Cognitive and Emotional Impairments in Acute Post-Stroke Patients—A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Variables, Data Sources, and Measurements
2.3. Bias
2.4. Statistical Analysis
2.4.1. Sample Size
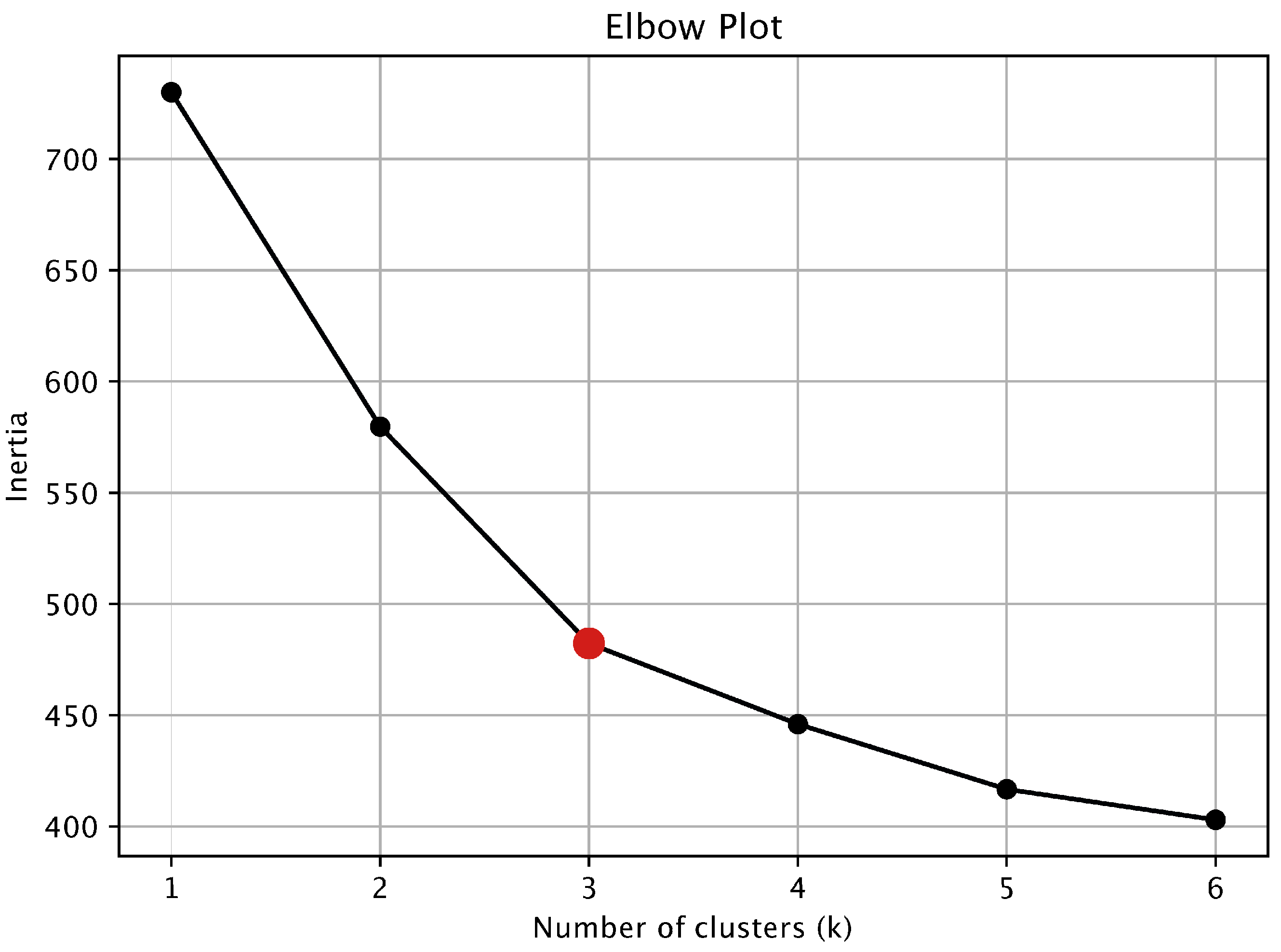
2.4.2. Cluster Analysis
2.4.3. Linear Regression
2.5. Ethics
3. Results
3.1. Sampling and Demographic Data
3.2. Patients’ Symptom Profiles
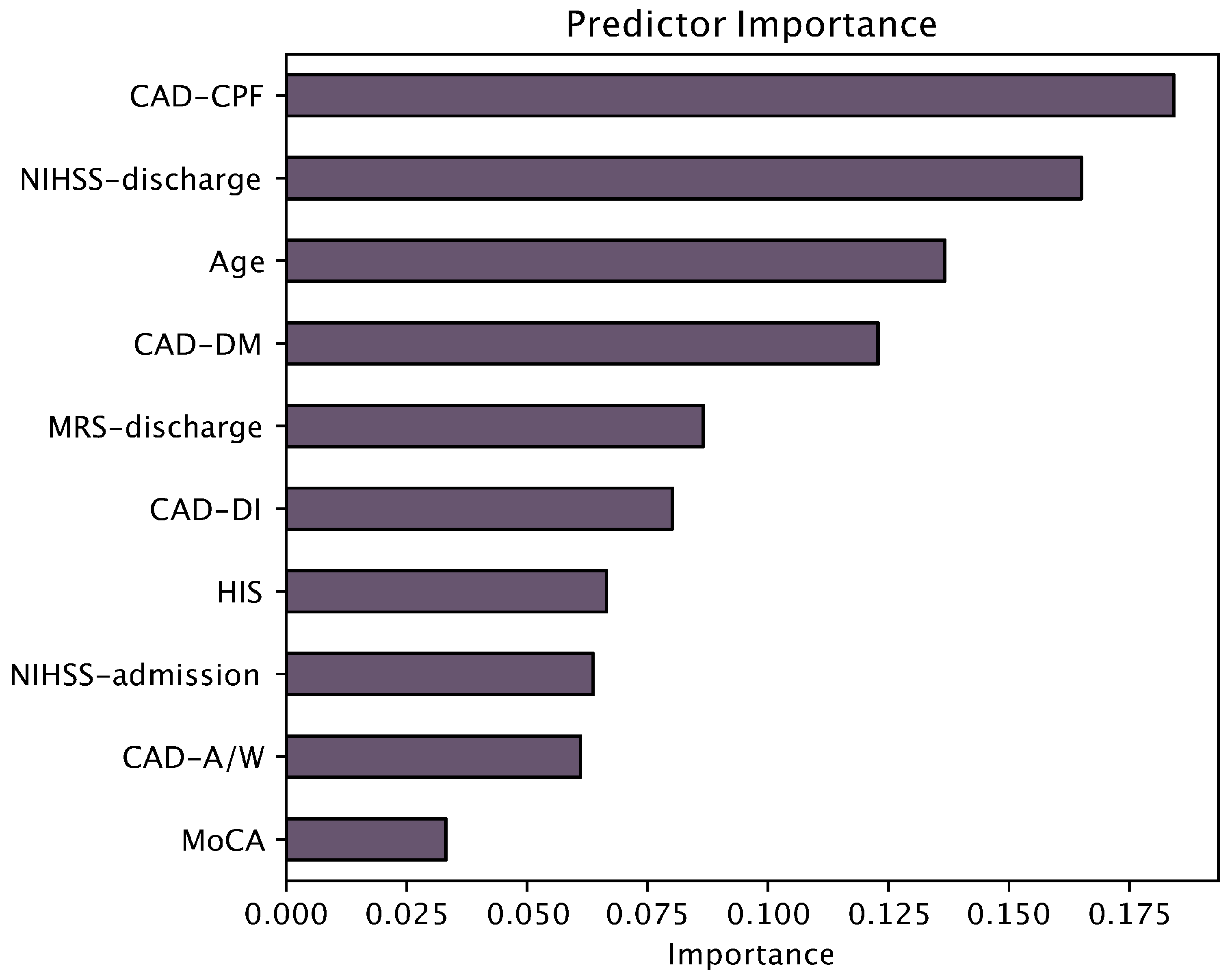
3.3. Linear Regression
4. Discussion
4.1. Patients’ Profiles Following Stroke
4.2. Incidence of Neuropsychiatric Symptoms
4.3. Clinical Implementations of Predictive Factors for Vascular Cognitive Impairment and Depression
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIHSS | National Institutes of Health Stroke Scale |
| mRS | modified Rankin Scale |
| MoCA | Montreal Cognitive Assessment |
| HIS | Hachinski Ischemic Score |
| CAD-DM | Cognitive and Affective Disorders scale—Depressed Mood subscale |
| CAD-A/W | Cognitive and Affective Disorders scale—Anxiety/Worry subscale |
| CAD-DI | Cognitive and Affective Disorders scale—Disinterest subscale |
| CAD-CPF | Cognitive and Affective Disorders scale—Cognitive and Physical Fatigue subscale |
| VCI | Vascular Cognitive Impairment |
| DP | Depressive Profile |
| MIP | Mild Impairment Profile |
Appendix A
Appendix A.1. Post Hoc Sample Size Power Analysis
Appendix A.2. Cluster Analysis
Appendix A.3. Distributions of MoCA and CAD Scores
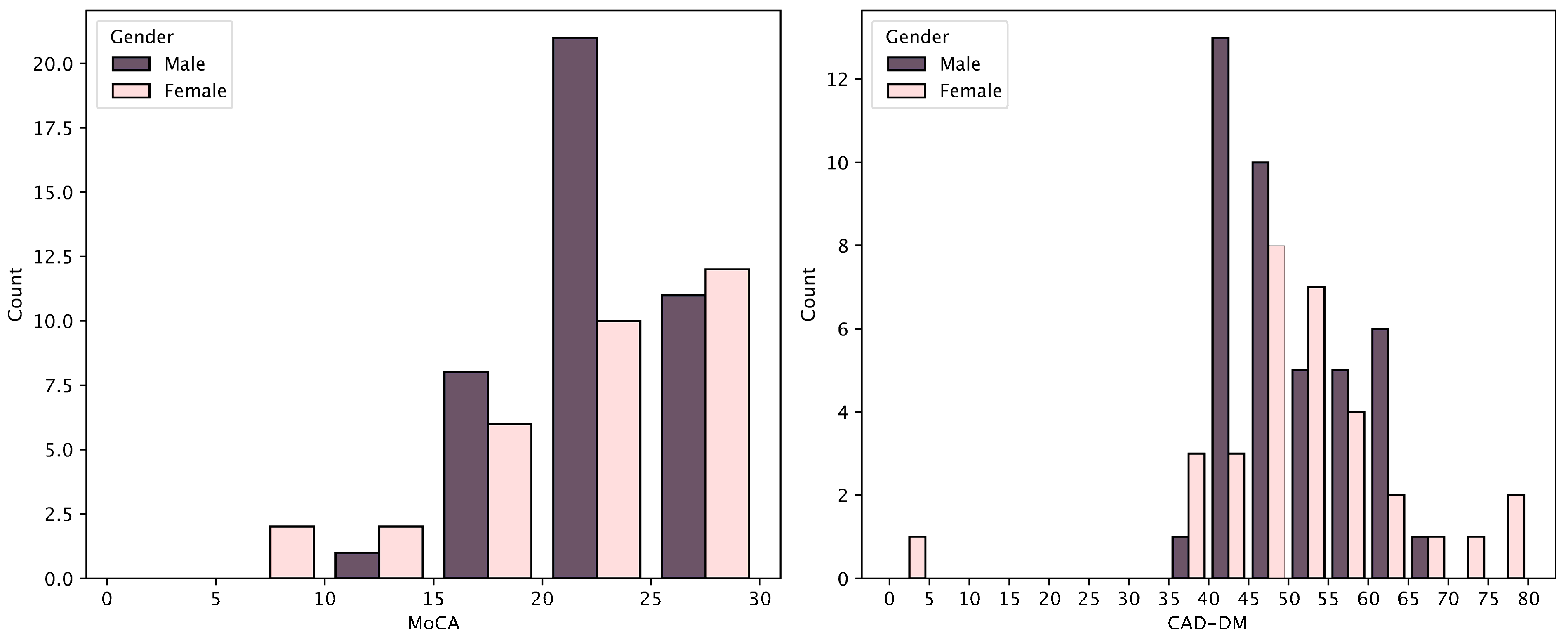
References
- Feigin, V.L.; Abate, M.D.; Abate, Y.H.; Abd ElHafeez, S.; Abd-Allah, F.; Abdelalim, A.; Abdelkader, A.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdi, P.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 973–1003. [Google Scholar] [CrossRef] [PubMed]
- Ovčar Štante, K.; Potočnik, J.; Rakuša, M. Vascular cognitive impairment and vascular dementia. Zdr. Vestn. 2017, 86, 331–345. [Google Scholar]
- Sadlonova, M.; Wasser, K.; Nagel, J.; Weber-Krüger, M.; Gröschel, S.; Uphaus, T.; Liman, J.; Hamann, G.F.; Kermer, P.; Gröschel, K.; et al. Health-related quality of life, anxiety and depression up to 12 months post-stroke: Influence of sex, age, stroke severity and atrial fibrillation—A longitudinal subanalysis of the Find-AFRANDOMISED trial. J. Psychosom. Res. 2021, 142, 110353. [Google Scholar] [CrossRef]
- Zhou, J.; Fangma, Y.; Chen, Z.; Zheng, Y. Post-Stroke Neuropsychiatric Complications: Types, Pathogenesis, and Therapeutic Intervention. Aging Dis. 2023, 14, 2127–2152. [Google Scholar] [CrossRef]
- Sobreiro, M.F.M.; Terroni, L.; Guajardo, V.D.; Mattos, P.F.; Leite, C.d.C.; Amaro, E.; Tinone, G.; Iosifescu, D.V.; Fraguas, R. The Impact of Post-Stroke Depressive Symptoms on Cognitive Performance in Women and in Men: A 4 Month Prospective Study. Life 2023, 13, 1554. [Google Scholar] [CrossRef]
- Lei, X.-Y.; Chen, L.-H.; Qian, L.-Q.; Lu, X.-D. Psychological interventions for post-stroke anxiety and depression: Current approaches and future perspectives. World J. Psychiatry 2025, 15, 103270. [Google Scholar] [CrossRef]
- Liu, L.; Xu, M.; Marshall, I.J.; Wolfe, C.D.A.; Wang, Y.; O’Connell, M.D. Prevalence and natural history of depression after stroke: A systematic review and meta-analysis of observational studies. PLoS Med. 2023, 20, e1004200. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Sun, H.; Jin, X.; Chen, B.; Zhou, J.; Zhao, J.; Liang, X.; Shen, W.; Zhang, Y.; Chan, P. Cognitive recovery in patients with post-stroke subjective cognitive complaints. Front. Neurol. 2022, 13, 977641. [Google Scholar] [CrossRef]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef]
- Butsing, N.; Zauszniewski, J.A.; Ruksakulpiwat, S.; Griffin, M.T.Q.; Niyomyart, A. Association between post-stroke depression and functional outcomes: A systematic review. PLoS ONE 2024, 19, e0309158. [Google Scholar] [CrossRef]
- Ignacio, K.H.D.; Muir, R.T.; Diestro, J.D.B.; Singh, N.; Yu, M.H.L.L.; El Omari, O.; Abdalrahman, R.; Barker-Collo, S.L.; Hackett, M.L.; Dukelow, S.P.; et al. Prevalence of depression and anxiety symptoms after stroke in young adults: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2024, 33, 107732. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zheng, Y.; Yuan, Z.; Guo, J.; Fan, C.; Li, C.; Deng, J.; Song, S.; Qiao, J.; Wang, J. The characteristics of brain network in patient with post-stroke depression under cognitive task condition. Front. Neurosci. 2023, 17, 1242543. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Geng, X.; Fan, F.; Fu, X.; He, S.; Li, T. The efficacy of therapies for post-stroke depression in aging: An umbrella review. Front. Aging Neurosci. 2022, 14, 993250. [Google Scholar] [CrossRef]
- Fischer, S.; Linseisen, J.; Kirchberger, I.; Zickler, P.; Ertl, M.; Naumann, M.; Meisinger, C. Association of post-stroke-depression and health-related quality of life three months after the stroke event. Results from the Stroke Cohort Augsburg (SCHANA) study. Psychol. Health Med. 2023, 28, 1148–1159. [Google Scholar] [CrossRef]
- Milosevich, E.; Kusec, A.; Pendlebury, S.T.; Demeyere, N. Domain-specific cognitive impairments, mood and quality of life 6 months after stroke. Disabil. Rehabil. 2025, 47, 435–444. [Google Scholar] [CrossRef]
- Quinn, T.J.; Richard, E.; Teuschl, Y.; Gattringer, T.; Hafdi, M.; O’Brien, J.T.; Merriman, N.; Gillebert, C.; Huygelier, H.; Verdelho, A.; et al. European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur. J. Neurol. 2021, 28, 3883–3920. [Google Scholar] [CrossRef]
- Potocnik, J.; Ovcar Stante, K.; Rakusa, M. The validity of the Montreal cognitive assessment (MoCA) for the screening of vascular cognitive impairment after ischemic stroke. Acta Neurol. Belg. 2020, 120, 681–685. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.; Qu, Y.; Zhang, Q. The trajectory of depression-related symptom clusters following stroke: A network and latent class analysis. J. Affect. Disord. 2025, 389, 119659. [Google Scholar] [CrossRef]
- Shi, Y.; Lenze, E.J.; Mohr, D.C.; Lee, J.-M.; Hu, L.; Metts, C.L.; Fong, M.W.M.; Wong, A.W.K. Post-stroke Depressive Symptoms and Cognitive Performances: A Network Analysis. Arch. Phys. Med. Rehabil. 2024, 105, 892–900. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.; Adams, H.P.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.T.L.; Hareendran, A.; Hendry, A.; Potter, J.; Bone, I.; Muir, K.W. Reliability of the modified Rankin Scale across multiple raters: Benefits of a structured interview. Stroke 2005, 36, 777–781. [Google Scholar] [CrossRef]
- Novak, A.; Vizjak, K.; Gacnik, A.; Rakusa, M. Cognitive impairment in people with epilepsy: Montreal Cognitive Assessment (MoCA) as a screening tool. Acta Neurol. Belg. 2023, 123, 451–456. [Google Scholar] [CrossRef]
- Lucza, T.; Karádi, K.; Kállai, J.; Weintraut, R.; Janszky, J.; Makkos, A.; Komoly, S.; Kovács, N. Screening Mild and Major Neurocognitive Disorders in Parkinson’s Disease. Behav. Neurol. 2015, 2015, 983606. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Dong, Y.; Zhu, R.; Jin, Z.; Li, Z.; Wang, Y.; Zhao, X.; Sachdev, P.; Zhang, W.; Wang, Y. Screening for cognitive impairment with the Montreal Cognitive Assessment in Chinese patients with acute mild stroke and transient ischaemic attack: A validation study. BMJ Open 2016, 6, e011310. [Google Scholar] [CrossRef]
- Johnson, L.A.; Cushing, B.; Rohlfing, G.; Edwards, M.; Davenloo, H.; D’Agostino, D.; Hall, J.R.; O’Bryant, S.E. The Hachinski Ischemic Scale and cognition: The influence of ethnicity. Age Ageing 2014, 43, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V. Optimizing the Hachinski Ischemic Scale. Arch. Neurol. 2012, 69, 169. [Google Scholar] [CrossRef]
- Bracken, B.A.; Keith, L.K. CAB, Clinical Assessment of Behavior: Professional Manual; PAR, Psychological Assessment Resources: Lutz, FL, USA, 2004. [Google Scholar]
- Berra, K.; Nguyen, C.; Bota, P. Comparison of self-rating of cognition and depression in patients with major depressive disorder. Ment. Illn. 2020, 12, 31–33. [Google Scholar] [CrossRef]
- Holmberg, J.; Jondell, B.; Abzhandadze, T.; Sunnerhagen, K.S. Very Early Cognitive Screening and Self-Reported Feeling of Fatigue Three Months After Stroke. Front. Hum. Neurosci. 2021, 15, 742105. [Google Scholar] [CrossRef]
- Hinkle, J.L.; Becker, K.J.; Kim, J.S.; Choi-Kwon, S.; Saban, K.L.; McNair, N.; Mead, G.E. Poststroke Fatigue: Emerging Evidence and Approaches to Management: A Scientific Statement for Healthcare Professionals From the American Heart Association. Stroke 2017, 48, e159–e170. [Google Scholar] [CrossRef]
- Etoom, M.; Battat MAl Hanafi, I.; Manocchio, N.; Foti, C.; Alghwiri, A. Post stroke fatigue: Analysis of subtypes and associated factors. J. Clin. Neurosci. 2025, 139, 111418. [Google Scholar] [CrossRef]
- Huang, H.; Lu, M.; Zhong, J.; Xu, Y.; Dong, Y.; Liu, X.; Sun, W. Prevalence, Trajectory, and Predictors of Poststroke Fatigue in Older Adults. Arch. Phys. Med. Rehabil. 2025, 106, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, L.; Sperber, C.; Guggisberg, A.G.; Kaller, C.P.; Heldner, M.R.; Monsch, A.U.; Hakim, A.; Silimon, N.; Fischer, U.; Arnold, M.; et al. Post-stroke cognitive impairment remains highly prevalent and disabling despite state-of-the-art stroke treatment. Int. J. Stroke 2024, 19, 888–897. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, D.; Zeng, Y.; Wu, W. Risk Factors for Post-stroke Depression: A Meta-analysis. Front. Aging Neurosci. 2017, 9, 218. [Google Scholar] [CrossRef]
- Chang Wong, E.; Chang Chui, H. Vascular Cognitive Impairment and Dementia. Continuum 2022, 28, 750–780. [Google Scholar] [CrossRef]
- Juárez-Belaúnde, A.; Soto-León, V.; Dileone, M.; Orcajo, E.; León-Álvarez, N.; Muñoz, A.; Tornero, J.; Oliviero, A. Early poststroke clinically significant fatigue predicts functional independence: A prospective longitudinal study. Front. Neurol. 2024, 15, 1364446. [Google Scholar] [CrossRef]
- Ghafar, M.Z.A.A.; Miptah, H.N.; O’Caoimh, R. Cognitive screening instruments to identify vascular cognitive impairment: A systematic review. Int. J. Geriatr. Psychiatry 2019, 34, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, M.; Ren, M.; Xu, W. The effects of educational background on Montreal Cognitive Assessment screening for vascular cognitive impairment, no dementia, caused by ischemic stroke. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2013, 20, 1406–1410. [Google Scholar] [CrossRef]
- Chaurasia, R.N.; Sharma, J.; Pathak, A.; Mishra, V.N.; Joshi, D. Poststroke Cognitive Decline: A Longitudinal Study from a Tertiary Care Center. J. Neurosci. Rural. Pract. 2019, 10, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Altendorff, S.; Healy, C.; Werring, D.J.; Cipolotti, L. The test accuracy of the Montreal Cognitive Assessment (MoCA) by stroke lateralisation. J. Neurol. Sci. 2017, 373, 100–104. [Google Scholar] [CrossRef]
- Towfighi, A.; Ovbiagele, B.; El Husseini, N.; Hackett, M.L.; Jorge, R.E.; Kissela, B.M.; Mitchell, P.H.; Skolarus, L.E.; Whooley, M.A.; Williams, L.S. Poststroke Depression: A Scientific Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2017, 48, e30–e43. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, P. Prevalence and Predictive factors of Post-Stroke Depression in Patients with Acute Cerebral Infarction. Alpha Psychiatry 2024, 25, 592–597. [Google Scholar] [CrossRef] [PubMed]
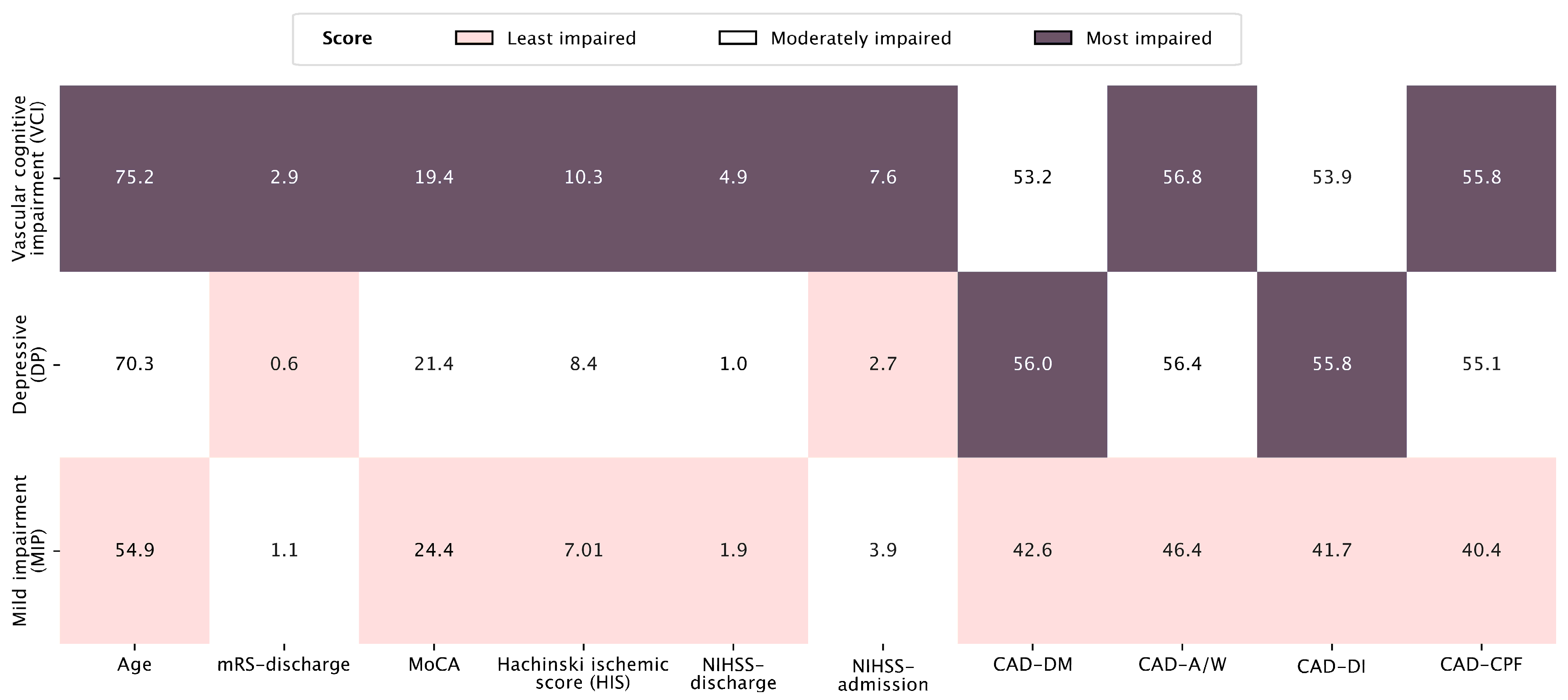
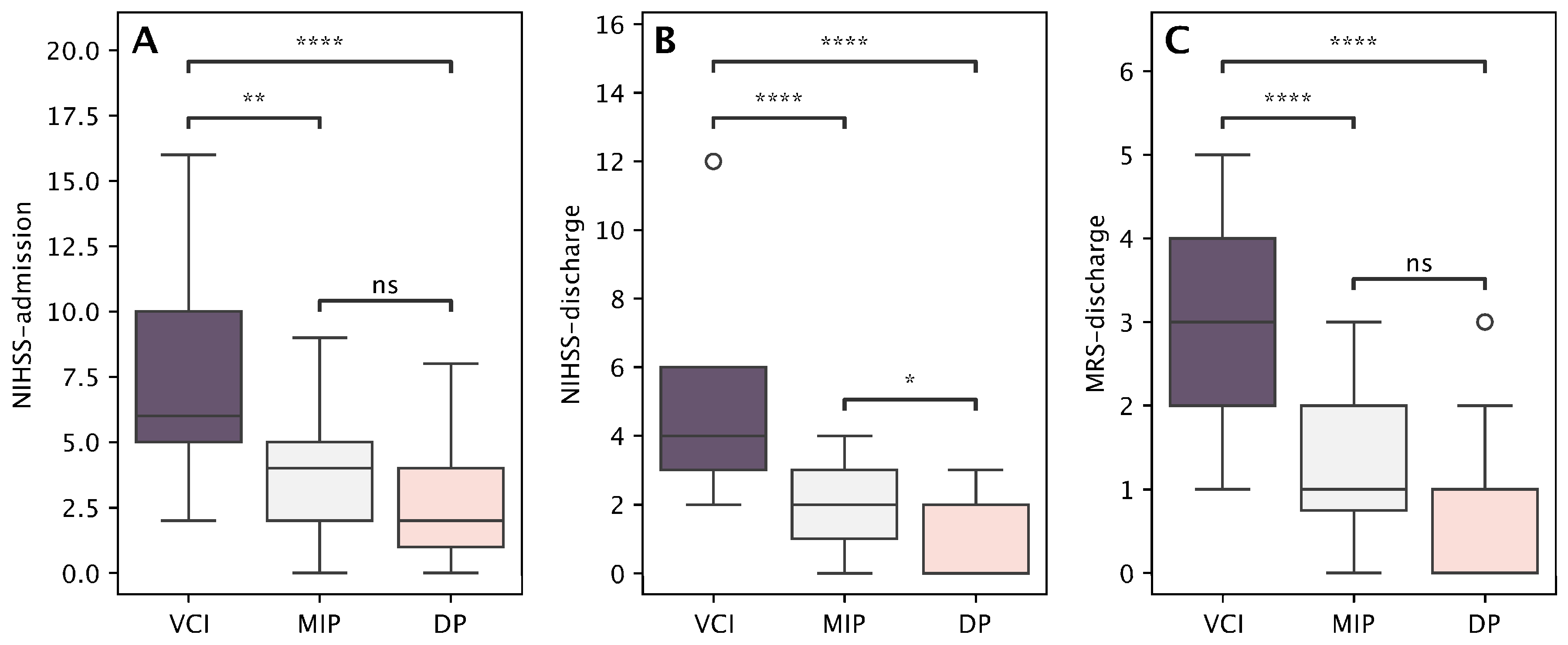
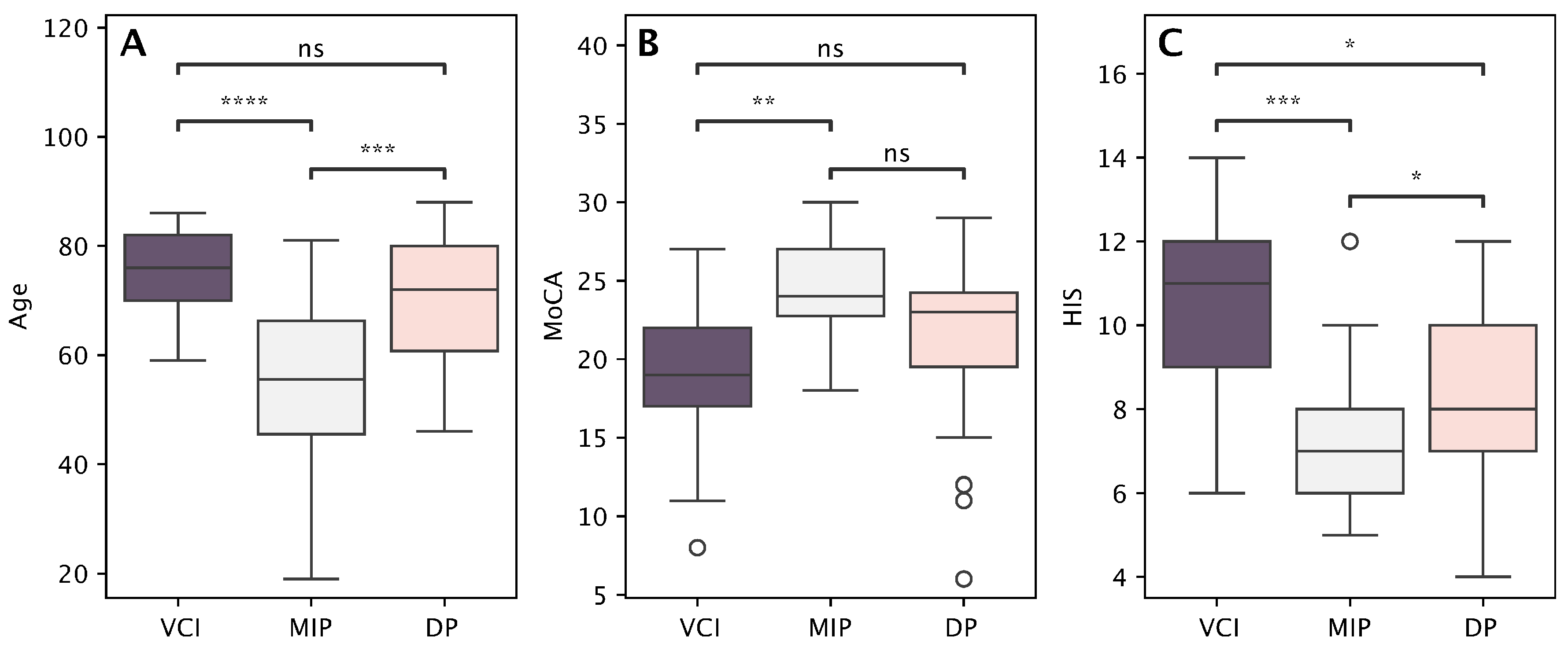
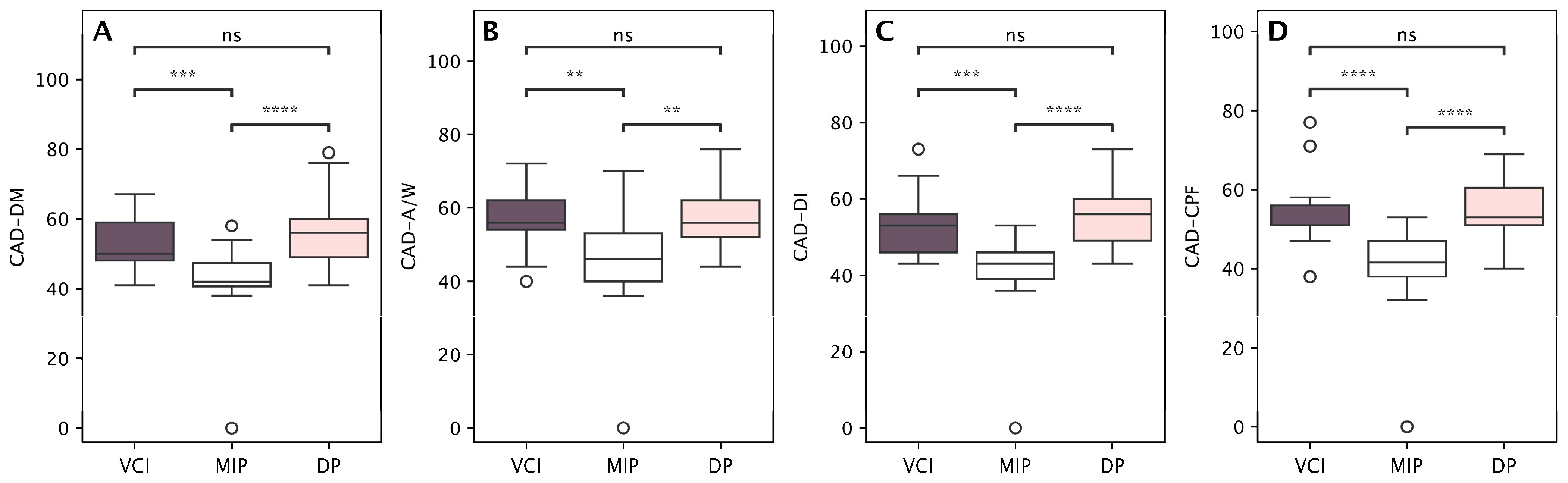
| Vascular Cognitive Impairment Profile (n = 17) | Depressive Profile (n = 28) | Mild Impairment Profile (n = 28) | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| Incidence | 23% | 38% | 38% | ||||
| Mean | SD | mean | SD | mean | SD | ||
| Age | 75.2 | 8.2 | 70.3 | 11.5 | 54.9 | 14.6 | <0.001 |
| Years of education | 11.4 | 2.7 | 11.5 | 2.4 | 12.4 | 2.0 | ns |
| NIHSS at admission | 7.6 | 4.4 | 2.7 | 2.1 | 3.9 | 2.3 | <0.001 |
| NIHSS at discharge | 4.9 | 2.3 | 1.0 | 1.2 | 1.9 | 1.2 | <0.001 |
| mRS at discharge | 2.9 | 1.1 | 0.6 | 0.8 | 1.1 | 0.9 | <0.001 |
| MoCA | 19.4 | 5.1 | 21.4 | 5.3 | 24.4 | 3.1 | 0.020 |
| HIS | 10.3 | 2.3 | 8.4 | 1.9 | 7.1 | 1.7 | <0.001 |
| CAD—DM | 53.2 | 7.5 | 56.0 | 9.1 | 42.6 | 9.8 | <0.001 |
| CAD—A/W | 56.8 | 7.6 | 56.4 | 8.0 | 46.4 | 12.4 | <0.001 |
| CAD—DI | 53.9 | 9.9 | 55.8 | 7.9 | 41.7 | 9.6 | <0.001 |
| CAD—CPF | 55.8 | 9.6 | 55.1 | 7.6 | 40.4 | 9.8 | <0.001 |
| Predictor | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept a | 22.5990 | 4.6902 | 4.818 | <0.001 |
| Age (years) | −0.1000 | 0.0386 | −2.592 | 0.012 |
| CAD-DM T score | −0.0112 | 0.0462 | −0.243 | 0.809 |
| Education (years) | 0.5847 | 0.2282 | 2.562 | 0.013 |
| HIS | −0.1564 | 0.2451 | −0.638 | 0.526 |
| NIHSS at admission | 0.1988 | 0.1950 | 1.019 | 0.312 |
| NIHSS at the discharge | −0.7180 | 0.3008 | −2.387 | 0.020 |
| Lesion location | ||||
| Left hemisphere—cerebellum | 1.6140 | 1.4718 | 1.097 | 0.277 |
| Right hemisphere—cerebellum | 2.5769 | 1.5239 | 1.691 | 0.096 |
| Predictor | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept a | 39.8294 | 13.930 | 2.8592 | 0.006 |
| Age (years) | 0.1143 | 0.109 | 1.0524 | 0.297 |
| Education (years) | 0.2460 | 0.647 | 0.3804 | 0.705 |
| HIS | 0.4714 | 0.662 | 0.7124 | 0.479 |
| NIHSS at admission | 0.0477 | 0.531 | 0.0898 | 0.929 |
| NIHSS at the discharge | −0.4314 | 0.847 | −0.5096 | 0.612 |
| MoCA | −0.0819 | 0.338 | −0.2427 | 0.809 |
| Lesion location | ||||
| Left hemisphere—cerebellum | −2.5795 | 4.001 | −0.6448 | 0.521 |
| Right hemisphere—cerebellum | −0.9311 | 4.207 | −0.2213 | 0.826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibic, M.; Miklič, L.; Rakusa, S.; Zmazek, J.; Menih, M.; Caf, K.; Rakusa, M. Cognitive and Emotional Impairments in Acute Post-Stroke Patients—A Cross-Sectional Study. Medicina 2025, 61, 1739. https://doi.org/10.3390/medicina61101739
Ibic M, Miklič L, Rakusa S, Zmazek J, Menih M, Caf K, Rakusa M. Cognitive and Emotional Impairments in Acute Post-Stroke Patients—A Cross-Sectional Study. Medicina. 2025; 61(10):1739. https://doi.org/10.3390/medicina61101739
Chicago/Turabian StyleIbic, Maja, Lara Miklič, Sofia Rakusa, Jan Zmazek, Marija Menih, Kim Caf, and Martin Rakusa. 2025. "Cognitive and Emotional Impairments in Acute Post-Stroke Patients—A Cross-Sectional Study" Medicina 61, no. 10: 1739. https://doi.org/10.3390/medicina61101739
APA StyleIbic, M., Miklič, L., Rakusa, S., Zmazek, J., Menih, M., Caf, K., & Rakusa, M. (2025). Cognitive and Emotional Impairments in Acute Post-Stroke Patients—A Cross-Sectional Study. Medicina, 61(10), 1739. https://doi.org/10.3390/medicina61101739








