Nano-Encapsulated Berberine Is a Potential Therapeutic Agent for Adipose Tissue Browning in C57BL/6J Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Treatment Preparation and Characterization
2.4. Nano-BBR Stability
2.5. Tissue Homogenization, RNA Extraction, and Real-Time PCR
2.6. Protein Extraction and ELISA
2.7. Statistical Analysis
3. Results
3.1. Nano-BBR Exhibited a More Sustained Release Behavior and Stability Compared to the Free Form
3.2. BBR Treatment Provided Weight Control in the Recovery Group
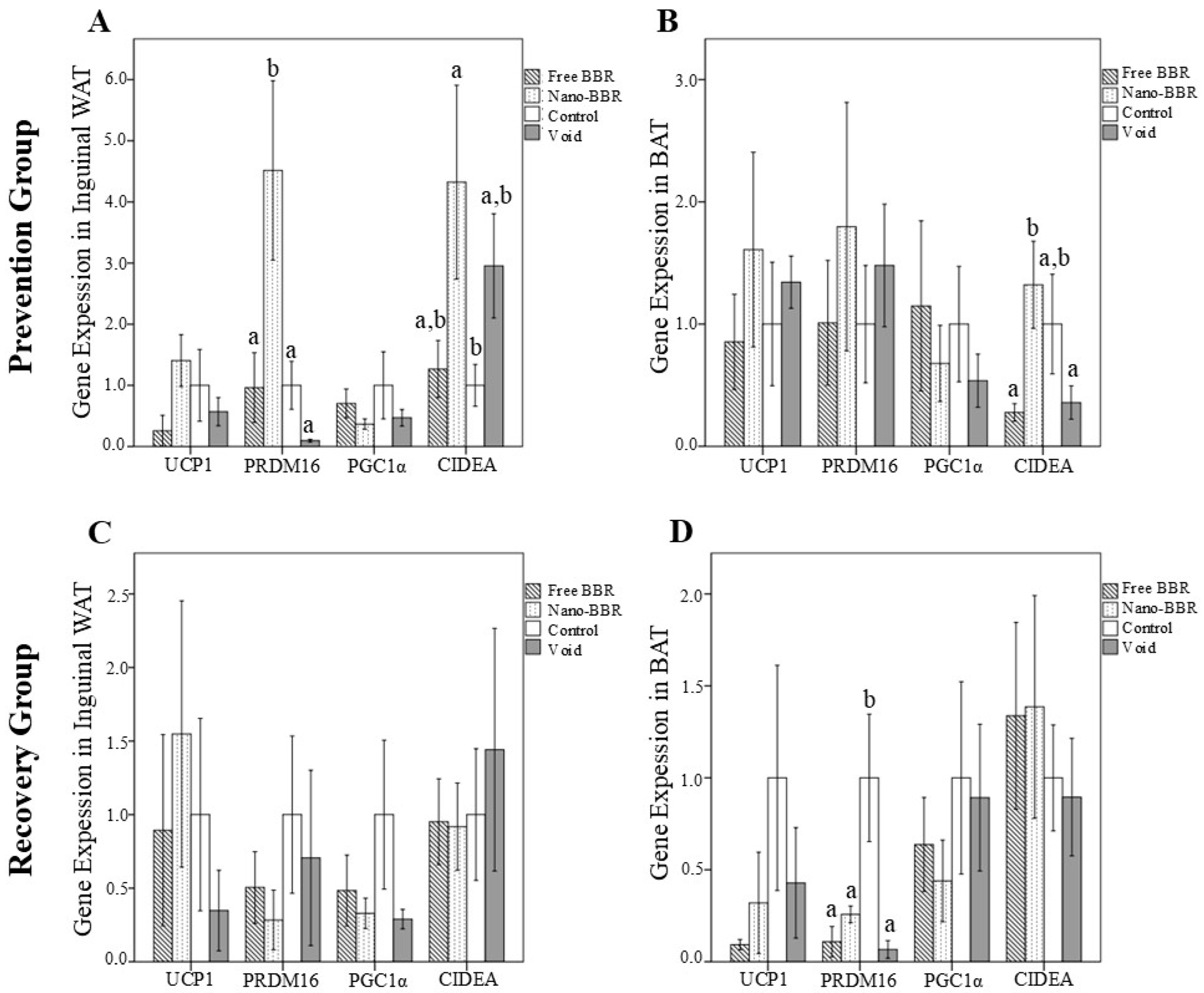
3.3. Gene Expression and Protein Levels of Browning Markers
3.4. Gene Expression and Protein Levels of Adipogenesis Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism 2019, 92, 121–135. [Google Scholar] [CrossRef]
- Upadhyay, J.; Farr, O.; Perakakis, N.; Ghaly, W.; Mantzoros, C. Obesity as a Disease. Med. Clin. N. Am. 2018, 102, 13–33. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2021; Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 July 2025).
- Gonzalez-Muniesa, P.; Martinez-Gonzalez, M.A.; Hu, F.B.; Despres, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Cypess, A.M. Reassessing Human Adipose Tissue. N. Engl. J. Med. 2022, 386, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Adipose Organ Development and Remodeling. Compr. Physiol. 2018, 8, 1357–1431. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhaes, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Wang, Y.; Zidichouski, J.A. Update on the Benefits and Mechanisms of Action of the Bioactive Vegetal Alkaloid Berberine on Lipid Metabolism and Homeostasis. Cholesterol 2018, 2018, 7173920. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guan, X.M.; Wang, R.Y.; Xie, Y.S.; Zhou, H.; Ni, W.J.; Tang, L.Q. Berberine mitigates high glucose-induced podocyte apoptosis by modulating autophagy via the mTOR/P70S6K/4EBP1 pathway. Life Sci. 2020, 243, 117277. [Google Scholar] [CrossRef]
- Feng, X.; Sureda, A.; Jafari, S.; Memariani, Z.; Tewari, D.; Annunziata, G.; Barrea, L.; Hassan, S.T.S.; Smejkal, K.; Malanik, M.; et al. Berberine in Cardiovascular and Metabolic Diseases: From Mechanisms to Therapeutics. Theranostics 2019, 9, 1923–1951. [Google Scholar] [CrossRef]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary Factors Promoting Brown and Beige Fat Development and Thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Cao, J.; Jiao, L.; Yang, S.; Zhang, L.; Lu, Y.; Du, G. Solubility and Stability Advantages of a New Cocrystal of Berberine Chloride with Fumaric Acid. ACS Omega 2020, 5, 8283–8292. [Google Scholar] [CrossRef]
- Goktas, Z.; Zu, Y.; Abbasi, M.; Galyean, S.; Wu, D.; Fan, Z.; Wang, S. Recent Advances in Nanoencapsulation of Phytochemicals to Combat Obesity and Its Comorbidities. J. Agric. Food Chem. 2020, 68, 8119–8131. [Google Scholar] [CrossRef]
- Panda, D.S.; Eid, H.M.; Elkomy, M.H.; Khames, A.; Hassan, R.M.; El-Ela, F.I.A.; Yassin, H.A. Berberine Encapsulated Lecithin-Chitosan Nanoparticles as Innovative Wound Healing Agent in Type II Diabetes. Pharmaceutics 2021, 13, 1197. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Somagoni, J.; Singh, M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 2014, 9, e89919. [Google Scholar] [CrossRef] [PubMed]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Azadi, R.; Mousavi, S.E.; Kazemi, N.M.; Yousefi-Manesh, H.; Rezayat, S.M.; Jaafari, M.R. Anti-inflammatory efficacy of Berberine Nanomicelle for improvement of cerebral ischemia: Formulation, characterization and evaluation in bilateral common carotid artery occlusion rat model. BMC Pharmacol. Toxicol. 2021, 22, 54. [Google Scholar] [CrossRef]
- Lin, Y.C.; Kuo, J.Y.; Hsu, C.C.; Tsai, W.C.; Li, W.C.; Yu, M.C.; Wen, H.W. Optimizing manufacture of liposomal berberine with evaluation of its antihepatoma effects in a murine xenograft model. Int. J. Pharm. 2013, 441, 381–388. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.E.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. J. Mater. Chem. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef]
- Ilyas, Z.; Perna, S.; Al-Thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The effect of Berberine on weight loss in order to prevent obesity: A systematic review. Biomed. Pharmacother. 2020, 127, 110137. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Feng, X.; Liu, X.; Deng, C.; Hu, C.H. Berberine Alleviates Olanzapine-Induced Adipogenesis via the AMPKalpha-SREBP Pathway in 3T3-L1 Cells. Int. J. Mol. Sci. 2016, 17, 1865. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Ma, S.R.; Zuo, Z.Y.; Wu, Y.B.; Kong, W.J.; Wang, A.P.; Jiang, J.D. Berberine inhibits adipocyte differentiation, proliferation and adiposity through down-regulating galectin-3. Sci. Rep. 2019, 9, 13415. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lv, X.; Li, J.; Meng, Z.; Wang, Q.; Chang, W.; Li, W.; Chen, L.; Liu, Y. Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis. Mol. Cell. Endocrinol. 2012, 363, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, N.; Cang, Z.; Zhu, C.; Zhao, L.; Nie, X.; Cheng, J.; Xia, F.; Zhai, H.; Lu, Y. Modulation of Microbiota-Gut-Brain Axis by Berberine Resulting in Improved Metabolic Status in High-Fat Diet-Fed Rats. Obes. Facts 2016, 9, 365–378. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Zhang, M.; Pang, X.; Xu, J.; Kang, C.; Li, M.; Zhang, C.; Zhang, Z.; Zhang, Y.; et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS ONE 2012, 7, e42529. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, T.; Ma, G.; Zheng, L.; Jiang, X.; Yang, F.; Wang, Z.; Li, N.; He, Z.; Song, X.; et al. Berberine modulates deacetylation of PPARgamma to promote adipose tissue remodeling and thermogenesis via AMPK/SIRT1 pathway. Int. J. Biol. Sci. 2021, 17, 3173–3187. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, Q.; Yu, Y.; Yang, F.; Bai, R.; Fan, X. Efficacy and underlying mechanisms of berberine against lipid metabolic diseases: A review. Front. Pharmacol. 2023, 14, 1283784. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Handschin, C.; Spiegelman, B.M. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005, 1, 361–370. [Google Scholar] [CrossRef]
- Ma, P.Y.; Li, X.Y.; Wang, Y.L.; Lang, D.Q.; Liu, L.; Yi, Y.K.; Liu, Q.; Shen, C.Y. Natural bioactive constituents from herbs and nutraceuticals promote browning of white adipose tissue. Pharmacol. Res. 2022, 178, 106175. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Oliver, P.; Palou, A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta 2013, 1831, 969–985. [Google Scholar] [CrossRef]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Mitani, Y.; Kurumisawa, K.; Nomura, K.; Wang, W.; Nakashima, K.I.; Inoue, M. Berberine stimulates fibroblast growth factor 21 by modulating the molecular clock component brain and muscle Arnt-like 1 in brown adipose tissue. Biochem. Pharmacol. 2019, 164, 165–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, X.; Feng, K.; Wang, T.; Li, W.; Yuan, T.; Sun, X.; Sun, Q.; Xiang, H.; Wang, H. Berberine Moderates Glucose and Lipid Metabolism through Multipathway Mechanism. Evid. Based Complement. Altern. Med. 2011, 2011, 924851. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, F.; Lu, R.; Jia, J. Berberine Inhibits Adipogenesis in Porcine Adipocytes via AMP-Activated Protein Kinase-Dependent and -Independent Mechanisms. Lipids 2019, 54, 667–678. [Google Scholar] [CrossRef]
- de Sa, P.M.; Richard, A.J.; Hang, H.; Stephens, J.M. Transcriptional Regulation of Adipogenesis. Compr. Physiol. 2017, 7, 635–674. [Google Scholar] [CrossRef]

| Before Intervention (g) | After Intervention (g) | Body Weight Changes (g) | ||
|---|---|---|---|---|
| Prevention group | Free BBR | 19.2 ± 1.56 | 24.5 ± 2.45 | 4.8 ± 1.88 |
| Nano-BBR | 18.3 ± 1.15 | 24.0 ± 1.64 | 5.7 ± 0.79 | |
| Void | 18.6 ± 1.02 | 23.1 ± 2.07 | 4.5 ± 1.93 | |
| Control | 18.5 ± 1.56 | 22.4 ± 1.80 | 3.9 ± 2.04 | |
| p = 0.325 | ||||
| Recovery group | Free BBR | 25.7 ± 2.25 | 24.5 ± 1.81 | −1.2 ± 1.40 a |
| Nano-BBR | 24.0 ± 2.47 | 24.3 ± 0.85 | 0.4 ± 2.16 | |
| Void | 22.6 ± 1.28 | 24.3 ± 1.10 | 1.6 ± 0.33 b | |
| Control | 24.4 ± 1.83 | 24.9 ± 1.36 | 0.5 ± 1.63 | |
| p = 0.042 | ||||
| UCP1 | PGC1α | PPARγ | |||
|---|---|---|---|---|---|
| Prevention Group | Inguinal WAT | Control | 2366.1 ± 616.56 | 595.8 ± 210.68 | 497.7 ± 211.60 |
| Void | 2326.9 ± 428.73 | 468.2 ± 119.04 | 633.6 ± 180.37 | ||
| Free BBR | 2305.8 ± 242.60 | 442.9 ± 115.78 | 530.3 ± 144.47 | ||
| Nano-BBR | 2209.9 ± 473.55 | 491.0 ± 197.75 | 507.0 ± 173.33 | ||
| p | 0.947 | 0.455 | 0.554 | ||
| BAT | Control | 2016.2 ± 417.89 | 500.9 ± 144.95 | 534.6 ± 218.63 | |
| Void | 2185.7 ± 338.18 | 493.9 ± 94.76 | 504.5 ± 140.95 | ||
| Free BBR | 1839.4 ± 578.98 | 487.0 ± 67.46 | 453.6 ± 13.31 | ||
| Nano-BBR | 1840.4 ± 588.04 | 486.6 ± 85.77 | 480.2 ± 134.07 | ||
| p | 0.581 | 0.995 | 0.830 | ||
| Serum | Control | 6912.1 ± 3599.91 | 555.7 ± 335.61 | 2217.3 ± 1189.36 | |
| Void | 8331.5 ± 3253.07 | 771.2 ± 494.86 | 2637.8 ± 1393.68 | ||
| Free BBR | 6544.4 ± 2538.78 | 527.5 ± 348.87 | 1958.8 ± 879.39 | ||
| Nano-BBR | 7358.5 ± 3657.19 | 637.3 ± 439.18 | 2628.8 ± 1353.45 | ||
| p | 0.821 | 0.753 | 0.757 | ||
| Recovery Group | Inguinal WAT | Control | 2589.7 ± 697.04 | 630.4 ± 166.41 a | 605.3 ± 249.78 |
| Void | 2636.2 ± 655.39 | 548.3 ± 101.51 a | 570.9 ± 126.82 | ||
| Free BBR | 2464.9 ± 395.70 | 390.8 ± 99.00 b | 500.6 ± 99.31 | ||
| Nano-BBR | 2266.4 ± 401.76 | 513.3 ± 122.45 | 545.8 ± 273.23 | ||
| p | 0.666 | 0.027 | 0.835 | ||
| BAT | Control | 1883.8 ± 478.19 | 457.7 ± 104.45 | 469.6 ± 172.57 a | |
| Void | 2185.8 ± 693.20 | 528.5 ± 171.23 | 714.0 ± 320.62 b | ||
| Free BBR | 2070.8 ± 744.33 | 496.0 ± 102.53 | 571.1 ± 131.98 a | ||
| Nano-BBR | 2123.5 ± 536.60 | 476.9 ± 134.24 | 404.6 ± 77.88 | ||
| p | 0.852 | 0.812 | 0.067 | ||
| Serum | Control | 6407.1 ± 2834.57 a | 496.8 ± 387.09 a | 1966.0 ± 1062.07 | |
| Void | 10,984.4 ± 5607.07 b | 1111.9 ± 699.75 b | 2930.2 ± 1378.26 a | ||
| Free BBR | 6028.4 ± 2485.50 a | 413.5 ± 263.18 a | 1655.9 ± 690.26 b | ||
| Nano-BBR | 7607.01 ± 2890.29 | 740.8 ± 500.99 | 2220.0 ± 896.72 | ||
| p | 0.113 | 0.093 | 0.211 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alpaslan, A.; Uçar Baş, K.; Örs Demet, E.D.; Tuğal Aslan, D.; Reçber, T.; Öztürk, S.C.; Gulsun, T.; Çelebier, M.; Göktaş, Z. Nano-Encapsulated Berberine Is a Potential Therapeutic Agent for Adipose Tissue Browning in C57BL/6J Mice. Medicina 2025, 61, 1738. https://doi.org/10.3390/medicina61101738
Alpaslan A, Uçar Baş K, Örs Demet ED, Tuğal Aslan D, Reçber T, Öztürk SC, Gulsun T, Çelebier M, Göktaş Z. Nano-Encapsulated Berberine Is a Potential Therapeutic Agent for Adipose Tissue Browning in C57BL/6J Mice. Medicina. 2025; 61(10):1738. https://doi.org/10.3390/medicina61101738
Chicago/Turabian StyleAlpaslan, Aslıhan, Kübra Uçar Baş, Elif Didem Örs Demet, Dilem Tuğal Aslan, Tuba Reçber, Süleyman Can Öztürk, Tugba Gulsun, Mustafa Çelebier, and Zeynep Göktaş. 2025. "Nano-Encapsulated Berberine Is a Potential Therapeutic Agent for Adipose Tissue Browning in C57BL/6J Mice" Medicina 61, no. 10: 1738. https://doi.org/10.3390/medicina61101738
APA StyleAlpaslan, A., Uçar Baş, K., Örs Demet, E. D., Tuğal Aslan, D., Reçber, T., Öztürk, S. C., Gulsun, T., Çelebier, M., & Göktaş, Z. (2025). Nano-Encapsulated Berberine Is a Potential Therapeutic Agent for Adipose Tissue Browning in C57BL/6J Mice. Medicina, 61(10), 1738. https://doi.org/10.3390/medicina61101738






