Sexual Health in Patients with Atopic Dermatitis: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Assessment of Sexual Dysfunction
2.3. Assessment of Quality of Life, Anxiety and Depression
2.4. Study Population
2.5. Statistical Analysis
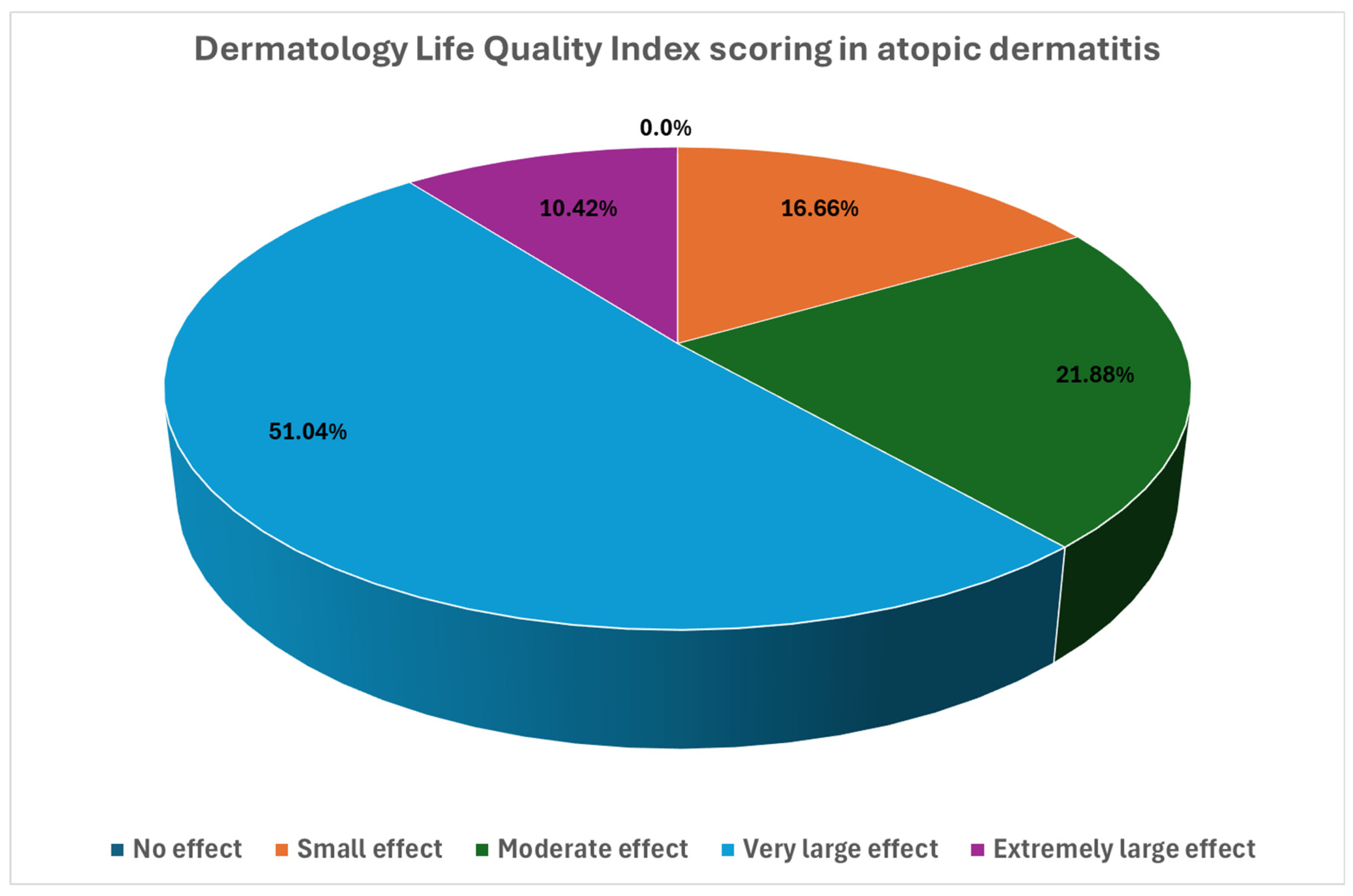
3. Results
| Patients with AD | Controls | p-Value | Cohen’s d with 95%CI | |
|---|---|---|---|---|
| DLQI | 12.3 ± 6.1 | 1.8 ± 3.1 | <0.001 | 2.20 [1.84; 2.5] |
| HADS Anxiety | 6.8 ± 3.6 | 5.0 ± 3.2 | <0.001 | 0.53 [0.24; 0.81] |
| HADS Depression | 5.2 ± 3.4 | 3.9 ± 2.9 | <0.01 | 0.42 [0.13; 0.69] |
| IIEF-5 | 21.3 ± 3.9 | 22.1 ± 4.3 | 0.38 | −0.18 [−0.59; 0.23] |
| FSFI | 24.8 ± 8.0 | 31.3 ± 3.0 | <0.001 | −1.11 [−1.52; 0.68] |
| SRSLQ | 15.0 ± 4.5 | 4.4 ± 4.7 | <0.001 | 1.69 [1.36; 2.01] |
| Men with AD | Women with AD | p-Value | Cohen’s d with 95%CI | |
|---|---|---|---|---|
| DLQI | 12.2 ± 6.6 | 12.4 ± 5.6 | 0.85 | N/A |
| HADS Anxiety | 6.0 ± 4.1 | 7.6 ± 2.9 | 0.03 | 0.46 [0.05; 0.86] |
| HADS Depression | 4.4 ± 3.5 | 5.9 ± 3.1 | 0.03 | 0.46 [0.06; 0.87] |
| SRSLQ | 13.8 ± 8.4 | 16.1 ± 6.4 | 0.14 | N/A |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murota, H.; Koike, Y.; Morisaki, H.; Matsumoto, M.; Takenaka, M. Exacerbating factors and disease burden in patients with atopic dermatitis. Allergol. Int. 2022, 71, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Bylund, S.; Kobyletzki, L.B.; Svalstedt, M.; Svensson, Å. Prevalence and incidence of atopic dermatitis: A systematic review. Acta Derm. Venereol. 2020, 100, adv00160. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.; Zink, A.; Arents, B.W.M.; Seitz, I.A.; Mensing, U.; Schielein, M.C.; Wettemann, N.; de Carlo, G.; Fink-Wagner, A. Atopic eczema: Burden of disease and individual suffering—Results from a large EU study in adults. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Laughter, M.R.; Maymone, M.B.C.; Mashayekhi, S.; Arents, B.W.M.; Karimkhani, C.; Langan, S.M.; Dellavalle, R.P.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef]
- Pojawa-Gołąb, M.; Reich, A. Skin pain in patients with atopic dermatitis or psoriasis: A web-based survey. Acta Derm. Venereol. 2020, 100, 5866. [Google Scholar] [CrossRef]
- Bawany, F.; Northcott, C.A.; Beck, L.A.; Pigeon, W.R. Sleep disturbances and atopic dermatitis: Relationships, methods for assessment, and therapies. J. Allergy Clin. Immunol. Pract. 2021, 9, 1488–1500. [Google Scholar] [CrossRef]
- Mann, C.; Dreher, M.; Weeß, H.-G.; Staubach, P. Sleep disturbance in patients with urticaria and atopic dermatitis: An underestimated burden. Acta Derm. Venereol. 2020, 100, 3416. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Garg, N.K.; Paller, A.S.; Fishbein, A.B.; Zee, P.C. Sleep disturbances in adults with eczema are associated with impaired overall health: A US population-based study. J. Investig. Dermatol. 2015, 135, 325. [Google Scholar] [CrossRef]
- Misery, L.; Seneschal, J.; Reguiai, Z.; Merhand, S.; Héas, S.; Huet, F.; Taieb, C.; Ezzedine, K. The impact of atopic dermatitis on sexual health. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 428–432. [Google Scholar] [CrossRef]
- Niemeier, V.; Winckelsesser, T.; Gieler, U. Hautkrankheit und Sexualität: Eine empirische Studie zum Sexualverhalten von Patienten mit Psoriasis vulgaris und Neurodermitis im Vergleich mit Hautgesunden. Hautarzt 1997, 48, 629–633. [Google Scholar] [CrossRef]
- Sampogna, F.; Abeni, D.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Titeca, G.; Lien, L.; Salek, M.-S.S. Impairment of sexual life in 3,485 dermatological outpatients from a multicentre study in 13 European countries. Acta Derm. Venereol. 2017, 97, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Astafieva, N.; Eremina, M.; Kobzev, D. Atopic dermatitis and sexual behavior of women. Allergy 2013, 68, 614–615. [Google Scholar]
- Chung, S.-D.; Keller, J.J.; Lin, H.-C. Association of erectile dysfunction with atopic dermatitis: A population-based case-control study. J. Sex. Med. 2012, 9, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.F. Severity scoring of atopic dermatitis: The SCORAD index. Consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1993, 186, 23–31. [Google Scholar] [CrossRef]
- Rosen, R.; Brown, C.; Heiman, J.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R. The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J. Sex. Marital. Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef]
- Rosen, R.C.; Riley, A.; Wagner, G.; Osterloh, I.H.; Kirkpatrick, J.; Mishra, A. The international index of erectile function (IIEF): A multidimensional scale for assessment of erectile dysfunction. Urology 1997, 49, 822–830. [Google Scholar] [CrossRef]
- Kędra, K.; Reich, A. Skin-Related Sexual Life Questionnaire (SRSLQ): Creation and validation of the questionnaire. Medicina 2023, 59, 2023. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)—A simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Rajka, G. Diagnostic features of atopic dermatitis. Acta Derm. Venereol. 1980, 92, 44–47. [Google Scholar] [CrossRef]
- World Health Organization. Health Topics, Sexual Health. Available online: https://www.who.int/health-topics/sexual-health#tab=tab_2 (accessed on 18 December 2024).
- Misery, L.; Seneschal, J.; Corgibet, F.; Halioua, B.; Marquié, A.; Merhand, S.; Lefur, G.; Staumont-Salle, D.; Bergqvist, C.; Taieb, C.; et al. Impact of atopic dermatitis on patients and their partners. Acta Derm. Venereol. 2023, 103, adv5285. [Google Scholar] [CrossRef] [PubMed]
- Misery, L.; Finlay, A.Y.; Martin, N.; Boussetta, S.; Nguyen, C.; Myon, E.; Taieb, C. Atopic dermatitis: Impact on the quality of life of patients and their partners. Dermatology 2007, 215, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Long, C.C.; Funnell, C.M.; Collard, R.; Finlay, A.Y. What do members of the National Eczema Society really want? Clin. Exp. Dermatol. 1993, 18, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Schonmann, Y.; Mansfield, K.E.; Hayes, J.F.; Abuabara, K.; Roberts, A.; Smeeth, L.; Langan, S.M. Atopic eczema in adulthood and risk of depression and anxiety: A population-based cohort study. J. Allergy Clin. Immunol. Pract. 2020, 8, 248. [Google Scholar] [CrossRef]
- Fougerousse, A.C.; Alexandre, M.; Darrigade, A.S.; Merhand, S.; Marquié, A.; Hamza, M.; Le Fur, G.; Jachiet, M.; Bursztejn, A.C.; Taieb, C. Impact of atopic dermatitis on adult women’s lives: A survey of 1,009 French women. Acta Derm. Venereol. 2024, 104, adv10321. [Google Scholar] [CrossRef]
- Kaundinya, T.; Rakita, U.; Silverberg, J.I. Prevalence, predictors, and longitudinal course of sexual dysfunction in adults with atopic dermatitis. Dermat. Contact Atopic Occup. Drug. 2023, 34, 233–240. [Google Scholar] [CrossRef]
- Woo, Y.R.; Han, Y.; Lee, J.H.; Lee, Y.B.; Kim, J.E.; Kim, M.; Park, C.J.; Lee, J.H.; Cho, S.H. Real-world prevalence and burden of genital eczema in atopic dermatitis: A multicenter questionnaire-based study. J. Dermatol. 2021, 48, 625–632. [Google Scholar] [CrossRef]
- Holm, E.A.; Esmann, S.; Jemec, G.B. Does visible atopic dermatitis affect quality of life more in women than in men? Gend. Med. 2004, 1, 125–130. [Google Scholar] [CrossRef]
- Ludwig, C.M.; Fernandez, J.M.; Hsiao, J.L.; Shi, V.Y. The interplay of atopic dermatitis and sexual health. Dermatitis 2020, 31, 303–308. [Google Scholar] [CrossRef]
| SRSLQ | |
|---|---|
| Pearson’s Correlation | |
| Age (years) | r = 0.09, p = 0.36 |
| Disease duration (years) | r = −0.09, p = 0.39 |
| SCORAD (points) | r = 0.33, p = 0.001 |
| Itch intensity (points) | r = 0.28, p = 0.005 |
| Sleeping problems (points) | r = 0.37, p < 0.001 |
| DLQI (points) | r = 0.52, p < 0.001 |
| HADS Anxiety (points) | r = 0.52, p < 0.001 |
| HADS Depression (points) | r = 0.51, p < 0.001 |
| IIEF-5 (points) | r = −0.45, p < 0.001 |
| FSFI (points) | r = −0.26, p = 0.07 |
| n | SRSLQ (Points) | p-Values | ||
|---|---|---|---|---|
| Mean ± SD | ||||
| Gender | Males | 47 | 13.83 ± 8.42 | p = 0.141 |
| Females | 49 | 16.10 ± 6.38 | ||
| Disease severity acc. to SCORAD | Mild atopic dermatitis | 18 | 11.17 ± 1.67 | p = 0.0025 |
| Moderate atopic dermatitis | 45 | 14.13 ± 1.06 | ||
| Severe atopic dermatitis | 33 | 18.24 ± 1.24 | ||
| Employment | Employed | 60 | 15.12 ± 0.97 | p = 0.37 |
| Unemployed | 9 | 17.11 ± 2.5 | ||
| Student | 17 | 12.47 ± 1.82 | ||
| Retired | 10 | 16.6 ± 2.37 | ||
| Education | Primary school | 3 | 13.33 ± 4.64 | p = 0.675 |
| Secondary school | 13 | 15.0 ± 12.14 | ||
| High school | 34 | 14.06 ± 1.32 | ||
| University | 39 | 16.23 ± 1.24 | ||
| Marital status | Married | 43 | 14.79 ± 1.11 | p = 0.9 |
| Single | 43 | 15.19 ± 1.16 | ||
| Divorced | 8 | 15.88 ± 2.68 | ||
| Widower | 2 | 11.5 ± 5.37 | ||
| Atopic lesions within genital area | Yes | 33 | 18.09 ± 7.78 | p = 0.005 |
| No | 62 | 13.42 ± 6.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juśko, N.; Masajada, M.; Żabówka, A.; Ćmiel, A.; Brzewski, P.; Reich, A. Sexual Health in Patients with Atopic Dermatitis: A Cross-Sectional Study. Medicina 2025, 61, 1782. https://doi.org/10.3390/medicina61101782
Juśko N, Masajada M, Żabówka A, Ćmiel A, Brzewski P, Reich A. Sexual Health in Patients with Atopic Dermatitis: A Cross-Sectional Study. Medicina. 2025; 61(10):1782. https://doi.org/10.3390/medicina61101782
Chicago/Turabian StyleJuśko, Natalia, Magdalena Masajada, Anna Żabówka, Adam Ćmiel, Paweł Brzewski, and Adam Reich. 2025. "Sexual Health in Patients with Atopic Dermatitis: A Cross-Sectional Study" Medicina 61, no. 10: 1782. https://doi.org/10.3390/medicina61101782
APA StyleJuśko, N., Masajada, M., Żabówka, A., Ćmiel, A., Brzewski, P., & Reich, A. (2025). Sexual Health in Patients with Atopic Dermatitis: A Cross-Sectional Study. Medicina, 61(10), 1782. https://doi.org/10.3390/medicina61101782







