The Emerging Role of the Cancerous Inhibitor of Protein Phosphatase 2A in Pulmonary Diseases
Abstract
1. Introduction
Global Impact of Respiratory Diseases
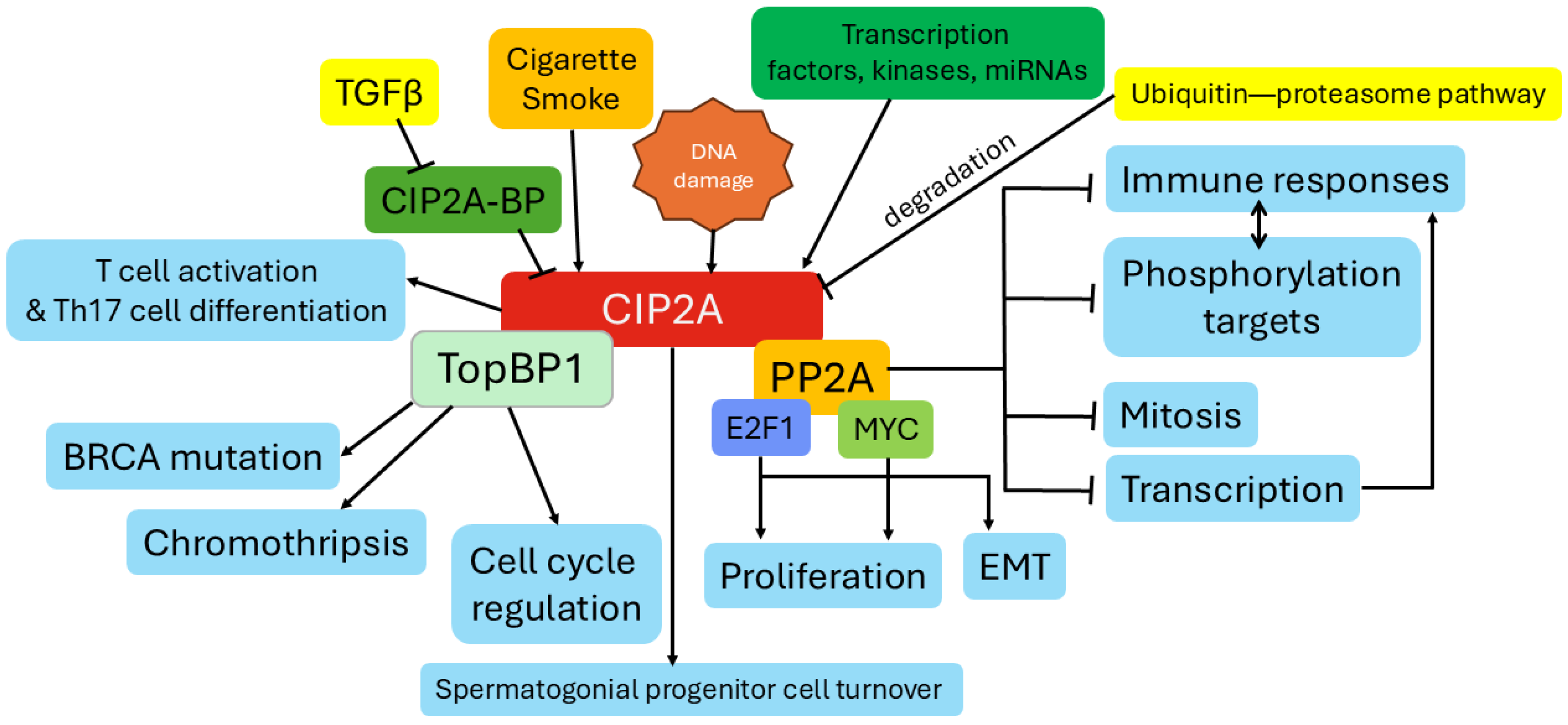
2. The Function of CIP2A
2.1. CIP2A Regulation of PP2A Activity
2.2. CIP2A Regulation of MYC Stabilization
2.3. CIP2A’s Role in Cell Cycle and Senescence
2.4. Emerging Evidence of CIP2A Regulation of EMT in Cancerous and Non-Cancerous Cells
2.5. CIP2A Signaling Linked to Immune Responses
2.6. Evidence for CIP2A Regulation of TGFβ Signaling
2.7. Other CIP2A Functions
3. Regulation of CIP2A Gene Expression and Protein Stabilization
3.1. CIP2A Transcriptional Regulation
3.2. CIP2A Protein Stability
4. CIP2A Expression in Pulmonary Diseases
4.1. Lung Cancer
4.2. Chronic Obstructive Pulmonary Disease (COPD)
4.3. CIP2A Responses in Fibrosis
4.4. CIP2A Regulation in Other Pulmonary Diseases
5. Therapeutic Targeting of CIP2A
5.1. Targeting CIP2A Expression
5.2. Targeting CIP2A Protein for Inactivation or Degradation
5.3. Indirect Targeting CIP2A Signaling
| Drug/Compound | Approval Status | Mode of Action | Therapeutic Relevance |
|---|---|---|---|
| Erlotinib (Tarceva®) | FDA-approved (NSCLC) | Reduces CIP2A levels, restoring PP2A activity, via inhibition of EGFR signaling [119] | Enhances sensitivity to EGFR-targeted therapies, overcoming resistance in NSCLC |
| Erlotinib derivatives, e.g., TD-19 and TD-52 | Experimental | Downregulation of CIP2A expression and p-AKT expression and increased PP2A activity; reduced EGFR involvement [124,125] | Inhibit CIP2A expression independent of EGFR |
| Bortezomib (Velcade®) | FDA-approved (Multiple Myeloma) | Downregulates CIP2A expression, reducing Akt activity [133,134] | Promotes apoptosis and tumor growth inhibition in hematologic cancers |
| Lapatinib (Tykerb®) | FDA-approved (Breast Cancer) | Inhibits CIP2A, leading to Akt inactivation [126] | Reduces tumor proliferation in ErbB2-positive breast cancer |
| Afatinib (Gilotrif) | FDA-approved (NSCLC) | Reduces Elk-1 binding to the CIP2A promoter and suppresses CIP2A expression [76] | Reduces tumor cell proliferation and triggers cell death, especially in tumors with EGFR mutations |
| Fingolimod (FTY720) | FDA-approved (Multiple Sclerosis) | Activates PP2A, potentially modulating CIP2A indirectly [144] | Shows promise in enhancing anticancer signaling and reducing inflammation |
| Celastrol | Experimental | Reduces CIP2A stability via proteasomal degradation [77] | Demonstrates antiproliferative effects in preclinical cancer models |
| FTY720 derivatives (e.g., FTY720-C2, FTY720-Mitoxy, FTY720 vinylphosphonate, and FTY720 methyl ether) | Experimental | Disrupt SET-PP2A complex, reducing CIP2A activity | Enhance PP2A activation, showing promise in preclinical cancer studies |
| Metformin | FDA-approved (Diabetes) | Reduces CIP2A levels through proteasomal degradation [137] | Potential application in neurodegenerative diseases and cancers with CIP2A involvement |
| Penfluridol (PF) | FDA-approved (Antipsychotic) | Directly targets CIP2A, promoting its degradation via the ubiquitin-proteasome pathway by enhancing CIP2A interaction with its E3 ligase VHL [131] | Shows potential in treating melanoma and metastases, particularly in the brain and lungs, by reactivating PP2A and disrupting oncogenic pathways like Akt and MYC |
| Temsirolimus + Cetuximab | FDA-approved (Temsirolimus: RCC, Cetuximab: EGFR+ cancers) | Temsirolimus suppresses CIP2A transcription and promotes its degradation via lysosomal autophagy; Cetuximab blocks EGFR activation, inhibiting downstream oncogenic signaling [127] | Synergistic effects demonstrated in colon cancer models, reducing tumor growth, inducing apoptosis, and improving patient outcomes by targeting CIP2A and EGFR pathways |
| Gambogenic acid | Experimental | Induces CIP2A degradation via the ubiquitin-proteasome pathway, inhibiting MYC and p-Akt signaling [133] | Enhances sensitivity to anticancer agents in hepatocellular carcinoma and potentiates bortezomib-induced apoptosis in multiple myeloma cells |
| Ethoxysanguinarine | Experimental | Inhibits CIP2A activity, disrupting oncogenic signaling pathways associated with CIP2A overexpression [4] | Derived from Macleaya cordata, shows potential as a CIP2A-targeting agent in preclinical studies for cancer treatment |
| Small molecular activators of PP2A, e.g., ATUX-792, DBK-1154, and ATUX-1215 | Experimental | Inhibit CIP2A expression in cancer cell lines; bind directly to PP2A, but the mechanism for the inhibition of CIP2A expression is unknown [99] | Potential utility in diseases where CIP2A is increased and PP2A activity is subdued |
| Tenuigenin | Experimental | Inhibits via CIP2A and NFκB signaling, via PP2A [12] | A bioactive ingredient from Polygala tenuifolia; has potential to reduce NFκB signaling and inflammation |
| Cucurbitacin B | Experimental | Promotes the lysosomal degradation of EGFR and the downregulation of the CIP2A/Akt and activation of PP2A [147] | A natural tetracyclic triterpenoid compound mainly found in Cucurbitaceae |
6. Systemic Targeting of CIP2A Responses
6.1. Limitations and Risks of Targeting CIP2A
6.2. Tolerability of CIP2A Inhibitors
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIP2A | Cancerous inhibitor of PP2A |
| PP2A | Protein phosphatase 2A |
| NSCLC | Non-small cell lung cancer |
| SCLC | Small cell lung cancer |
| COPD | Chronic obstructive pulmonary disease |
| EMT | Epithelial-to-mesenchymal transition |
| NFκB | Nuclear factor kappa B |
| IPF | Idiopathic pulmonary fibrosis |
| ALI | Acute lung injury |
| ARDS | Acute respiratory distress syndrome |
| CRD | Chronic respiratory diseases |
| DAPk | Death-associated protein kinase |
| ECM | Extracellular matrix |
| MAPK | Mitogen-activated protein kinases |
| JNK | c-Jun N-terminal kinase |
| AGK | Acylglycerol kinase |
| STAT3 | Signal transducer and activator of transcription 3 |
| LINC00665 | Long intergenic noncoding RNA 00665 |
| TAK1 | TGFβ-activated kinase-1 |
| PKM2 | Pyruvate kinase M2 |
| miRNA | microRNA |
| ATF | Activated transcription factor |
| TFCP2 | Transcription factor CP2 |
| HDAC1 | Histone deacetylase 1 |
| Oct4 | Octamer-binding transcription factor 4 |
| PLK1 | Polo-like kinase 1 |
| CREB | cAMP response element-binding protein |
| HBE | Human bronchial epithelial |
| RNAi | RNA interference |
| PF | Penfluridol |
| VHL | von Hippel–Lindau |
| GEA | Gambogenic acid |
| FTY720 | Fingolimod |
| Dab2 | Disabled-2 |
| SHC1 | Src homology domain-containing transforming protein 1 |
| eNOS | Endothelial nitric oxide synthase |
| TGFβ | Transforming growth factor beta |
| Akt | Protein kinase B |
| CDK | Cyclin-dependent kinase |
| IL | Interleukin |
| COVID-19 | Coronavirus disease 2019 |
| Plzf | Promyelocytic leukemia zinc finger |
| Oct-4 | Octamer-binding transcription factor 4 |
| B56α | PP2A regulatory subunit B′ alpha-isoform |
| UNC5H2 | unc-5 homolog B |
| P27kip1 | Cyclin-dependent kinase inhibitor 1B |
| MYC | Myelocytomatosis oncogene |
| CDC25C | Cell division cycle 25C |
| E2F1 | E2F transcription factor 1 |
| K-Ras | Kirsten rat sarcoma viral oncogene homolog |
| MEK | Mitogen-activated protein kinase |
| Slug | Zinc-finger protein SNAI2 |
| Snail | Zinc-finger transcription factor |
| ROS | Reactive oxygen species |
| CD | Cluster of differentiation |
| CIP2A-BP | CIP2A binding protein |
| Bcl2 | B-cell lymphoma 2 |
| ETS | ETS proto-oncogene, transcription factor |
| CHK1 | Checkpoint kinase 1 |
| SP1 | Specificity protein 1 |
| RNA-DPPA2 | mRNA transcript of the developmental pluripotency-associated 2 gene |
| cAMP | Cyclic adenosine monophosphate |
| PI3K | Phosphoinositide 3-kinase |
| EGFR | Epidermal growth factor receptor |
| TP53TG1 | Tumor protein p53 target gene 1 |
| TopBP1 | Topoisomerase II binding protein 1 |
| mTOR | Mechanistic target of rapamycin |
| MMP | Matrix metalloproteinase |
| FDA | Food and drug administration |
| HER2 | Human epidermal growth factor receptor 2 |
| SET | SE translocation |
| HbA1C | Hemoglobin A1c |
| APACHE II | Acute physiology and chronic health evaluation II |
References
- Junttila, M.R.; Puustinen, P.; Niemela, M.; Ahola, R.; Arnold, H.; Bottzauw, T.; Ala-aho, R.; Nielsen, C.; Ivaska, J.; Taya, Y.; et al. CIP2A inhibits PP2A in human malignancies. Cell 2007, 130, 51–62. [Google Scholar] [CrossRef]
- Nath, S.; Ohlmeyer, M.; Salathe, M.A.; Poon, J.; Baumlin, N.; Foronjy, R.F.; Geraghty, P. Chronic Cigarette Smoke Exposure Subdues PP2A Activity by Enhancing Expression of the Oncogene CIP2A. Am. J. Respir. Cell Mol. Biol. 2018, 59, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Das, D.N.; Puthusseri, B.; Gopu, V.; Krishnan, V.; Bhagavath, A.K.; Bolla, S.; Saini, Y.; Criner, G.J.; Marchetti, N.; Tang, H.; et al. Caveolin-1-derived peptide attenuates cigarette smoke-induced airway and alveolar epithelial injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2023, 325, L689–L708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhao, X.; Li, Y.; Zhang, B. CIP2A promotes bronchiolitis obliterans by activating the NF-κB pathway. Mol. Med. Report. 2025, 31, 108. [Google Scholar] [CrossRef]
- Ma, L.; Wen, Z.S.; Liu, Z.; Hu, Z.; Ma, J.; Chen, X.Q.; Liu, Y.Q.; Pu, J.X.; Xiao, W.L.; Sun, H.D.; et al. Overexpression and small molecule-triggered downregulation of CIP2A in lung cancer. PLoS ONE 2011, 6, e20159. [Google Scholar] [CrossRef]
- Dong, Q.Z.; Wang, Y.; Dong, X.J.; Li, Z.X.; Tang, Z.P.; Cui, Q.Z.; Wang, E.H. CIP2A is overexpressed in non-small cell lung cancer and correlates with poor prognosis. Ann. Surg. Oncol. 2011, 18, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xie, K.; Hu, H.; Yang, L.; Bai, Y. Expression and clinical significance of CIP2A in small cell lung cancer patients. Zhonghua Zhong Liu Za Zhi 2015, 37, 517–520. [Google Scholar]
- Guo, B.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Wang, Y.; Zhang, S.; Wu, R.; Lu, J.; et al. Micropeptide CIP2A-BP encoded by LINC00665 inhibits triple-negative breast cancer progression. EMBO J. 2020, 39, e102190. [Google Scholar] [CrossRef]
- Pillai, M.; Lafortune, P.; Dabo, A.; Yu, H.; Park, S.S.; Taluru, H.; Ahmed, H.; Bobrow, D.; Sattar, Z.; Jundi, B.; et al. Small-Molecule Activation of Protein Phosphatase 2A Counters Bleomycin-Induced Fibrosis in Mice. ACS Pharmacol. Transl. Sci. 2023, 6, 1659–1672. [Google Scholar] [CrossRef]
- Chang, Y.; Li, J.Y.; Jayakumar, T.; Hung, S.H.; Lee, W.C.; Manubolu, M.; Sheu, J.R.; Hsu, M.J. Ketamine, a Clinically Used Anesthetic, Inhibits Vascular Smooth Muscle Cell Proliferation via PP2A-Activated PI3K/Akt/ERK Inhibition. Int. J. Mol. Sci. 2017, 18, 2545. [Google Scholar] [CrossRef]
- Wang, Q. PP2A: A new link between peroxynitrite and endothelial barrier dysfunction? Cardiovasc. Res. 2009, 81, 5–6. [Google Scholar] [CrossRef]
- Yang, S.; Liu, S.; Dai, Z. Tenuigenin inhibits osteosarcoma growth via CIP2A/PP2A/NF-kappaB axis. Cancer Chemother. Pharmacol. 2024, 95, 15. [Google Scholar] [CrossRef]
- Bockelman, C.; Lassus, H.; Hemmes, A.; Leminen, A.; Westermarck, J.; Haglund, C.; Butzow, R.; Ristimaki, A. Prognostic role of CIP2A expression in serous ovarian cancer. Br. J. Cancer 2011, 105, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Come, C.; Laine, A.; Chanrion, M.; Edgren, H.; Mattila, E.; Liu, X.; Jonkers, J.; Ivaska, J.; Isola, J.; Darbon, J.M.; et al. CIP2A is associated with human breast cancer aggressivity. Clin. Cancer Res. 2009, 15, 5092–5100. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Alliance Against Chronic Respiratory Disease—About GARD. Available online: https://www.who.int/groups/global-alliance-against-chronic-respiratory-diseases-(gard) (accessed on 16 January 2025).
- European Respiratory Society. The economic burden of lung disease. In European Lung White Book; European Respiratory Society: Lausanne, Switzerland, 2025; Volume 2025. [Google Scholar]
- Ganti, A.K.; Klein, A.B.; Cotarla, I.; Seal, B.; Chou, E. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients with Non-Small Cell Lung Cancer in the US. JAMA Oncol. 2021, 7, 1824–1832. [Google Scholar] [CrossRef]
- WHO. WHO Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed on 16 January 2025).
- Anees Ur, R.; Ahmad Hassali, M.A.; Muhammad, S.A.; Shah, S.; Abbas, S.; Hyder Ali, I.A.B.; Salman, A. The economic burden of chronic obstructive pulmonary disease (COPD) in the USA, Europe, and Asia: Results from a systematic review of the literature. Expert Rev. Pharmacoecon. Outcomes Res. 2020, 20, 661–672. [Google Scholar] [CrossRef]
- Boers, E.; Barrett, M.; Su, J.G.; Benjafield, A.V.; Sinha, S.; Kaye, L.; Zar, H.J.; Vuong, V.; Tellez, D.; Gondalia, R.; et al. Global Burden of Chronic Obstructive Pulmonary Disease Through 2050. JAMA Netw. Open 2023, 6, e2346598. [Google Scholar] [CrossRef]
- CDC. Health Effects of Cigarettes: Chronic Obstructive Pulmonary Disease (COPD). Available online: https://www.cdc.gov/tobacco/about/cigarettes-and-copd.html (accessed on 16 January 2025).
- Hutchinson, J.; Fogarty, A.; Hubbard, R.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef]
- Zheng, Q.; Cox, I.A.; Campbell, J.A.; Xia, Q.; Otahal, P.; de Graaff, B.; Corte, T.J.; Teoh, A.K.Y.; Walters, E.H.; Palmer, A.J. Mortality and survival in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. ERJ Open Res. 2022, 8, 00591–2021. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Humbert, M.; Souza, R.; Idrees, M.; Kawut, S.M.; Sliwa-Hahnle, K.; Jing, Z.C.; Gibbs, J.S. A global view of pulmonary hypertension. Lancet Respir. Med. 2016, 4, 306–322. [Google Scholar] [CrossRef]
- Reinders, S.; Didden, E.M.; Ong, R. Survival, morbidity, and quality of life in pulmonary arterial hypertension patients: A systematic review of outcomes reported by population-based observational studies. Respir. Res. 2024, 25, 373. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Zheng, J.; Li, Y.; Kong, X.; Guo, J. Exploring immune-related pathogenesis in lung injury: Providing new insights Into ALI/ARDS. Biomed. Pharmacother. 2024, 175, 116773. [Google Scholar] [CrossRef]
- Nagase, T.; Kikuno, R.; Ishikawa, K.; Hirosawa, M.; Ohara, O. Prediction of the coding sequences of unidentified human genes. XVII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2000, 7, 143–150. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Jiang, C.; Ding, Y. PP2A-Mediated Anticancer Therapy. Gastroenterol. Res. Pract. 2013, 2013, 675429. [Google Scholar] [CrossRef] [PubMed]
- De Marco Zompit, M.; Esteban, M.T.; Mooser, C.; Adam, S.; Rossi, S.E.; Jeanrenaud, A.; Leimbacher, P.A.; Fink, D.; Shorrocks, A.K.; Blackford, A.N.; et al. The CIP2A-TOPBP1 complex safeguards chromosomal stability during mitosis. Nat. Commun. 2022, 13, 4143. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.; Nagelli, S.G.; Farrington, C.; Butt, U.; Cvrljevic, A.N.; Vainonen, J.P.; Feringa, F.M.; Gronroos, T.J.; Gautam, P.; Khan, S.; et al. CIP2A Interacts with TopBP1 and Drives Basal-Like Breast Cancer Tumorigenesis. Cancer Res. 2021, 81, 4319–4331. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.F.; Hu, Q.; Mazzagatti, A.; Valle-Inclan, J.E.; Maurais, E.G.; Dahiya, R.; Guyer, A.; Sanders, J.T.; Engel, J.L.; Nguyen, G.; et al. Mitotic clustering of pulverized chromosomes from micronuclei. Nature 2023, 618, 1041–1048. [Google Scholar] [CrossRef]
- Ventela, S.; Come, C.; Makela, J.A.; Hobbs, R.M.; Mannermaa, L.; Kallajoki, M.; Chan, E.K.; Pandolfi, P.P.; Toppari, J.; Westermarck, J. CIP2A promotes proliferation of spermatogonial progenitor cells and spermatogenesis in mice. PLoS ONE 2012, 7, e33209. [Google Scholar] [CrossRef]
- Schonthal, A.H. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 2001, 170, 1–13. [Google Scholar] [CrossRef]
- Wang, J.; Okkeri, J.; Pavic, K.; Wang, Z.; Kauko, O.; Halonen, T.; Sarek, G.; Ojala, P.M.; Rao, Z.; Xu, W.; et al. Oncoprotein CIP2A is stabilized via interaction with tumor suppressor PP2A/B56. EMBO Rep. 2017, 18, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Pavic, K.; Gupta, N.; Omella, J.D.; Derua, R.; Aakula, A.; Huhtaniemi, R.; Maatta, J.A.; Hofflin, N.; Okkeri, J.; Wang, Z.; et al. Structural mechanism for inhibition of PP2A-B56alpha and oncogenicity by CIP2A. Nat. Commun. 2023, 14, 1143. [Google Scholar] [CrossRef]
- Janssens, V.; Rebollo, A. The role and therapeutic potential of Ser/Thr phosphatase PP2A in apoptotic signalling networks in human cancer cells. Curr. Mol. Med. 2012, 12, 268–287. [Google Scholar] [CrossRef]
- Widau, R.C.; Jin, Y.; Dixon, S.A.; Wadzinski, B.E.; Gallagher, P.J. Protein phosphatase 2A (PP2A) holoenzymes regulate death-associated protein kinase (DAPK) in ceramide-induced anoikis. J. Biol. Chem. 2010, 285, 13827–13838. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.M.; Liu, C.Y.; Chang, K.C.; Chu, P.Y.; Shiau, C.W.; Chen, K.F. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 2012, 14, R68. [Google Scholar] [CrossRef]
- Guenebeaud, C.; Goldschneider, D.; Castets, M.; Guix, C.; Chazot, G.; Delloye-Bourgeois, C.; Eisenberg-Lerner, A.; Shohat, G.; Zhang, M.; Laudet, V.; et al. The dependence receptor UNC5H2/B triggers apoptosis via PP2A-mediated dephosphorylation of DAP kinase. Mol. Cell 2010, 40, 863–876. [Google Scholar] [CrossRef]
- Nader, C.P.; Cidem, A.; Verrills, N.M.; Ammit, A.J. Protein phosphatase 2A (PP2A): A key phosphatase in the progression of chronic obstructive pulmonary disease (COPD) to lung cancer. Respir. Res. 2019, 20, 222. [Google Scholar] [CrossRef]
- Yu, H.; Zaveri, S.; Sattar, Z.; Schaible, M.; Perez Gandara, B.; Uddin, A.; McGarvey, L.R.; Ohlmeyer, M.; Geraghty, P. Protein Phosphatase 2A as a Therapeutic Target in Pulmonary Diseases. Medicina 2023, 59, 1552. [Google Scholar] [CrossRef] [PubMed]
- Kauko, O.; Imanishi, S.Y.; Kulesskiy, E.; Yetukuri, L.; Laajala, T.D.; Sharma, M.; Pavic, K.; Aakula, A.; Rupp, C.; Jumppanen, M.; et al. Phosphoproteome and drug-response effects mediated by the three protein phosphatase 2A inhibitor proteins CIP2A, SET, and PME-1. J. Biol. Chem. 2020, 295, 4194–4211. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qiu, H.; Song, Y.; Liu, Y.; Wang, H.; Lu, M.; Deng, M.; Gu, Y.; Yin, J.; Luo, K.; et al. Cip2a promotes cell cycle progression in triple-negative breast cancer cells by regulating the expression and nuclear export of p27Kip1. Oncogene 2017, 36, 1952–1964. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Bockelman, C.; Hemmes, A.; Junttila, M.R.; Wiksten, J.P.; Lundin, M.; Junnila, S.; Murphy, D.J.; Evan, G.I.; Haglund, C.; et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J. Natl. Cancer Inst. 2009, 101, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Laine, A.; Sihto, H.; Come, C.; Rosenfeldt, M.T.; Zwolinska, A.; Niemela, M.; Khanna, A.; Chan, E.K.; Kahari, V.M.; Kellokumpu-Lehtinen, P.L.; et al. Senescence sensitivity of breast cancer cells is defined by positive feedback loop between CIP2A and E2F1. Cancer Discov. 2013, 3, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Soofiyani, S.R.; Hejazi, M.S.; Baradaran, B. The role of CIP2A in cancer: A review and update. Biomed. Pharmacother. 2017, 96, 626–633. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, L.; Zang, Y.; Ren, J.; Xu, Z. CIP2A Promotes Proliferation, Invasion and Chemoresistance to Cisplatin in Renal Cell Carcinoma. J. Cancer 2018, 9, 4029–4038. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, L.; Delgado, M.D.; Leon, J. MYC Oncogene Contributions to Release of Cell Cycle Brakes. Genes. 2019, 10, 244. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, H.; Qiao, L.; Zhang, W.; Zheng, J.; Zhao, W.; Chen, J.J.; Zhang, W. CIP2A facilitates the G1/S cell cycle transition via B-Myb in human papillomavirus 16 oncoprotein E6-expressing cells. J. Cell. Mol. Med. 2018, 22, 4150–4160. [Google Scholar] [CrossRef]
- Lei, N.; Peng, B.; Zhang, J.Y. CIP2A regulates cell proliferation via the AKT signaling pathway in human lung cancer. Oncol. Rep. 2014, 32, 1689–1694. [Google Scholar] [CrossRef]
- Puustinen, P.; Rytter, A.; Mortensen, M.; Kohonen, P.; Moreira, J.M.; Jaattela, M. CIP2A oncoprotein controls cell growth and autophagy through mTORC1 activation. J. Cell Biol. 2014, 204, 713–727. [Google Scholar] [CrossRef]
- Jeong, A.L.; Yang, Y. PP2A function toward mitotic kinases and substrates during the cell cycle. BMB Rep. 2013, 46, 289–294. [Google Scholar] [CrossRef]
- Sur, S.; Agrawal, D.K. Phosphatases and kinases regulating CDC25 activity in the cell cycle: Clinical implications of CDC25 overexpression and potential treatment strategies. Mol. Cell. Biochem. 2016, 416, 33–46. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, A.L.; Park, J.S.; Han, S.; Jang, C.Y.; Kim, K.I.; Kim, Y.; Park, J.H.; Lim, J.S.; Lee, M.S.; et al. IK-guided PP2A suppresses Aurora B activity in the interphase of tumor cells. Cell. Mol. Life Sci. 2016, 73, 3375–3386. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Hegazy, A.M.; Zhang, S.; Liu, X.; Tian, J.; Wu, J.; Shi, F.; Li, L.; Niu, X.; et al. Depletion of CIP2A inhibits the proliferation, migration, invasion and epithelial-mesenchymal transition of glioma cells. Brain Res. Bull. 2021, 173, 14–21. [Google Scholar] [CrossRef]
- Pang, X.; Fu, X.; Chen, S.; Zhu, X.; Qi, H.; Li, Y.; Li, F.; Tan, W. Overexpression of CIP2A promotes bladder cancer progression by regulating EMT. Clin. Transl. Oncol. 2016, 18, 289–295. [Google Scholar] [CrossRef]
- Wu, Y.; Gu, T.T.; Zheng, P.S. CIP2A cooperates with H-Ras to promote epithelial-mesenchymal transition in cervical-cancer progression. Cancer Lett. 2015, 356, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wang, Q.; Zeng, G.; Li, Q.; Jiang, T.; Zhang, Z.; Zheng, W.; Wang, K. Overexpression of CIP2A in clear cell renal cell carcinoma promotes cellular epithelial-mesenchymal transition and is associated with poor prognosis. Oncol. Rep. 2015, 34, 2515–2522. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fang, D.; Ou, X.; Sun, K.; Zhou, X.; Li, Y.; Shi, P.; Zhao, Z.; He, Y.; Peng, J.; Xu, J. m6A modification-mediated lncRNA TP53TG1 inhibits gastric cancer progression by regulating CIP2A stability. Cancer Sci. 2022, 113, 4135–4150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, T.; Ergashev, A.; Bo, Z.; Wang, J.; Shi, F.; Pan, Z.; Xie, H.; Chen, G.; Ma, F.; et al. CIP2A inhibitors TD52 and Ethoxysanguinarine promote macrophage autophagy and alleviates acute pancreatitis by modulating the AKT-mTOR pathway. Phytomedicine 2025, 136, 156263. [Google Scholar] [CrossRef]
- Zeng, R.; Jin, C.; Zheng, C.; Li, S.; Qian, S.; Pan, J.; Wang, L.; Zhao, J.; Qin, L. OCT4 Represses Inflammation and Cell Injury During Orchitis by Regulating CIP2A Expression. Front. Cell Dev. Biol. 2021, 9, 683209. [Google Scholar] [CrossRef]
- Shentu, Y.P.; Hu, W.T.; Zhang, Q.; Huo, Y.; Liang, J.W.; Liuyang, Z.Y.; Zhou, H.; Wei, H.; Ke, D.; Wang, X.C.; et al. CIP2A-promoted astrogliosis induces AD-like synaptic degeneration and cognitive deficits. Neurobiol. Aging 2019, 75, 198–208. [Google Scholar] [CrossRef]
- Peng, B.; Chai, Y.; Li, Y.; Liu, X.; Zhang, J. CIP2A overexpression induces autoimmune response and enhances JNK signaling pathway in human lung cancer. BMC Cancer 2015, 15, 895. [Google Scholar] [CrossRef]
- Come, C.; Cvrljevic, A.; Khan, M.M.; Treise, I.; Adler, T.; Aguilar-Pimentel, J.A.; Au-Yeung, B.; Sittig, E.; Laajala, T.D.; Chen, Y.; et al. CIP2A Promotes T-Cell Activation and Immune Response to Listeria monocytogenes Infection. PLoS ONE 2016, 11, e0152996. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ullah, U.; Khan, M.H.; Kong, L.; Moulder, R.; Valikangas, T.; Bhosale, S.D.; Komsi, E.; Rasool, O.; Chen, Z.; et al. CIP2A Constrains Th17 Differentiation by Modulating STAT3 Signaling. iScience 2020, 23, 100947. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Xie, Z.; Shen, J.; Jia, Y.; Duan, S. LINC00665: An Emerging Biomarker for Cancer Diagnostics and Therapeutics. Cells 2022, 11, 1540. [Google Scholar] [CrossRef]
- SHIMIZU, S.; TERAMACHI, J.; HARADA, T.; HIASA, M.; TENSHIN, H.; ODA, A.; SEKI, A.; INOUE, Y.; TANIMOTO, K.; HIGA, Y.; et al. Aberrant upregulation of the endogenous PP2A inhibitor CIP2A is vital for myeloma cell growth and survival. Int. J. Myeloma 2022, 12, 14–23. [Google Scholar]
- Liang, L.J.; Yang, F.Y.; Wang, D.; Zhang, Y.F.; Yu, H.; Wang, Z.; Sun, B.B.; Liu, Y.T.; Wang, G.Z.; Zhou, G.B. CIP2A induces PKM2 tetramer formation and oxidative phosphorylation in non-small cell lung cancer. Cell Discov. 2024, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Valikangas, T.; Khan, M.H.; Moulder, R.; Ullah, U.; Bhosale, S.D.; Komsi, E.; Butt, U.; Qiao, X.; Westermarck, J.; et al. Protein interactome of the Cancerous Inhibitor of protein phosphatase 2A (CIP2A) in Th17 cells. Curr. Res. Immunol. 2020, 1, 10–22. [Google Scholar] [CrossRef]
- Myant, K.; Qiao, X.; Halonen, T.; Come, C.; Laine, A.; Janghorban, M.; Partanen, J.I.; Cassidy, J.; Ogg, E.L.; Cammareri, P.; et al. Serine 62-Phosphorylated MYC Associates with Nuclear Lamins and Its Regulation by CIP2A Is Essential for Regenerative Proliferation. Cell Rep. 2015, 12, 1019–1031. [Google Scholar] [CrossRef]
- Nagelli, S.; Westermarck, J. CIP2A coordinates phosphosignaling, mitosis, and the DNA damage response. Trends Cancer 2024, 10, 52–64. [Google Scholar] [CrossRef]
- Tarek, M.M.; Yahia, A.; El-Nakib, M.M.; Elhefnawi, M. Integrative assessment of CIP2A overexpression and mutational effects in human malignancies identifies possible deleterious variants. Comput. Biol. Med. 2021, 139, 104986. [Google Scholar] [CrossRef]
- Chao, T.T.; Wang, C.Y.; Chen, Y.L.; Lai, C.C.; Chang, F.Y.; Tsai, Y.T.; Chao, C.H.; Shiau, C.W.; Huang, Y.C.; Yu, C.J.; et al. Afatinib induces apoptosis in NSCLC without EGFR mutation through Elk-1-mediated suppression of CIP2A. Oncotarget 2015, 6, 2164–2179. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Wen, Z.S.; Hu, Z.; Wu, F.Q.; Li, W.; Liu, J.; Zhou, G.B. Cancerous inhibitor of PP2A is targeted by natural compound celastrol for degradation in non-small-cell lung cancer. Carcinogenesis 2014, 35, 905–914. [Google Scholar] [CrossRef]
- Wang, X.; Gao, P.; Wang, M.; Liu, J.; Lin, J.; Zhang, S.; Zhao, Y.; Zhang, J.; Pan, W.; Sun, Z.; et al. Feedback between E2F1 and CIP2A regulated by human papillomavirus E7 in cervical cancer: Implications for prognosis. Am. J. Transl. Res. 2017, 9, 2327–2339. [Google Scholar]
- Wiegering, A.; Pfann, C.; Uthe, F.W.; Otto, C.; Rycak, L.; Mader, U.; Gasser, M.; Waaga-Gasser, A.M.; Eilers, M.; Germer, C.T. CIP2A influences survival in colon cancer and is critical for maintaining Myc expression. PLoS ONE 2013, 8, e75292. [Google Scholar] [CrossRef]
- Mathiasen, D.P.; Egebjerg, C.; Andersen, S.H.; Rafn, B.; Puustinen, P.; Khanna, A.; Daugaard, M.; Valo, E.; Tuomela, S.; Bottzauw, T.; et al. Identification of a c-Jun N-terminal kinase-2-dependent signal amplification cascade that regulates c-Myc levels in ras transformation. Oncogene 2012, 31, 390–401. [Google Scholar] [CrossRef][Green Version]
- Song, J.; Yan, S.; Ren, X. IDDF2023-ABS-0024 TFCP2 promotes EMT, invasion and metastasis of gastric cancer by regulating the expression of CIP2A. Gut 2023, 72, A61–A62. [Google Scholar] [CrossRef]
- Khanna, A.; Kauko, O.; Bockelman, C.; Laine, A.; Schreck, I.; Partanen, J.I.; Szwajda, A.; Bormann, S.; Bilgen, T.; Helenius, M.; et al. Chk1 targeting reactivates PP2A tumor suppressor activity in cancer cells. Cancer Res. 2013, 73, 6757–6769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Ma, S.; Zhang, N.; Yang, D.; Wang, L.; Ye, S.; Zhang, Q.; Ruan, J.; Ma, J.; et al. ChK1 activation induces reactive astrogliosis through CIP2A/PP2A/STAT3 pathway in Alzheimer’s disease. FASEB J. 2022, 36, e22209. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.M.; Harris, R.J.; Holcroft, A.K.; Scott, L.J.; Carmell, N.; McDonald, E.; Polydoros, F.; Clark, R.E. Second generation tyrosine kinase inhibitors prevent disease progression in high-risk (high CIP2A) chronic myeloid leukaemia patients. Leukemia 2015, 29, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Balliu, M.; Cellai, C.; Lulli, M.; Laurenzana, A.; Torre, E.; Vannucchi, A.M.; Paoletti, F. HDAC1 controls CIP2A transcription in human colorectal cancer cells. Oncotarget 2016, 7, 25862–25871. [Google Scholar] [CrossRef]
- Brink, H.J.; van Senten, J.R.; De Vries-van Leeuwen, I.J.; da Costa Pereira, D.; Piersma, S.R.; Jimenez, C.R.; Centorrino, F.; Ottmann, C.; Siderius, M.; Smit, M.J.; et al. Fusicoccin-A Targets Cancerous Inhibitor of Protein Phosphatase 2A by Stabilizing a C-Terminal Interaction with 14-3-3. ACS Chem. Biol. 2022, 17, 2972–2978. [Google Scholar] [CrossRef]
- Jung, H.M.; Patel, R.S.; Phillips, B.L.; Wang, H.; Cohen, D.M.; Reinhold, W.C.; Chang, L.J.; Yang, L.J.; Chan, E.K. Tumor suppressor miR-375 regulates MYC expression via repression of CIP2A coding sequence through multiple miRNA-mRNA interactions. Mol. Biol. Cell 2013, 24, 1638–1648. [Google Scholar] [CrossRef]
- Liu, X.; Chai, Y.; Li, J.; Ren, P.; Liu, M.; Dai, L.; Qian, W.; Li, W.; Zhang, J.Y. Autoantibody response to a novel tumor-associated antigen p90/CIP2A in breast cancer immunodiagnosis. Tumour Biol. 2014, 35, 2661–2667. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y. miR-548b-3p Regulates Proliferation, Apoptosis, and Mitochondrial Function by Targeting CIP2A in Hepatocellular Carcinoma. Biomed. Res. Int. 2018, 2018, 7385426. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gao, X.; Zang, S.; Li, Y.; Feng, X.; Yuan, X. MicroRNA-383-5p acts as a prognostic marker and inhibitor of cell proliferation in lung adenocarcinoma by cancerous inhibitor of protein phosphatase 2A. Oncol. Lett. 2017, 14, 3573–3579. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chen, D.; Luo, K.; Lu, M.; Gu, Y.; Zeng, S.; Chen, X.; Song, Y.; Zhang, Z.; Zheng, G.; et al. Cip2a/miR-301a feedback loop promotes cell proliferation and invasion of triple-negative breast cancer. J. Cancer 2019, 10, 5964–5974. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Bu, X.; Kan, A.; Luo, L.; Xu, Y.; Chen, H.; Lin, X.; Lai, Z.; Wen, D.; Huang, L.; et al. SP1-induced lncRNA DUBR promotes stemness and oxaliplatin resistance of hepatocellular carcinoma via E2F1-CIP2A feedback. Cancer Lett. 2022, 528, 16–30. [Google Scholar] [CrossRef]
- Sung, W.W.; Wang, Y.C.; Lin, P.L.; Cheng, Y.W.; Chen, C.Y.; Wu, T.C.; Lee, H. IL-10 promotes tumor aggressiveness via upregulation of CIP2A transcription in lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 4092–4103. [Google Scholar] [CrossRef]
- Ventela, S.; Sittig, E.; Mannermaa, L.; Makela, J.A.; Kulmala, J.; Loyttyniemi, E.; Strauss, L.; Carpen, O.; Toppari, J.; Grenman, R.; et al. CIP2A is an Oct4 target gene involved in head and neck squamous cell cancer oncogenicity and radioresistance. Oncotarget 2015, 6, 144–158. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Z.; Deng, J.; Liu, J.; Ma, K.; Si, Y.; Zhang, T.; Feng, T.; Liu, Y.; Tan, Y. Polyphyllin I inhibits invasion and epithelial-mesenchymal transition via CIP2A/PP2A/ERK signaling in prostate cancer. Int. J. Oncol. 2018, 53, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.W.; Pan, J.H.; Cai, Y.X.; Rupa, D.; Huang, T.S.; Kuo, T.C.; Lin, C.W.; Chen, C.W.; Lin, C.C.; Lee, H.S.; et al. Reciprocal regulation of CIP2A and AR expression in prostate cancer cells. Discov. Oncol. 2022, 13, 87. [Google Scholar] [CrossRef]
- Choi, Y.A.; Koo, J.S.; Park, J.S.; Park, M.Y.; Jeong, A.L.; Oh, K.S.; Yang, Y. Estradiol enhances CIP2A expression by the activation of p70 S6 kinase. Endocr. Relat. Cancer 2014, 21, 189–202. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, E.J.; Oh, J.S.; Park, I.C.; Hwang, S.G. CIP2A modulates cell-cycle progression in human cancer cells by regulating the stability and activity of Plk1. Cancer Res. 2013, 73, 6667–6678. [Google Scholar] [CrossRef]
- Bownes, L.V.; Marayati, R.; Quinn, C.H.; Beierle, A.M.; Hutchins, S.C.; Julson, J.R.; Erwin, M.H.; Stewart, J.E.; Mroczek-Musulman, E.; Ohlmeyer, M.; et al. Pre-Clinical Study Evaluating Novel Protein Phosphatase 2A Activators as Therapeutics for Neuroblastoma. Cancers 2022, 14, 1952. [Google Scholar] [CrossRef]
- Cazzoli, R.; Romeo, F.; Pallavicini, I.; Peri, S.; Romanenghi, M.; Perez-Valencia, J.A.; Hagag, E.; Ferrucci, F.; Elgendy, M.; Vittorio, O.; et al. Endogenous PP2A inhibitor CIP2A degradation by chaperone-mediated autophagy contributes to the antitumor effect of mitochondrial complex I inhibition. Cell Rep. 2023, 42, 112616. [Google Scholar] [CrossRef]
- Khanna, A.; Pimanda, J.E.; Westermarck, J. Cancerous inhibitor of protein phosphatase 2A, an emerging human oncoprotein and a potential cancer therapy target. Cancer Res. 2013, 73, 6548–6553. [Google Scholar] [CrossRef]
- Chen, B.; Hu, H.; Chen, X. From Basic Science to Clinical Practice: The Role of Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A)/p90 in Cancer. Front. Genet. 2023, 14, 1110656. [Google Scholar] [CrossRef] [PubMed]
- Chun-Yu, L.; Tzu-Ting, H.; Yi-Ting, C.; Ji-Lin, C.; Pei-Yi, C.; Chun-Teng, H.; Wan-Lun, W.; Ka-Yi, L.; Ming-Shen, D.; Chung-Wai, S.; et al. Targeting SET to restore PP2A activity disrupts an oncogenic CIP2A-feedforward loop and impairs triple negative breast cancer progression. eBioMedicine 2019, 40, 263–275. [Google Scholar] [CrossRef]
- Feng, F.; Cheng, P.; Wang, C.; Wang, Y.; Wang, W. Polyphyllin I and VII potentiate the chemosensitivity of A549/DDP cells to cisplatin by enhancing apoptosis, reversing EMT and suppressing the CIP2A/AKT/mTOR signaling axis. Oncol. Lett. 2019, 18, 5428–5436. [Google Scholar] [CrossRef]
- Doherty, D.F.; Nath, S.; Poon, J.; Foronjy, R.F.; Ohlmeyer, M.; Dabo, A.J.; Salathe, M.; Birrell, M.; Belvisi, M.; Baumlin, N.; et al. Protein Phosphatase 2A Reduces Cigarette Smoke-induced Cathepsin S and Loss of Lung Function. Am. J. Respir. Crit. Care Med. 2019, 200, 51–62. [Google Scholar] [CrossRef]
- Soto, B.; Ahmed, H.; Pillai, M.; Park, S.S.; Ploszaj, M.; Reece, J.; Taluru, H.; Bobrow, D.; Yu, H.; Lafortune, P.; et al. Evaluating Novel Protein Phosphatase 2A Activators as Therapeutics for Emphysema. Am. J. Respir. Cell Mol. Biol. 2023, 69, 533–544. [Google Scholar] [CrossRef]
- Nair, P.M.; Starkey, M.R.; Haw, T.J.; Liu, G.; Collison, A.M.; Mattes, J.; Wark, P.A.; Morris, J.C.; Verrills, N.M.; Clark, A.R.; et al. Enhancing tristetraprolin activity reduces the severity of cigarette smoke-induced experimental chronic obstructive pulmonary disease. Clin. Transl. Immunol. 2019, 8, e01084. [Google Scholar] [CrossRef]
- Geraghty, P.; Eden, E.; Pillai, M.; Campos, M.; McElvaney, N.G.; Foronjy, R.F. alpha1-Antitrypsin activates protein phosphatase 2A to counter lung inflammatory responses. Am. J. Respir. Crit. Care Med. 2014, 190, 1229–1242. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Shen, Q.; Ouyang, X.; Zhou, Z.; Luo, H.; Peng, H. CSE regulates LINC000665/XBP-1 in the progress of pulmonary fibrosis. Tob. Induc. Dis. 2023, 21, 170. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Seeman, J.; Hong, J.; Hergert, P.; Bodem, V.; Jessurun, J.; Smith, K.; Nho, R.; Kahm, J.; Gaillard, P.; et al. Low α2β1 integrin function enhances the proliferation of fibroblasts from patients with idiopathic pulmonary fibrosis by activation of the β-catenin pathway. Am. J. Pathol. 2012, 181, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Collison, A.M.; Li, J.; de Siqueira, A.P.; Lv, X.; Toop, H.D.; Morris, J.C.; Starkey, M.R.; Hansbro, P.M.; Zhang, J.; Mattes, J. TRAIL signals through the ubiquitin ligase MID1 to promote pulmonary fibrosis. BMC Pulm. Med. 2019, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Sun, X.; Chen, X.; Wei, K.; Luo, D.; Yang, S.; Zhang, C.; Xu, J.; Deng, Z. CIP2A promotes inflammation and exacerbates osteoarthritis by targeting CEMIP. Cell. Mol. Biol. Lett. 2025, 30, 67. [Google Scholar] [CrossRef]
- Ji, N.; Chen, Z.; Wang, Z.; Sun, W.; Yuan, Q.; Zhang, X.; Jia, X.; Wu, J.; Jiang, J.; Song, M.; et al. LincR-PPP2R5C Promotes Th2 Cell Differentiation Through PPP2R5C/PP2A by Forming an RNA-DNA Triplex in Allergic Asthma. Allergy Asthma Immunol. Res. 2024, 16, 71–90. [Google Scholar] [CrossRef]
- Reza, M.I.; Kumar, A.; Pabelick, C.M.; Britt, R.D., Jr.; Prakash, Y.S.; Sathish, V. Downregulation of protein phosphatase 2Aalpha in asthmatic airway smooth muscle. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 326, L651–L659. [Google Scholar] [CrossRef]
- Theoharis, C.T.; Konstantinos-Dionysios, A.; Asimenia, A.; Danae-Anastasia, D.; Nikolaos, S.; Bodi, Z.; Shahrzad, A.; Magdalini, V.; Zuyi, W.; Alexandra, M.; et al. Mast cells and inflammation. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Kranias, G.; Watt, L.F.; Carpenter, H.; Holst, J.; Ludowyke, R.; Strack, S.; Sim, A.T.; Verrills, N.M. Protein phosphatase 2A carboxymethylation and regulatory B subunits differentially regulate mast cell degranulation. Cell. Signal. 2010, 22, 1882–1890. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Mercado, N.; Barnes, P.J.; Ito, K. Defects of protein phosphatase 2A causes corticosteroid insensitivity in severe asthma. PLoS ONE 2011, 6, e27627. [Google Scholar] [CrossRef]
- Nair, P.M.; Starkey, M.R.; Haw, T.J.; Liu, G.; Horvat, J.C.; Morris, J.C.; Verrills, N.M.; Clark, A.R.; Ammit, A.J.; Hansbro, P.M. Targeting PP2A and proteasome activity ameliorates features of allergic airway disease in mice. Allergy 2017, 72, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, J.; Hahn, W.C. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol. Med. 2008, 14, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wu, G.; Wang, X.; Zou, X.; Zhang, G.; Xiao, R.; Yuan, Y.; Long, D.; Yang, J.; Wu, Y.; et al. CIP2A is a predictor of survival and a novel therapeutic target in bladder urothelial cell carcinoma. Med. Oncol. 2013, 30, 406. [Google Scholar] [CrossRef]
- Mendelsohn, J.; Baselga, J. Epidermal growth factor receptor targeting in cancer. Semin. Oncol. 2006, 33, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.; Wolf, J.L.; Rusk, J.; Beard, S.E.; Clark, G.M.; Witt, K.; Cagnoni, P.J. Effects of smoking on the pharmacokinetics of erlotinib. Clin. Cancer Res. 2006, 12, 2166–2171. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chao, T.T.; Chang, F.Y.; Chen, Y.L.; Tsai, Y.T.; Lin, H.I.; Huang, Y.C.; Shiau, C.W.; Yu, C.J.; Chen, K.F. CIP2A mediates erlotinib-induced apoptosis in non-small cell lung cancer cells without EGFR mutation. Lung Cancer 2014, 85, 152–160. [Google Scholar] [CrossRef]
- Chao, T.T.; Wang, C.Y.; Lai, C.C.; Chen, Y.L.; Tsai, Y.T.; Chen, P.T.; Lin, H.I.; Huang, Y.C.; Shiau, C.W.; Yu, C.J.; et al. TD-19, an erlotinib derivative, induces epidermal growth factor receptor wild-type nonsmall-cell lung cancer apoptosis through CIP2A-mediated pathway. J. Pharmacol. Exp. Ther. 2014, 351, 352–358. [Google Scholar] [CrossRef]
- Yu, H.C.; Hung, M.H.; Chen, Y.L.; Chu, P.Y.; Wang, C.Y.; Chao, T.T.; Liu, C.Y.; Shiau, C.W.; Chen, K.F. Erlotinib derivative inhibits hepatocellular carcinoma by targeting CIP2A to reactivate protein phosphatase 2A. Cell Death Dis. 2014, 5, e1359. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hu, M.H.; Hsu, C.J.; Huang, C.T.; Wang, D.S.; Tsai, W.C.; Chen, Y.T.; Lee, C.H.; Chu, P.Y.; Hsu, C.C.; et al. Lapatinib inhibits CIP2A/PP2A/p-Akt signaling and induces apoptosis in triple negative breast cancer cells. Oncotarget 2016, 7, 9135–9149. [Google Scholar] [CrossRef]
- Wang, H.-W.; Yang, S.-H.; Huang, G.-D.; Lin, J.-K.; Chen, W.-S.; Jiang, J.-K.; Lan, Y.-T.; Lin, C.-C.; Hwang, W.-L.; Tzeng, C.-H.; et al. Temsirolimus enhances the efficacy of cetuximab in colon cancer through a CIP2A-dependent mechanism. J. Cancer Res. Clin. Oncol. 2014, 140, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Reungwetwattana, T.; Molina, J.R.; Mandrekar, S.J.; Allen-Ziegler, K.; Rowland, K.M.; Reuter, N.F.; Luyun, R.F.; Dy, G.K.; Marks, R.S.; Schild, S.E.; et al. Brief report: A phase II “window-of-opportunity” frontline study of the MTOR inhibitor, temsirolimus given as a single agent in patients with advanced NSCLC, an NCCTG study. J. Thorac. Oncol. 2012, 7, 919–922. [Google Scholar] [CrossRef]
- Zhao, M.; Howard, E.W.; Parris, A.B.; Guo, Z.; Zhao, Q.; Ma, Z.; Xing, Y.; Liu, B.; Edgerton, S.M.; Thor, A.D.; et al. Activation of cancerous inhibitor of PP2A (CIP2A) contributes to lapatinib resistance through induction of CIP2A-Akt feedback loop in ErbB2-positive breast cancer cells. Oncotarget 2017, 8, 58847–58864. [Google Scholar] [CrossRef] [PubMed]
- Saafan, H.; Alahdab, A.; Michelet, R.; Gohlke, L.; Ziemann, J.; Holdenrieder, S.; McLaughlin, K.-M.; Wass, M.N.; Cinatl, J.; Michaelis, M.; et al. Constitutive Cell Proliferation Regulating Inhibitor of Protein Phosphatase 2A (CIP2A) Mediates Drug Resistance to Erlotinib in an EGFR Activating Mutated NSCLC Cell Line. Cells 2021, 10, 716. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, J.; Ai, M.; Zhang, T.; Ming, Y.; Li, C.; Pu, W.; Yang, Y.; Li, Z.; Qi, Y.; et al. Penfluridol inhibits melanoma growth and metastasis through enhancing von Hippel–Lindau tumor suppressor-mediated cancerous inhibitor of protein phosphatase 2A (CIP2A) degradation. MedComm 2024, 5, e758. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, L.; Wen, Z.S.; Cheng, Y.X.; Zhou, G.B. Ethoxysanguinarine Induces Inhibitory Effects and Downregulates CIP2A in Lung Cancer Cells. ACS Med. Chem. Lett. 2014, 5, 113–118. [Google Scholar] [CrossRef][Green Version]
- Yu, X.J.; Zhao, Q.; Wang, X.B.; Zhang, J.X.; Wang, X.B. Gambogenic acid induces proteasomal degradation of CIP2A and sensitizes hepatocellular carcinoma to anticancer agents. Oncol. Rep. 2016, 36, 3611–3618. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, H.; Liu, P.; Wu, X.; Chen, B. Gambogenic acid synergistically potentiates bortezomib-induced apoptosis in multiple myeloma cells. J. Cancer 2017, 8, 839–849. [Google Scholar] [CrossRef]
- Yu, B.; Qiao, J.; Shen, Y.; Li, L. Protective effects of tenuigenin on Staphylococcus aureus-induced pneumonia in mice. Microb. Pathog. 2017, 110, 385–389. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes Medications as Monotherapy or Metformin-Based Combination Therapy for Type 2 Diabetes: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef]
- Elgendy, M.; Ciro, M.; Hosseini, A.; Weiszmann, J.; Mazzarella, L.; Ferrari, E.; Cazzoli, R.; Curigliano, G.; DeCensi, A.; Bonanni, B.; et al. Combination of Hypoglycemia and Metformin Impairs Tumor Metabolic Plasticity and Growth by Modulating the PP2A-GSK3beta-MCL-1 Axis. Cancer Cell 2019, 35, 798–815.e5. [Google Scholar] [CrossRef] [PubMed]
- Hitchings, A.W.; Lai, D.; Jones, P.W.; Baker, E.H. Metformin in severe exacerbations of chronic obstructive pulmonary disease: A randomised controlled trial. Thorax 2016, 71, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Liu, F.; Liu, J.; Xu, J.; Wu, Q.; Li, X. The effect of metformin on lung cancer risk and survival in patients with type 2 diabetes mellitus: A meta-analysis. J. Clin. Pharm. Ther. 2020, 45, 783–792. [Google Scholar] [CrossRef]
- Saylor, J.; Derian, N.; Lamichhane, R.; Megri, M.A. Respiratory outcomes of metformin users in patients with copd: A retrospective cohort study. Chest 2024, 166, A4646. [Google Scholar] [CrossRef]
- Liang, Z.; Yang, M.; Xu, C.; Zeng, R.; Dong, L. Effects and safety of metformin in patients with concurrent diabetes mellitus and chronic obstructive pulmonary disease: A systematic review and meta-analysis. Endocr. Connect. 2022, 11, e220289. [Google Scholar] [CrossRef] [PubMed]
- Stafman, L.L.; Williams, A.P.; Marayati, R.; Aye, J.M.; Stewart, J.E.; Mroczek-Musulman, E.; Beierle, E.A. PP2A activation alone and in combination with cisplatin decreases cell growth and tumor formation in human HuH6 hepatoblastoma cells. PLoS ONE 2019, 14, e0214469. [Google Scholar] [CrossRef]
- Kauko, O.; O’Connor, C.M.; Kulesskiy, E.; Sangodkar, J.; Aakula, A.; Izadmehr, S.; Yetukuri, L.; Yadav, B.; Padzik, A.; Laajala, T.D.; et al. PP2A inhibition is a druggable MEK inhibitor resistance mechanism in KRAS-mutant lung cancer cells. Sci. Transl. Med. 2018, 10, eaaq1093. [Google Scholar] [CrossRef]
- Luque, M.; Cristobal, I.; Sanz-Alvarez, M.; Santos, A.; Zazo, S.; Eroles, P.; Arpi, O.; Rovira, A.; Albanell, J.; Madoz-Gurpide, J.; et al. CIP2A as a Key Regulator for AKT Phosphorylation Has Partial Impact Determining Clinical Outcome in Breast Cancer. J. Clin. Med. 2022, 11, 1610. [Google Scholar] [CrossRef]
- Chen, K.C.; Chen, H.Y.; Chen, C.Y. Potential Protein Phosphatase 2A Agents from Traditional Chinese Medicine against Cancer. Evid. Based Complement. Alternat. Med. 2014, 2014, 436863. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.; Peng, R.; Lee, Y.; Lee, Z.; Liu, Y. Efficacy of Xuebijing injection on pulmonary ventilation improvement in acute pancreatitis: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1549419. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiang, Y.; Liu, X.; Zhang, T.; Yang, R.; Chen, S.; Xu, L.; Yu, Q.; Zhao, H.; Zhang, L.; et al. Cucurbitacin B Induces the Lysosomal Degradation of EGFR and Suppresses the CIP2A/PP2A/Akt Signaling Axis in Gefitinib-Resistant Non-Small Cell Lung Cancer. Molecules 2019, 24, 647. [Google Scholar] [CrossRef]
- Adam, S.; Rossi, S.E.; Moatti, N.; De Marco Zompit, M.; Xue, Y.; Ng, T.F.; Alvarez-Quilon, A.; Desjardins, J.; Bhaskaran, V.; Martino, G.; et al. The CIP2A-TOPBP1 axis safeguards chromosome stability and is a synthetic lethal target for BRCA-mutated cancer. Nat. Cancer 2021, 2, 1357–1371. [Google Scholar] [CrossRef]
- Sacharidou, A.; Chambliss, K.L.; Ulrich, V.; Salmon, J.E.; Shen, Y.M.; Herz, J.; Hui, D.Y.; Terada, L.S.; Shaul, P.W.; Mineo, C. Antiphospholipid antibodies induce thrombosis by PP2A activation via apoER2-Dab2-SHC1 complex formation in endothelium. Blood 2018, 131, 2097–2110. [Google Scholar] [CrossRef]
- Yin, S.; Han, C.; Xia, Y.; Wan, F.; Hu, J.; Kou, L.; Sun, Y.; Wu, J.; Li, Y.; Zhou, Q.; et al. Cancerous Inhibitor of Protein Phosphatase 2A (CIP2A): Could It Be a Promising Biomarker and Therapeutic Target in Parkinson’s Disease? Mol. Neurobiol. 2022, 59, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
| Drug/Compound | Approved for the Treatment of | Reported Side Effects |
|---|---|---|
| Erlotinib (Tarceva®) | Metastatic NSCLC; advanced pancreatic cancer | Common side effects: rash, dry and itching skin, thinning hair, brittle and inflamed nails, diarrhea, nausea, vomiting, loss of appetite, weight loss, mouth sores, cough, shortness of breath, fatigue, headache, numbness or tingling in hands and feet, bone or muscle pain, eye irritation, redness, or dryness |
| Bortezomib (Velcade®) | Multiple Myeloma; Mantle Cell Lymphoma | Common side effects: peripheral neuropathy, nausea, vomiting, diarrhea, constipation, fatigue, low blood cell counts, and rash Serious side effects: heart and lung complications and development of posterior reversible encephalopathy syndrome |
| Lapatinib (Tykerb®) | HER2-positive advanced or metastatic breast cancer | Common side effects: diarrhea, hand–foot syndrome, nausea, vomiting, fatigue, and rash Serious side effects: interstitial lung disease development and cardiovascular complications |
| Afatinib (Gilotrif) | Metastatic NSCLC; metastatic squamous NSCLC | Common side effects: diarrhea and rash |
| Fingolimod (FTY720) | Relapsing forms of multiple sclerosis (MS), such as clinically isolated syndrome, relapsing–remitting disease, active secondary progressive disease | Common side effects: headache, changes in liver function tests, and increased susceptibility to infections Serious side effects: Progressive Multifocal Leukoencephalopathy, macular edema, liver injury, and potential worsening of MS symptoms |
| Metformin | Type 2 diabetes | Common side effects: diarrhea, nausea, gas, stomach pain, fatigue, and weight loss Serious side effects: lactic acidosis, vitamin B12 deficiency, and hypoglycemia |
| Penfluridol | Chronic schizophrenia and other related psychotic disorders | Common side effects: dizziness, restlessness, sedation, weight gain, gastrointestinal issues, tremors, and rigidity |
| Temsirolimus | Advanced renal cell carcinoma | Common side effects: rash, pimples, dry skin, skin blemishes, mouth irritation or sores, constipation, diarrhea, stomach pain, upset stomach, decreased appetite, fatigue, headache, back pain, muscle or joint pain, difficulty sleeping, changes in taste, weight loss, nosebleeds, runny or stuffy nose, and throat irritation Serious side effects: hives, rash, itching, difficulty breathing or swallowing, flushing, chest pain, shortness of breath, abdominal pain, abdominal swelling, chills, fever, constipation, nausea, vomiting, increased infection, fever, sore throat, chills, cough, nausea, confusion, dizziness or faintness, weakness or numbness of an arm or leg, red blood in stools, and decrease in the amount of urine |
| Cetuximab | Colorectal and head and neck cancers | Common side effects: skin rash, dry skin, itching, and nail issues Serious side effects: infusion reactions, heart complications, and electrolyte imbalances |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, H.; Nirmal, D.; Pappas, S.; Ebubechukwu, U.; Gill, S.; Al-Ajam, A.; Ohlmeyer, M.; Geraghty, P. The Emerging Role of the Cancerous Inhibitor of Protein Phosphatase 2A in Pulmonary Diseases. Medicina 2025, 61, 1740. https://doi.org/10.3390/medicina61101740
Hamza H, Nirmal D, Pappas S, Ebubechukwu U, Gill S, Al-Ajam A, Ohlmeyer M, Geraghty P. The Emerging Role of the Cancerous Inhibitor of Protein Phosphatase 2A in Pulmonary Diseases. Medicina. 2025; 61(10):1740. https://doi.org/10.3390/medicina61101740
Chicago/Turabian StyleHamza, Hamza, Dinesh Nirmal, Stephanie Pappas, Ugochukwu Ebubechukwu, Sunydip Gill, Adam Al-Ajam, Michael Ohlmeyer, and Patrick Geraghty. 2025. "The Emerging Role of the Cancerous Inhibitor of Protein Phosphatase 2A in Pulmonary Diseases" Medicina 61, no. 10: 1740. https://doi.org/10.3390/medicina61101740
APA StyleHamza, H., Nirmal, D., Pappas, S., Ebubechukwu, U., Gill, S., Al-Ajam, A., Ohlmeyer, M., & Geraghty, P. (2025). The Emerging Role of the Cancerous Inhibitor of Protein Phosphatase 2A in Pulmonary Diseases. Medicina, 61(10), 1740. https://doi.org/10.3390/medicina61101740







