Impact of COVID-19 Pandemic on Bronchiolitis Epidemiology in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Exclusion Criteria
2.3. Data Collection
2.4. Virus Identification
2.5. Assessment of Disease Severity
2.6. Data Analysis by Time Periods
2.7. Statistical Analysis
3. Results
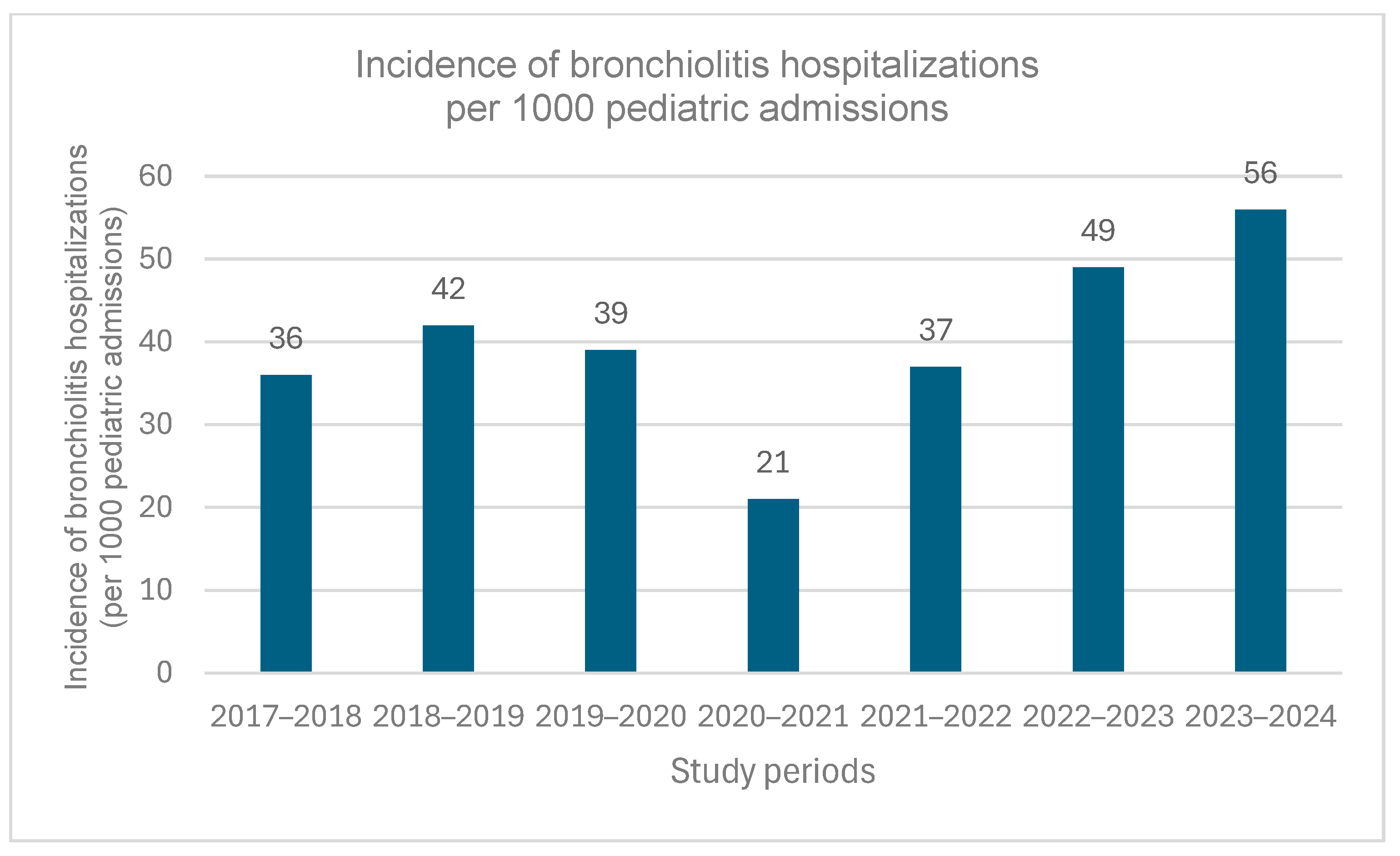
3.1. Study Population
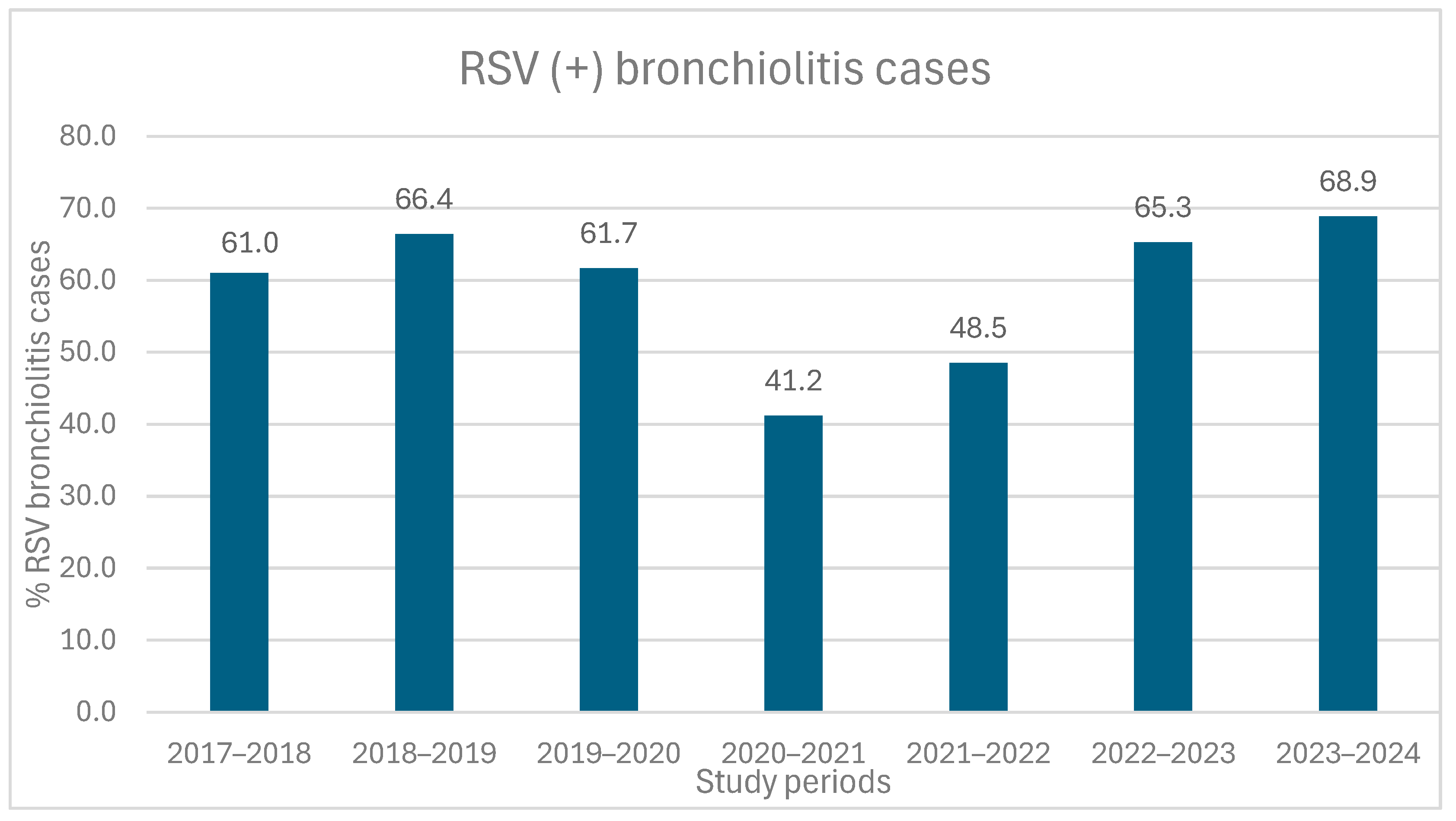
3.2. Viral Etiological Factors of Bronchiolitis Cases
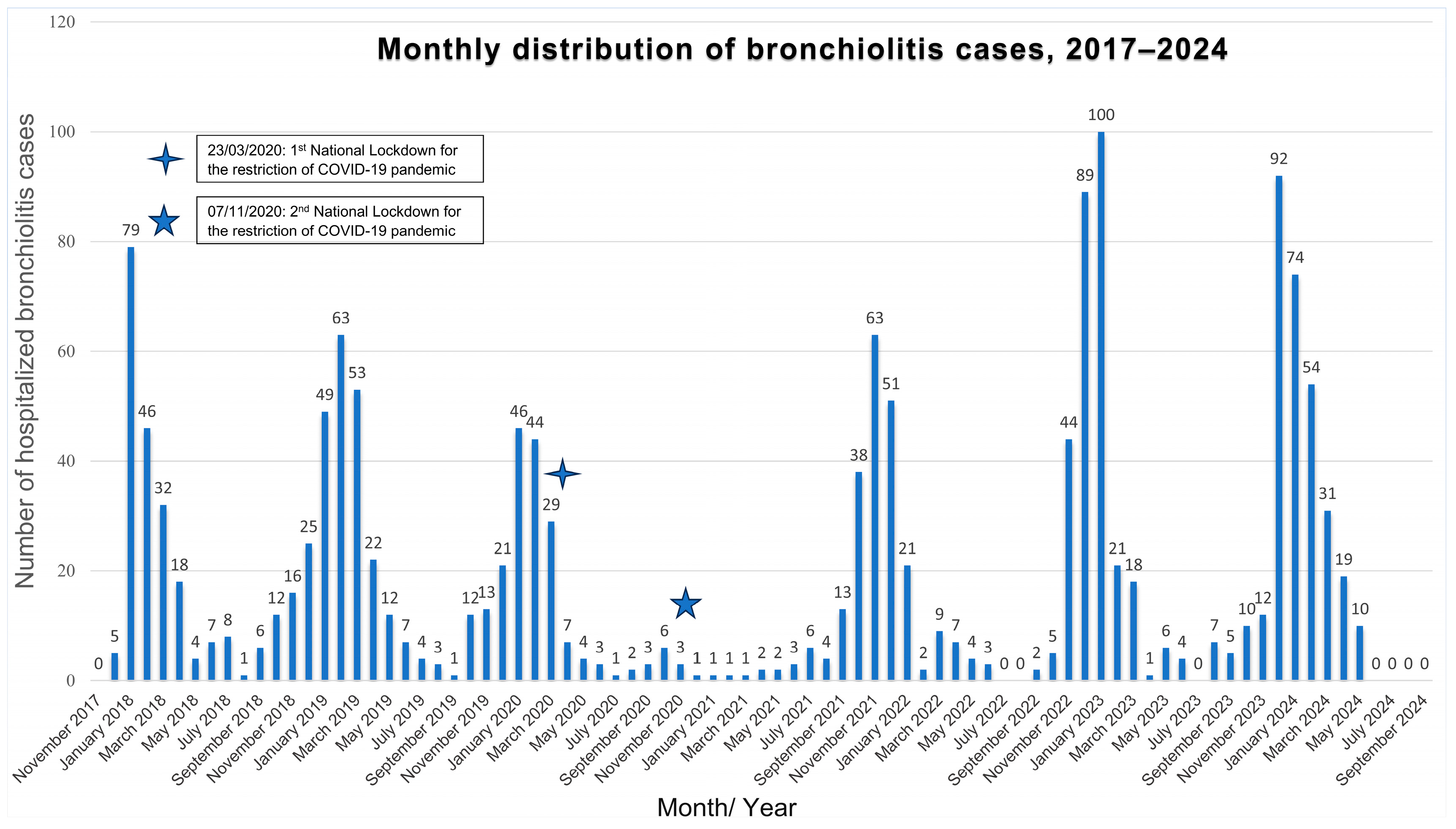
3.3. Seasonal Distribution
3.4. Age Distribution
3.5. Disease Severity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RSV | Respiratory Syncytial Virus |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| ED | Emergency Department |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| LFNO | Low-Flow Nasal Oxygen |
| HFNC | High-Flow Nasal Cannula |
| CPAP | Continuous Positive Airway Pressure |
| MV | Mechanical Ventilation |
| ICU | Intensive Care Unit |
| MTS | Modified Tal Score |
| SpO2 | Peripheral Capillary Oxygen Saturation |
| NICE | National Institute for Health and Care Excellence |
References
- WHO Coronavirus Disease (COVID-19) Pandemic_Timeline: WHO’s COVID-19 Response. n.d. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline (accessed on 22 December 2023).
- Greek National Public Health Organization. Greek National Public Health Organization_Press Releases 2020–2023. n.d. Available online: https://eody.gov.gr/category/deltia-typoy/ (accessed on 22 December 2023).
- Florin, T.A.; Plint, A.C.; Zorc, J.J. Viral bronchiolitis. Lancet 2017, 389, 211–224. [Google Scholar] [CrossRef]
- Pogka, V.; Kossivakis, A.; Kalliaropoulos, A.; Moutousi, A.; Sgouras, D.; Panagiotopoulos, T.; Chrousos, G.P.; Theodoridou, M.; Syriopoulou, V.P.; Mentis, A.F. Respiratory viruses involved in influenza-like illness in a Greek pediatric population during the winter period of the years 2005–2008. J. Med. Virol. 2011, 83, 1841–1848. [Google Scholar] [CrossRef]
- Moriyama, M.; Hugentobler, W.J.; Iwasaki, A. Seasonality of Respiratory Viral Infections. Annu. Rev. Virol. 2020, 7, 83–101. [Google Scholar] [CrossRef]
- Tsolia, M.N.; Kafetzis, D.; Danelatou, K.; Astra, H.; Kallergi, K.; Spyridis, P.; Karpathios, T.E. Epidemiology of respiratory syncytial virus bronchiolitis in hospitalized infants in Greece. Eur. J. Epidemiol. 2002, 18, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Van Brusselen, D.; De Troeyer, K.; ter Haar, E.; Vander Auwera, A.; Poschet, K.; Van Nuijs, S.; Bael, A.; Stobbelaar, K.; Verhulst, S.; Van Herendael, B.; et al. Bronchiolitis in COVID-19 times: A nearly absent disease? Eur. J. Pediatr. 2021, 180, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Markham, J.L.; Richardson, T.; DePorre, A.; Teufel, R.J.; Hersh, A.L.; Fleegler, E.W.; Antiel, R.M.; Williams, D.C.; Goldin, A.B.; Shah, S.S. Inpatient Use and Outcomes at Children’s Hospitals During the Early COVID-19 Pandemic. Pediatrics 2021, 147, e2020044735. [Google Scholar] [CrossRef] [PubMed]
- Chiapinotto, S.; Sarria, E.E.; Mocelin, H.T.; Lima, J.A.B.; Mattiello, R.; Fischer, G.B. Impact of non-pharmacological initiatives for COVID-19 on hospital admissions due to pediatric acute respiratory illnesses. Paediatr. Respir. Rev. 2021, 39, 3–8. [Google Scholar] [CrossRef]
- Stera, G.; Pierantoni, L.; Masetti, R.; Leardini, D.; Biagi, C.; Buonsenso, D.; Pession, A.; Lanari, M. Impact of SARS-CoV-2 Pandemic on Bronchiolitis Hospitalizations: The Experience of an Italian Tertiary Center. Children 2021, 8, 556. [Google Scholar] [CrossRef]
- Guedj, R.; Lorrot, M.; Lecarpentier, T.; Leger, P.; Corvol, H.; Carbajal, R. Infant bronchiolitis dramatically reduced during the second French COVID-19 outbreak. Acta Paediatr. 2021, 110, 1297–1299. [Google Scholar] [CrossRef]
- Cohen, R.; Ashman, M.; Taha, M.-K.; Varon, E.; Angoulvant, F.; Levy, C.; Rybak, A.; Ouldali, N.; Guiso, N.; Grimprel, E. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect. Dis. Now. 2021, 51, 418–423. [Google Scholar] [CrossRef]
- Baker, R.E.; Park, S.W.; Yang, W.; Vecchi, G.A.; Metcalf, C.J.E.; Grenfell, B.T. The impact of COVID-19 nonpharmaceutical interventions on the future dynamics of endemic infections. Proc. Natl. Acad. Sci. USA 2020, 117, 30547–30553. [Google Scholar] [CrossRef]
- Billard, M.-N.; Bont, L.J. Quantifying the RSV immunity debt following COVID-19: A public health matter. Lancet Infect. Dis. 2023, 23, 3–5. [Google Scholar] [CrossRef]
- Brisca, G.; Mariani, M.; Buratti, S.; Ferretti, M.; Pirlo, D.; Buffoni, I.; Mallamaci, M.; Salvati, P.; Tagliarini, G.; Piccotti, E.; et al. How has the SARS-CoV-2 pandemic changed the epidemiology and management of acute bronchiolitis? Pediatr. Pulmonol. 2023, 58, 1169–1177. [Google Scholar] [CrossRef]
- Camporesi, A.; Morello, R.; Ferro, V.; Pierantoni, L.; Rocca, A.; Lanari, M.; Trobia, G.L.; Sciacca, T.; Bellinvia, A.G.; De Ferrari, A.; et al. Epidemiology, Microbiology and Severity of Bronchiolitis in the First Post-Lockdown Cold Season in Three Different Geographical Areas in Italy: A Prospective, Observational Study. Children 2022, 9, 491. [Google Scholar] [CrossRef]
- Faraguna, M.C.; Lepri, I.; Clavenna, A.; Bonati, M.; Vimercati, C.; Sala, D.; Cattoni, A.; Melzi, M.L.; Biondi, A. The bronchiolitis epidemic in 2021–2022 during the SARS-CoV-2 pandemic: Experience of a third level centre in Northern Italy. Ital. J. Pediatr. 2023, 49, 26. [Google Scholar] [CrossRef] [PubMed]
- Vaux, S.; Viriot, D.; Forgeot, C.; Pontais, I.; Savitch, Y.; Barondeau-Leuret, A.; Smadja, S.; Valette, M.; Enouf, V.; Parent du Chatelet, I. Bronchiolitis epidemics in France during the SARS-CoV-2 pandemic: The 2020–2021 and 2021–2022 seasons. Infect. Dis. Now. 2022, 52, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Mrcela, D.; Markic, J.; Zhao, C.; Viskovic, D.V.; Milic, P.; Copac, R.; Li, Y. Changes following the Onset of the COVID-19 Pandemic in the Burden of Hospitalization for Respiratory Syncytial Virus Acute Lower Respiratory Infection in Children under Two Years: A Retrospective Study from Croatia. Viruses 2022, 14, 2746. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N. Resurgence of Respiratory Syncytial Virus Infections during COVID-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef]
- Ghirardo, S.; Cozzi, G.; Tonin, G.; Risso, F.M.; Dotta, L.; Zago, A.; Lupia, D.; Cogo, P.; Ullmann, N.; Coretti, A.; et al. Increased use of high-flow nasal cannulas after the pandemic in bronchiolitis: A more severe disease or a changed physician’s attitude? Eur. J. Pediatr. 2022, 181, 3931–3936. [Google Scholar] [CrossRef]
- Cardenas, J.; Pringle, C.; Filipp, S.L.; Gurka, M.J.; Ryan, K.A.; Avery, K.L. Changes in Critical Bronchiolitis After COVID-19 Lockdown. Cureus 2022, 14, e25064. [Google Scholar] [CrossRef]
- NICE. NICE Guideline NG9_Bronchiolitis: Diagnosis and Management of Bronchiolitis in Children, Methods, Evidence and Recommendations, June 2015-Updated August 2021. 2015. Available online: https://www.nice.org.uk/Guidance/NG9 (accessed on 15 June 2025).
- Golan-Tripto, I.; Goldbart, A.; Akel, K.; Dizitzer, Y.; Novack, V.; Tal, A. Modified Tal Score: Validated score for prediction of bronchiolitis severity. Pediatr. Pulmonol. 2018, 53, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xu, Y.; Wu, H.; Zhu, R.; Sun, Y.; Chen, D.M.; Wang, F.; Zhou, Y.T.; Guo, Q.; Wu, A.; et al. Changes in endemic patterns of respiratory syncytial virus infection in pediatric patients under the pressure of nonpharmaceutical interventions for COVID-19 in Beijing, China. J. Med. Virol. 2023, 95, e28411. [Google Scholar] [CrossRef] [PubMed]
- Camporesi, A.; Morello, R.; Pierucci, U.M.; Proli, F.; Lazzareschi, I.; Bersani, G.; Valentini, P.; Roland, D.; Buonsenso, D. 2021/22 and 2022/23 Post-Pandemic Bronchiolitis Seasons in Two Major Italian Cities: A Prospective Study. Children 2023, 10, 1081. [Google Scholar] [CrossRef]
- Juhn, Y.J.; Wi, C.-I.; Takahashi, P.Y.; Ryu, E.; King, K.S.; Hickman, J.A.; Yao, J.D.; Binnicker, M.J.; Natoli, T.L.; Evans, T.K.; et al. Incidence of Respiratory Syncytial Virus Infection in Older Adults Before and During the COVID-19 Pandemic. JAMA Netw. Open 2023, 6, e2250634. [Google Scholar] [CrossRef]
- Ogilvie, M.M.; Santhire Vathenen, A.; Radford, M.; Codd, J.; Key, S. Maternal antibody and respiratory syncytial virus infection in infancy. J. Med. Virol. 1981, 7, 263–271. [Google Scholar] [CrossRef]
- Graham, B.S. Protecting the Family to Protect the Child: Vaccination Strategy Guided by RSV Transmission Dynamics. J. Infect. Dis. 2014, 209, 1679–1681. [Google Scholar] [CrossRef]
- Ryan, L.; Plötz, F.B.; van den Hoogen, A.; Latour, J.M.; Degtyareva, M.; Keuning, M.; Klingenberg, C.; Reiss, I.K.M.; Giannoni, E.; Roehr, C.; et al. Neonates and COVID-19: State of the art: Neonatal Sepsis series. Pediatr. Res. 2022, 91, 432–439. [Google Scholar] [CrossRef]
- Principi, N.; Autore, G.; Ramundo, G.; Esposito, S. Epidemiology of Respiratory Infections during the COVID-19 Pandemic. Viruses 2023, 15, 1160. [Google Scholar] [CrossRef]
- Zee-Cheng, J.E.; McCluskey, C.K.; Klein, M.J.; Scanlon, M.C.; Rotta, A.T.; Shein, S.L.; Pineda, J.A.; Remy, K.E.; Carroll, C.L. Changes in Pediatric ICU Utilization and Clinical Trends During the Coronavirus Pandemic. Chest 2021, 160, 529–537. [Google Scholar] [CrossRef]
- Agha, R.; Avner, J.R. Delayed Seasonal RSV Surge Observed During the COVID-19 Pandemic. Pediatrics 2021, 148, e2021052089. [Google Scholar] [CrossRef]
- Berikopoulou, M.M.; Dessypris, N.; Kalogera, E.; Petridou, E.; Benetou, V.; Zahariadou, L.D.; Siahanidou, T.; Michos, A. Epidemiology of respiratory syncytial virus in hospitalized children before, during, and after the COVID-19 lockdown restriction measures in Greece. Epidemiol. Infect. 2024, 152, e94. [Google Scholar] [CrossRef]
- Cozzi, G.; Sovtic, A.; Garelli, D.; Krivec, U.; Silvagni, D.; Corsini, I.; Colombo, M.; Giangreco, M.; Giannattasio, A.; Milani, G.P.; et al. SARS-CoV-2-related bronchiolitis: A multicentre international study. Arch. Dis. Child. 2023, 108, e15. [Google Scholar] [CrossRef]
- Andina-Martinez, D.; Alonso-Cadenas, J.A.; Cobos-Carrascosa, E.; Bodegas, I.; Oltra-Benavent, M.; Plazaola, A.; Epalza, C.; Jimenez-García, R.; Moraleda, C.; Tagarro, A.; et al. SARS-CoV-2 acute bronchiolitis in hospitalized children: Neither frequent nor more severe. Pediatr. Pulmonol. 2022, 57, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Nunziata, F.; Salomone, S.; Catzola, A.; Poeta, M.; Pagano, F.; Punzi, L.; Lo Vecchio, A.; Guarino, A.; Bruzzese, E. Clinical Presentation and Severity of SARS-CoV-2 Infection Compared to Respiratory Syncytial Virus and Other Viral Respiratory Infections in Children Less than Two Years of Age. Viruses 2023, 15, 717. [Google Scholar] [CrossRef] [PubMed]
- Flores-Pérez, P.; Gerig, N.; Cabrera-López, M.I.; de Unzueta-Roch, J.L.; Del Rosal, T.; Calvo, C.; COVID-19 Study Group in Children. Acute bronchiolitis during the COVID-19 pandemic. Enfermedades Infecc. Y Microbiol. Clin. 2022, 40, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Presti, S.; Manti, S.; Gammeri, C.; Parisi, G.F.; Papale, M.; Leonardi, S. Epidemiological shifts in bronchiolitis patterns and impact of the COVID-19: A two-season comparative study. Pediatr. Pulmonol. 2024, 59, 1298–1304. [Google Scholar] [CrossRef]
- Dagan, R.; van der Beek, B.A.; Ben-Shimol, S.; Greenberg, D.; Shemer-Avni, Y.; Weinberger, D.M.; Danino, D. The COVID-19 pandemic as an opportunity for unravelling the causative association between respiratory viruses and pneumococcus-associated disease in young children: A prospective study. eBioMedicine 2023, 90, 104493. [Google Scholar] [CrossRef]
- Nagakumar, P.; Chadwick, C.-L.; Bush, A.; Gupta, A. Collateral impact of COVID-19: Why should children continue to suffer? Eur. J. Pediatr. 2021, 180, 1975–1979. [Google Scholar] [CrossRef]
- Azar, B.; Hashavya, S.; Ohana Sarna Cahan, L.; Reif, S.; Gross, I. Bronchiolitis Due to RSV and HMPV—Epidemiology, Clinical Course, and Prognosis: Experience of a Single Tertiary Center. Clin. Pediatr. 2023, 62, 1032–1039. [Google Scholar] [CrossRef]
- Betts, T.A.; Darby, A.E.; Hussain, F.; Edwards, M. Identifying the impact of non-pharmaceutical interventions on RSV transmission in a single-centre observational study. BMJ Paediatr. Open 2024, 8, e002603. [Google Scholar] [CrossRef]
- Abushahin, A.; Toma, H.; Alnaimi, A.; Abu-Hasan, M.; Alneirab, A.; Alzoubi, H.; Belavendra, A.; Janahi, I. Impact of COVID-19 pandemic restrictions and subsequent relaxation on the prevalence of respiratory virus hospitalizations in children. BMC Pediatr. 2024, 24, 91. [Google Scholar] [CrossRef] [PubMed]
- Vittucci, A.C.; Antilici, L.; Russo, C.; Musolino, A.M.C.; Cristaldi, S.; Cutrera, R.; Persia, S.; Di Maio, C.V.; Raponi, M.; Perno, C.F.; et al. Respiratory syncytial virus: Can we still believe that after pandemic bronchiolitis is not a critical issue for public health? Eur. J. Pediatr. 2023, 182, 5303–5313. [Google Scholar] [CrossRef] [PubMed]
- Nenna, R.; Pierangeli, A.; Matera, L.; Petrarca, L.; Conti, M.G.; Mancino, E.; di Mattia, G.; La Regina, D.P.; Virgili, F.; Papoff, P.; et al. Respiratory Syncytial Virus Bronchiolitis Before and After COVID-19 Pandemic: Has the Immunity Debt Been Paid Off? Pediatr. Infect. Dis. J. 2024, 43, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Brisca, G.; Strati, M.F.; Buratti, S.; Mariani, M.; Ferretti, M.; Pirlo, D.; Meleca, V.; Piccotti, E.; Castagnola, E.; Moscatelli, A. The increase of bronchiolitis severity in the 2022–2023 season in an Italian tertiary children’s hospital: An isolated phenomenon or a warning sign? Pediatr. Pulmonol. 2024, 59, 1236–1245. [Google Scholar] [CrossRef]
- Boccard, V.; Prevost, B.; Denamur, S.; Peulier-Maitre, E.; Nathan, N.; Corvol, H. Bronchiolitis: Increased severity in the post-COVID-19 era. Pediatr. Pulmonol. 2024, 59, 3197–3203. [Google Scholar] [CrossRef]
- Ghirardo, S.; Ullmann, N.; Zago, A.; Ghezzi, M.; Minute, M.; Madini, B.; D‘Auria, E.; Basile, C.; Castelletti, F.; Chironi, F.; et al. Increased bronchiolitis burden and severity after the pandemic: A national multicentric study. Ital. J. Pediatr. 2024, 50, 25. [Google Scholar] [CrossRef]
- Muller, W.J.; Madhi, S.A.; Seoane Nuñez, B.; Baca Cots, M.; Bosheva, M.; Dagan, R.; Hammitt, L.L.; Llapur, C.J.; Novoa, J.M.; Saez Llorens, X.; et al. Nirsevimab for Prevention of RSV in Term and Late-Preterm Infants. N. Engl. J. Med. 2023, 388, 1533–1534. [Google Scholar] [CrossRef]
- Madhi, S.A.; Polack, F.P.; Piedra, P.A.; Munoz, F.M.; Trenholme, A.A.; Simões, E.A.F.; Swamy, G.K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N. Engl. J. Med. 2020, 383, 426–439. [Google Scholar] [CrossRef]
- Sumsuzzman, D.M.; Wang, Z.; Langley, J.M.; Moghadas, S.M. Real-world effectiveness of nirsevimab against respiratory syncytial virus disease in infants: A systematic review and meta-analysis. Lancet Child Adolesc. Health 2025, 9, 393–403. [Google Scholar] [CrossRef]
- Karami, H.; Derakhshani, A.; Ghasemigol, M.; Fereidouni, M.; Miri-Moghaddam, E.; Baradaran, B.; Tabrizi, N.J.; Najafi, S.; Solimando, A.G.; Marsh, L.M.; et al. Weighted Gene Co-Expression Network Analysis Combined with Machine Learning Validation to Identify Key Modules and Hub Genes Associated with SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 3567. [Google Scholar] [CrossRef]
- Nenna, R.; Frassanito, A.; Petrarca, L.; Di Mattia, G.; Midulla, F. Age Limit in Bronchiolitis Diagnosis: 6 or 12 Months? Front. Pediatr. 2020, 8, 144. [Google Scholar] [CrossRef]
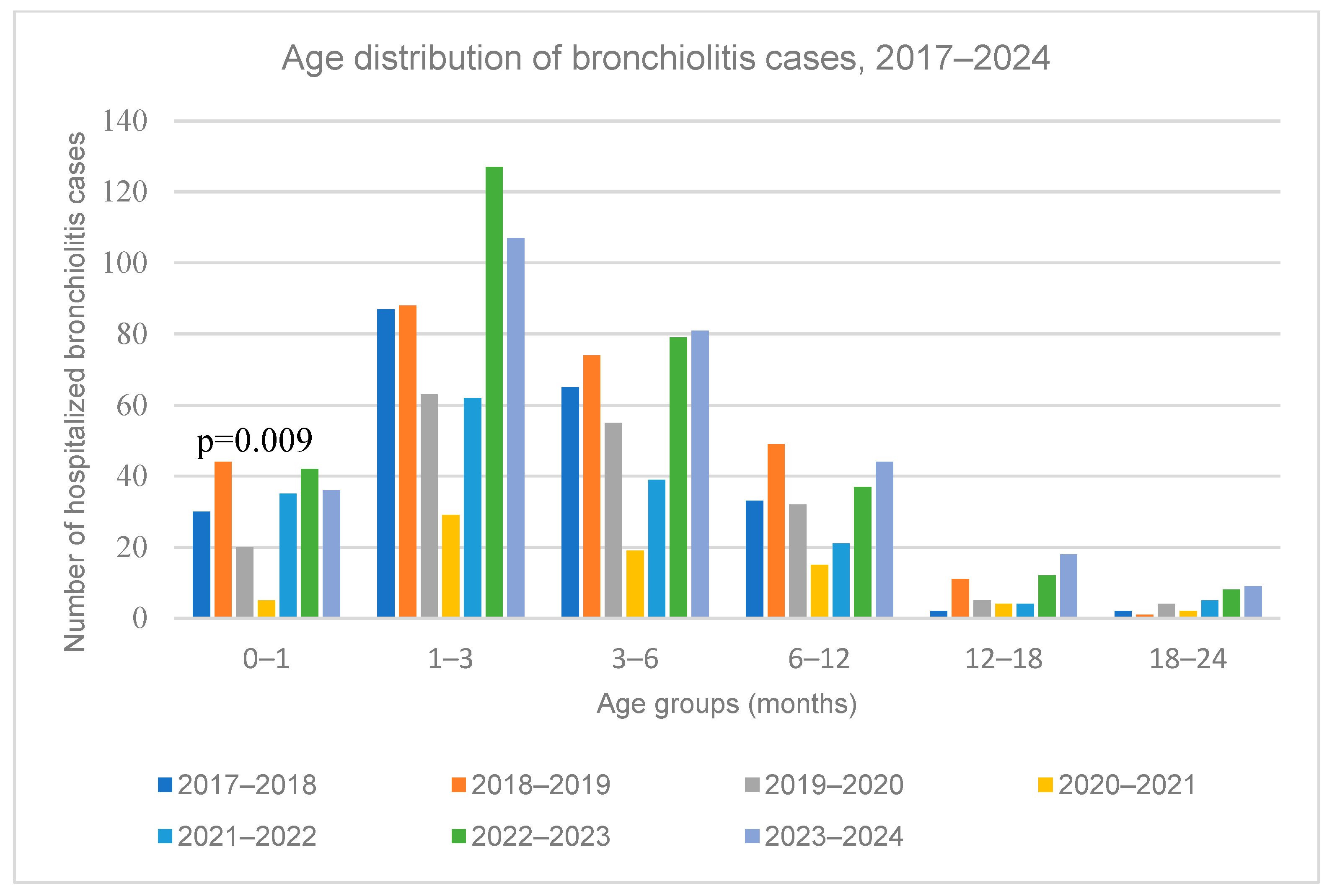
| RSV Seasons | ||||||||
|---|---|---|---|---|---|---|---|---|
| RSV seasons | 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 | 2021–2022 | 2022–2023 | 2023–2024 | 2017–2024 |
| Patients | ||||||||
| Total hospitalized bronchiolitis patients, (per 1000 pediatric admissions) | 219 (36) | 267 (42) | 179 (39) | 74 (21) | 166 (37) | 305 (49) | 295 (56) | 1505 (41) d |
| Total admissions in PED departments | 6047 | 6300 | 4572 | 3518 | 4531 | 6236 | 5222 | 36,426 |
| Age (months) | 2.8 (3.2) | 3 (4.2) | 3.1 (4.2) | 3 (6.0) | 2.63 (3.2) | 2.53 (3.9) | 3.13 (4.3) | 2.80 (3.7) |
| Gender (M/F) | 139/80 | 160/107 | 104/75 | 52/22 | 84/82 | 161/144 | 178/117 | 876/627 |
| Parity | 2 (2) | 2 (1) | 2 (1) | 2 (2) | 2 (1) | 2 (1) | 2 (2) | 2 (1) |
| Prematurity n, (%) | 47 (23.5) | 53 (20.5) | 20 (11.6) | 19 (26.4) | 36 (22.2) | 66 (21.6) | 54 (18.3) | 295/1505 (19.6) |
| Previous hospitalization for bronchiolitis n, (%) | 27 (12.9) | 37 (14) | 25 (14.5) | 9 (12.5) | 12(7.3) | 35 (11.5) | 40 (13.6) | 185/1505 (12.3) |
| Comorbidities n, (%) a | 14 (6.4) | 23 (8.6) | 12 (6.7) | 2 (2.7) | 8 (4.8) | 12 (3.9) | 18 (6.1) | 89/1505 (5.9) |
| Clinical presentation | ||||||||
| Respiratory distress b | 161 (74.2) | 199 (74.8) | 141 (79.2) | 66 (88.0) | 145 (87.3) | 259 (84.9) | 230 (78) | 1201 (79.8) |
| Respiratory Rate on admission | 53 (15) | 56 (17) | 56 (15) | 52 (15) | 56 (15) | 56 (10) | 52 (15) | 55 (15) |
| SpO2 (%) on admission | 97 (4) | 97 (4) | 96.5 (5) | 98 (5) | 97 (5) | 96 (4) | 97 (4) | 97 (5) |
| Heart Rate on admission | 150 (30) | 150 (24) | 150 (28) | 150 (22) | 154 (30) | 152 (29) | 154 (28) | 150 (29) |
| Fever >38 °C c (°C) | 82 (39.4) | 140 (53.2) | 98 (55.1) | 39 (52.7) | 73 (44.2) | 123 (40.3) | 95 (31.1) | 650 (43.2) |
| Maximum temperature c (°C) | 38.4 (1.1) | 38.4 (1.1) | 38.4 (1.2) | 38.6 (0.9) | 38.5 (1.1) | 38.5 (1.3) | 38.5 (1.1) | 38.5 (1.1) |
| Apnoea episodes c | 22 (10.2) | 14 (5.3) | 4 (2.3) | 0 | 9 (5.4) | 13 (4.3) | 5 (1.7) | 67 (4.5) |
| 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 | 2021–2022 | 2022–2023 | 2023–2024 | 2017–2024 | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 219 | 267 | 179 | 74 | 166 | 305 | 295 | 1505 | |
| Admission MTS | 5 (4) | 5 (3) | 5 (3) | 5 (4) | 5 (2) | 5 (2) | 5 (3) | 5 (2) | 0.52 |
| Peak MTS | 6 (3) | 6 (2) | 6 (3) | 5 (3) | 5 (3) | 6 (2) | 6 (3) | 6 (3) | 0.41 |
| Requirement for respiratory support n, (%) | 193 (88.9) | 228 (85.4) | 146 (81.6) | 58 (78.4) | 125 (75.8) | 256 (81.6) | 245 (83.1) | 1251 (83.1) | <0.001 |
| Type of respiratory support a n, (%) | |||||||||
| LFNO | 193 (100) | 228 (100) | 146 (100) | 58 (100) | 125 (100) | 256 (100) | 241 (98.4) | 1247 (99.7) | 0.7 |
| HFNC | 12 (6.2) | 8 (3.5) | 7 (4.8) | 5 (8.6) | 4 (3.2) | 15 (5.6) | 39 (15.9) | 90 (7.2) | <0.001 |
| CPAP | 5 (2.6) | 1 (0.4) | 1 (0.7) | 0 | 0 | 2 (0.8) | 0 | 9 (0.7) | 0.38 |
| MV | 1 (0.5) | 1 (0.4) | 2 (1.4) | 0 | 0 | 0 | 0 | 4 (0.3) | 0.82 |
| Duration of respiratory support (days) | 4 (4) | 4 (3) | 4 (3) | 3 (4) | 4 (4) | 3 (3) | 3 (3) | 4 (3) | 0.25 |
| Duration of HFNC use (days) | 3 (0) | 2 (0) | 2 (0) | 2,5(0) | 3 (0) | 4 (0) | 4 (4) | 3 (1) | <0.001 |
| Duration of hydration (days) | 3 (4) | 2 (3.7) | 2 (3) | 2 (3) | 2 (4) | 2 (3) | 3 (3) | 2 (3) | 0.31 |
| ICU admission n, (%) | 16 (7.5) | 9 (3.4) | 7 (3.9) | 6 (8.1) | 6 (3.6) | 9 (2.9) | 4 (1.4) | 57 (3.8) | 0.03 |
| Length of stay in ICU (days) | 4 (0) | 3 (0) | 4 (0) | 2.5(0) | 3 (0) | 4 (0) | 7 (0) | 3 (0) | 0.16 |
| Length of Hospital Stay (days) | 5 (4) | 4 (3) | 4 (3) | 5 (5) | 4 (3) | 4 (3) | 4 (3) | 4 (4) | 0.38 |
| Hospital Readmission b n, (%) | 1 (0.4) | 7 (2.6) | 4 (2.2) | 1 (1.3) | 1 (0.6) | 7 (2.3) | 3 (1) | 20 (1.7) | 0.68 |
| Chest X-ray performed n, (%) | 124 (56.6) | 116 (43.4) | 76 (42.5) | 29 (39.2) | 63 (37.9) | 105 (34.4) | 124 (42) | 637 (42.3) | <0.001 |
| Findings in Chest X-ray n, (%) c | 111 (89.5) | 101 (87.1) | 71 (93.4) | 27 (89.6) | 56 (88.9) | 96 (91.4) | 111 (89.5) | 573 (90) | 0.95 |
| Pulmonary infiltrates n, (%) c | 19 (15.3) | 24 (20.7) | 20 (26.3) | 7 (24.1) | 31 (49.2) | 34 (32.4) | 48 (38.7) | 183 (28.7) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koloi, A.; Dimopoulou, D.; Papakonstantinou, D.; Damianos, G.; Korentzelou, V.; Kotzamani, M.T.; Neofytou, A.; Paraschos, C.; Pasparaki, S.D.; Rizargioti, A.; et al. Impact of COVID-19 Pandemic on Bronchiolitis Epidemiology in Greece. Medicina 2025, 61, 1746. https://doi.org/10.3390/medicina61101746
Koloi A, Dimopoulou D, Papakonstantinou D, Damianos G, Korentzelou V, Kotzamani MT, Neofytou A, Paraschos C, Pasparaki SD, Rizargioti A, et al. Impact of COVID-19 Pandemic on Bronchiolitis Epidemiology in Greece. Medicina. 2025; 61(10):1746. https://doi.org/10.3390/medicina61101746
Chicago/Turabian StyleKoloi, Athina, Dimitra Dimopoulou, Dimitris Papakonstantinou, Georgios Damianos, Vasiliki Korentzelou, Marina Triantafyllia Kotzamani, Ariadni Neofytou, Christos Paraschos, Sofia D. Pasparaki, Agori Rizargioti, and et al. 2025. "Impact of COVID-19 Pandemic on Bronchiolitis Epidemiology in Greece" Medicina 61, no. 10: 1746. https://doi.org/10.3390/medicina61101746
APA StyleKoloi, A., Dimopoulou, D., Papakonstantinou, D., Damianos, G., Korentzelou, V., Kotzamani, M. T., Neofytou, A., Paraschos, C., Pasparaki, S. D., Rizargioti, A., Benetatou, K., Tampouratzi, M., Konidari, A., Soldatou, A., & Tsolia, M. N. (2025). Impact of COVID-19 Pandemic on Bronchiolitis Epidemiology in Greece. Medicina, 61(10), 1746. https://doi.org/10.3390/medicina61101746






