% | 0.038 |
| Discharge | AMA discharge 7% | AMA discharge 35% | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanu, A.-A.; Lazăr, D.Ș.; Popescu, C.P.; Lazăr, M.-I.; Nica, M.; Florescu, S.A. Characteristics of Infective Endocarditis in Intravenous Drug Users vs. Non-Users: A Retrospective Study Conducted in Bucharest, Romania. Medicina 2025, 61, 1785. https://doi.org/10.3390/medicina61101785
Nanu A-A, Lazăr DȘ, Popescu CP, Lazăr M-I, Nica M, Florescu SA. Characteristics of Infective Endocarditis in Intravenous Drug Users vs. Non-Users: A Retrospective Study Conducted in Bucharest, Romania. Medicina. 2025; 61(10):1785. https://doi.org/10.3390/medicina61101785
Chicago/Turabian StyleNanu, Adina-Alexandra, Dragos Ștefan Lazăr, Corneliu Petru Popescu, Miruna-Ioana Lazăr, Maria Nica, and Simin Aysel Florescu. 2025. "Characteristics of Infective Endocarditis in Intravenous Drug Users vs. Non-Users: A Retrospective Study Conducted in Bucharest, Romania" Medicina 61, no. 10: 1785. https://doi.org/10.3390/medicina61101785
APA StyleNanu, A.-A., Lazăr, D. Ș., Popescu, C. P., Lazăr, M.-I., Nica, M., & Florescu, S. A. (2025). Characteristics of Infective Endocarditis in Intravenous Drug Users vs. Non-Users: A Retrospective Study Conducted in Bucharest, Romania. Medicina, 61(10), 1785. https://doi.org/10.3390/medicina61101785








