Novel 1-(2-Aryl-2-adamantyl)piperazine Derivatives Exhibit In Vitro Anticancer Activity Across Various Human Cancer Cell Lines, with Selective Efficacy Against Melanoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cell Culture
2.2. Synthesis of 1-(2-Aryl-2-adamantyl)piperazine Derivatives
2.3. Cell Viability Assays
2.3.1. Sulforhodamine B (SRB)
2.3.2. Trypan Blue Exclusion
2.4. Electrophoresis and Western Blot Analysis
2.5. Clonogenic/Long-Term Assay
2.6. Statistical Analysis
3. Results
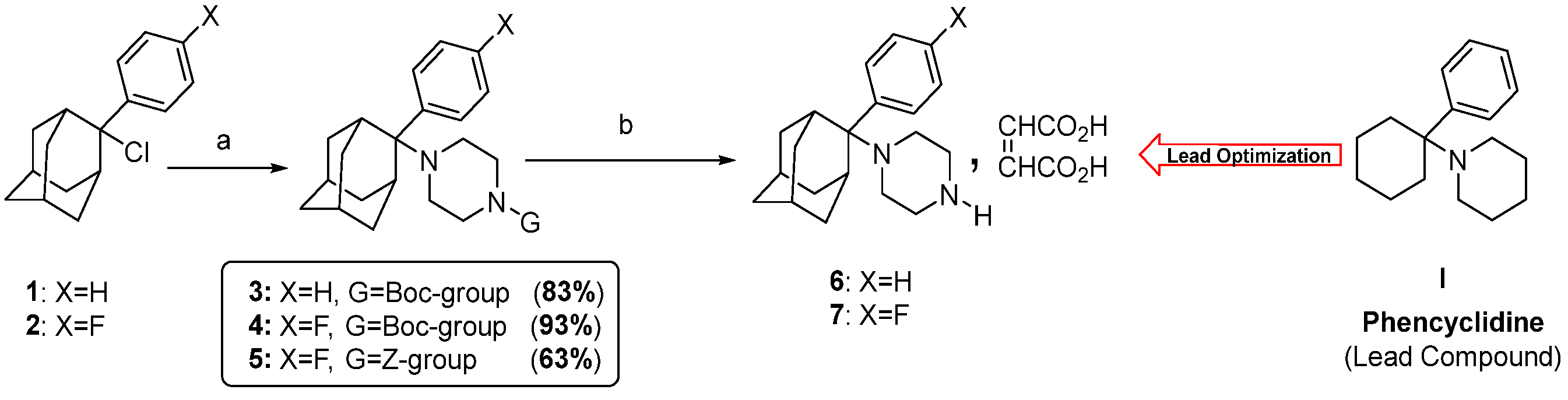
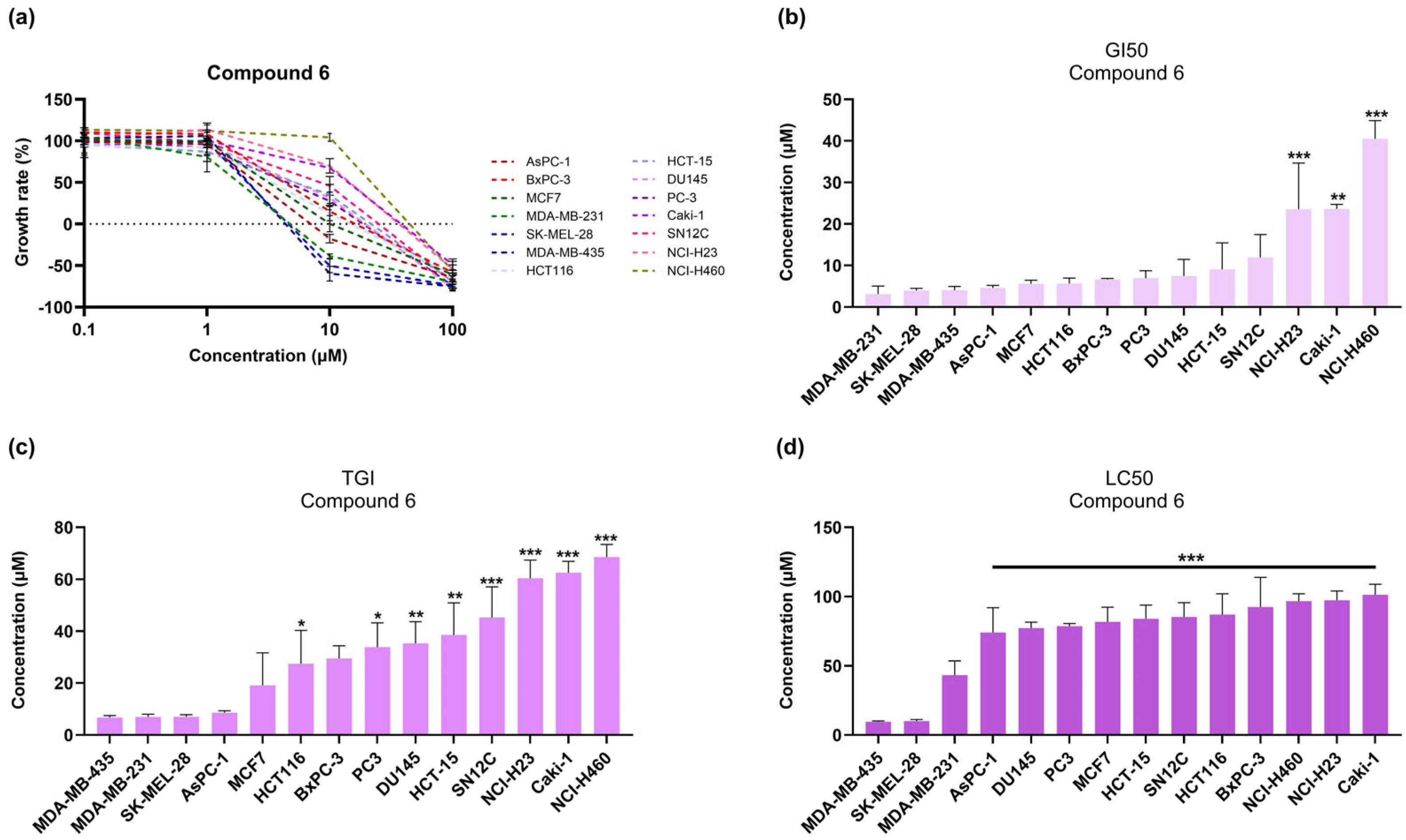
3.1. Synthesis of Compounds 1-(2-Phenyltricyclo[3.3.1.13,7]dec-2-yl)piperazine Maleate 6 and 1-[2-(4-Fluorophenyl)tricyclo[3.3.1.13,7]dec-2-yl]piperazine Maleate 7
3.2. 1-(2-Aryl-2-adamantyl)piperazine Derivatives Inhibit Cell Growth of Melanoma Cancer Cell Lines
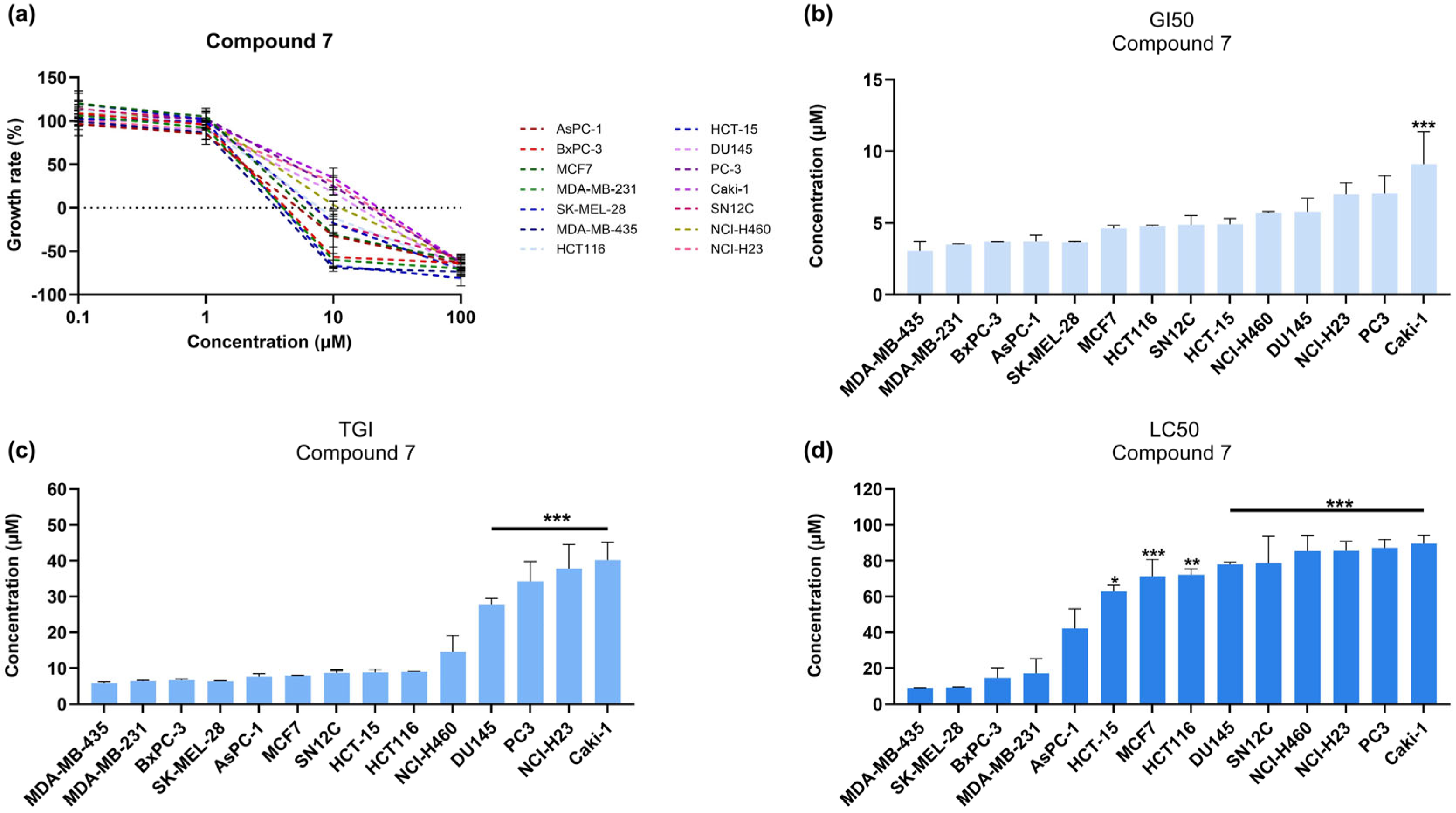
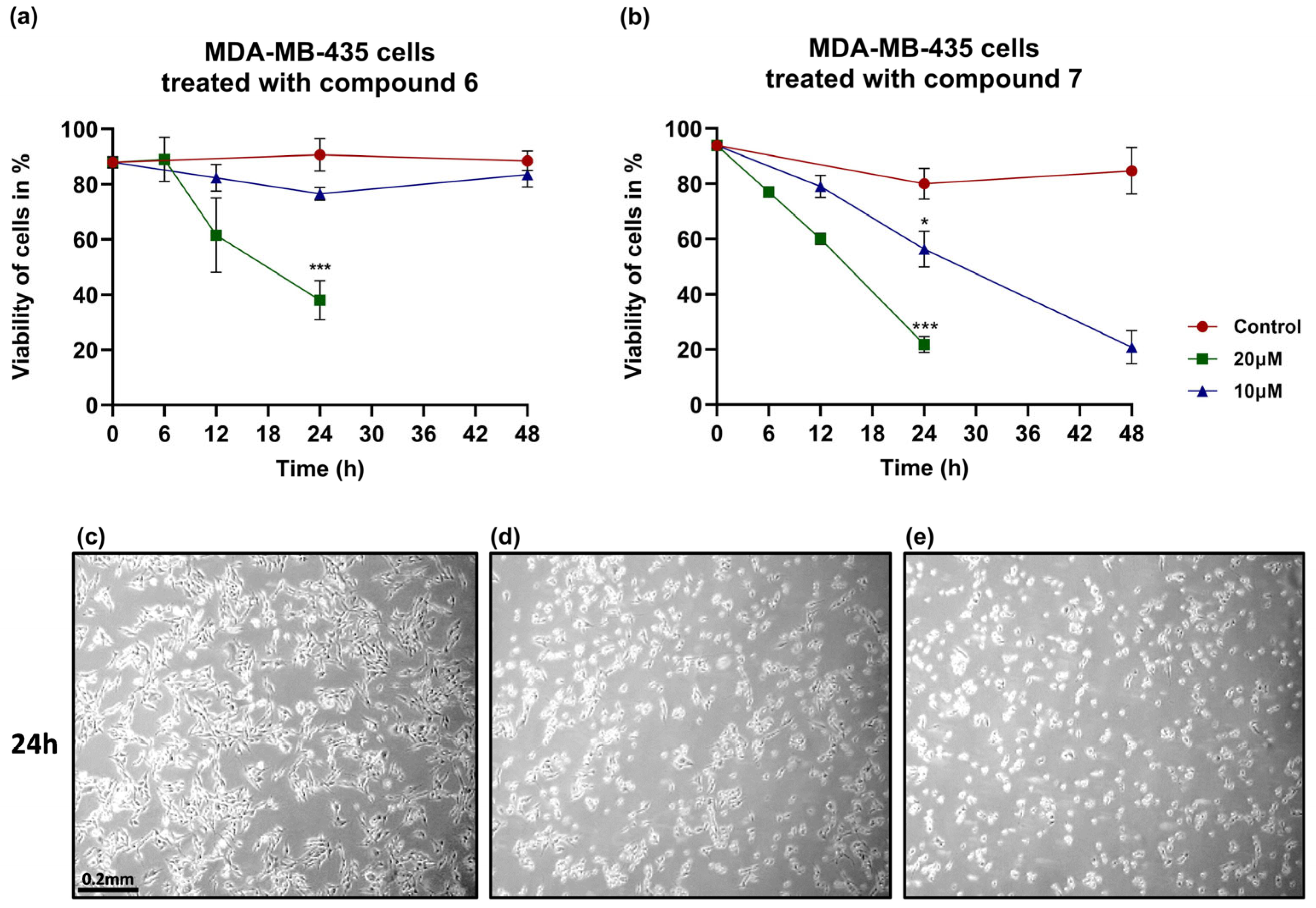
3.3. Compound 7 Induces Stronger and Sustained Cytotoxic Effects in Melanoma Cells Compared to Compound 6
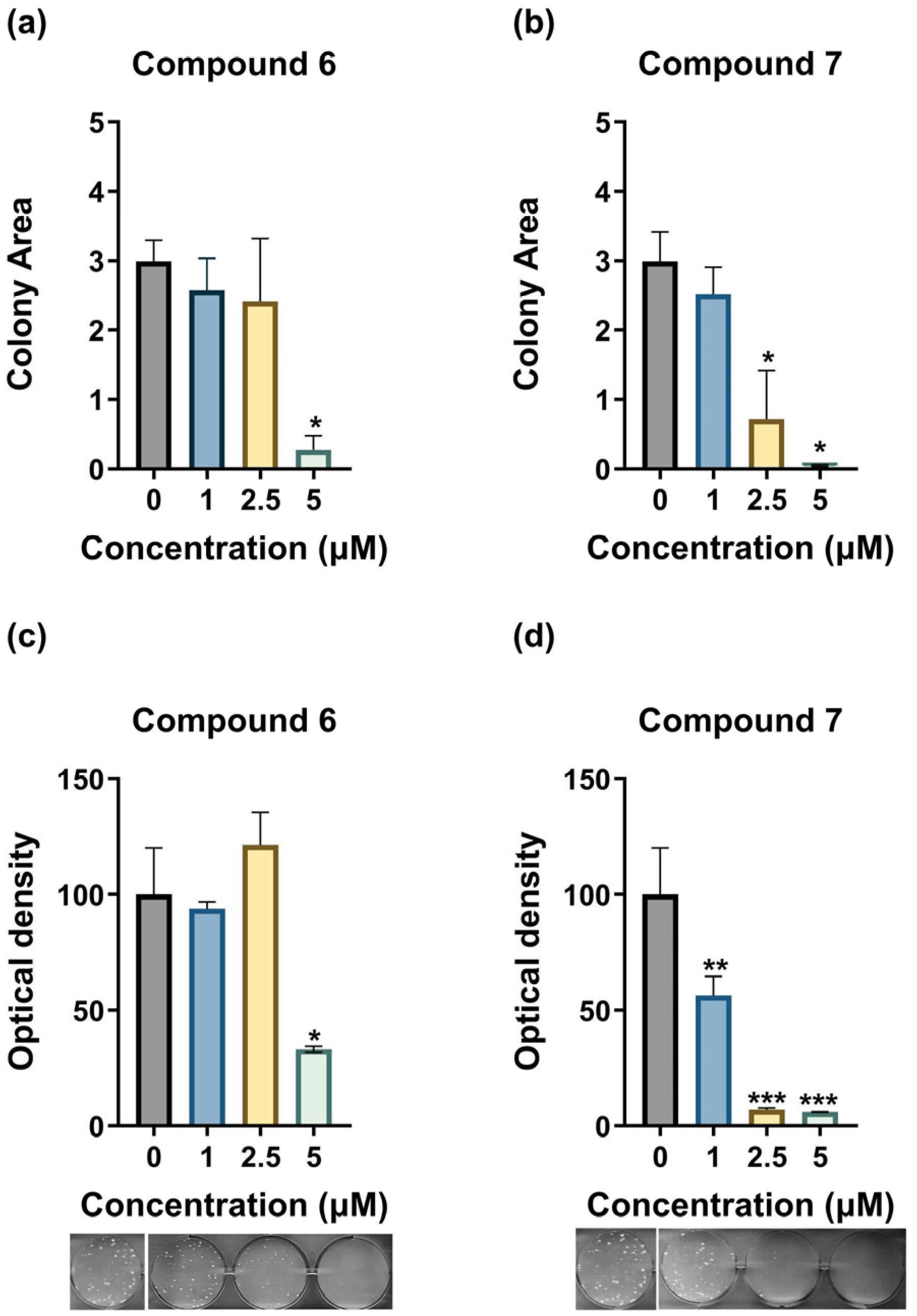
3.4. Compounds 6 and 7 Inhibit the Clonogenicity of MDA-MB-435 Cells
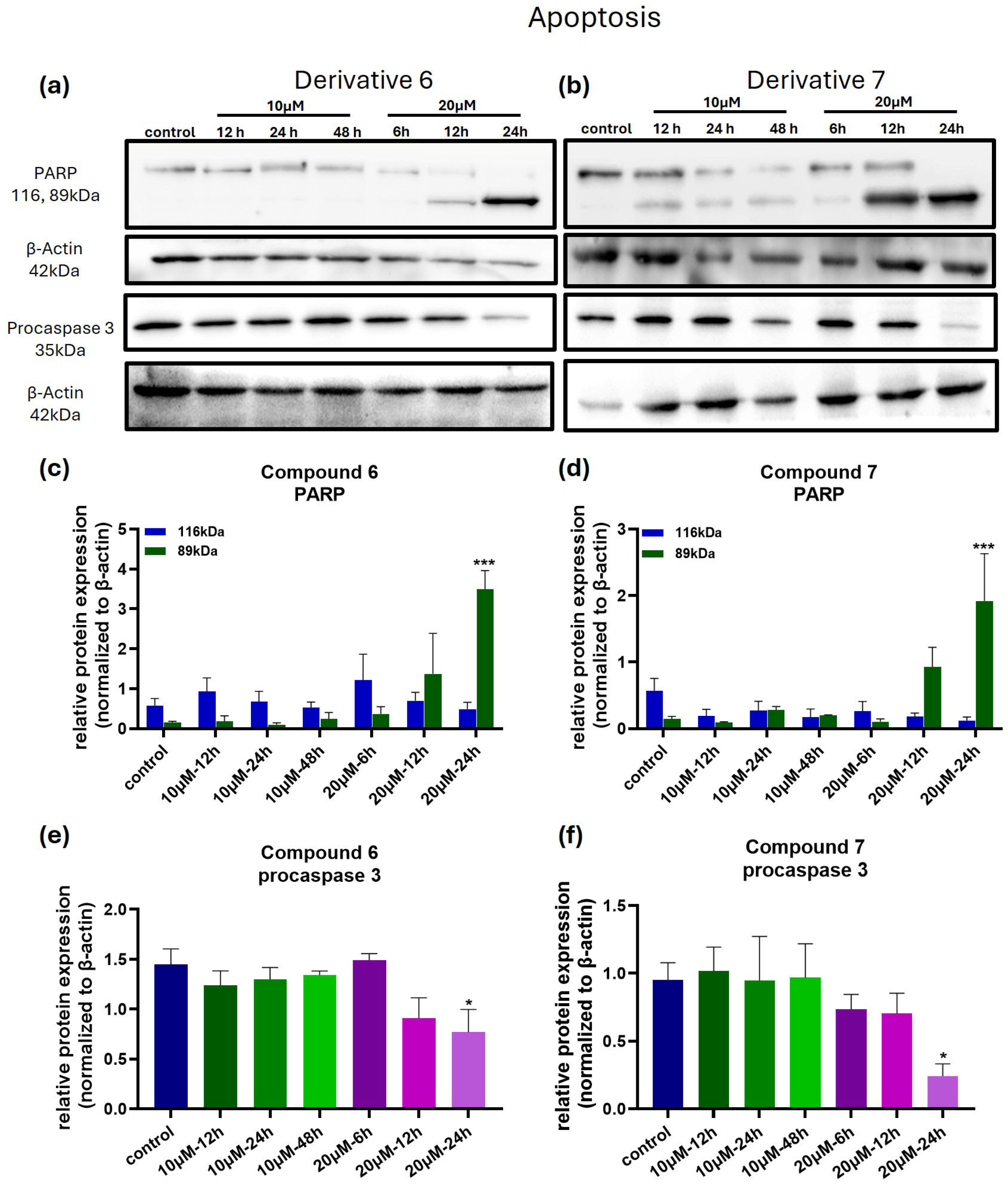
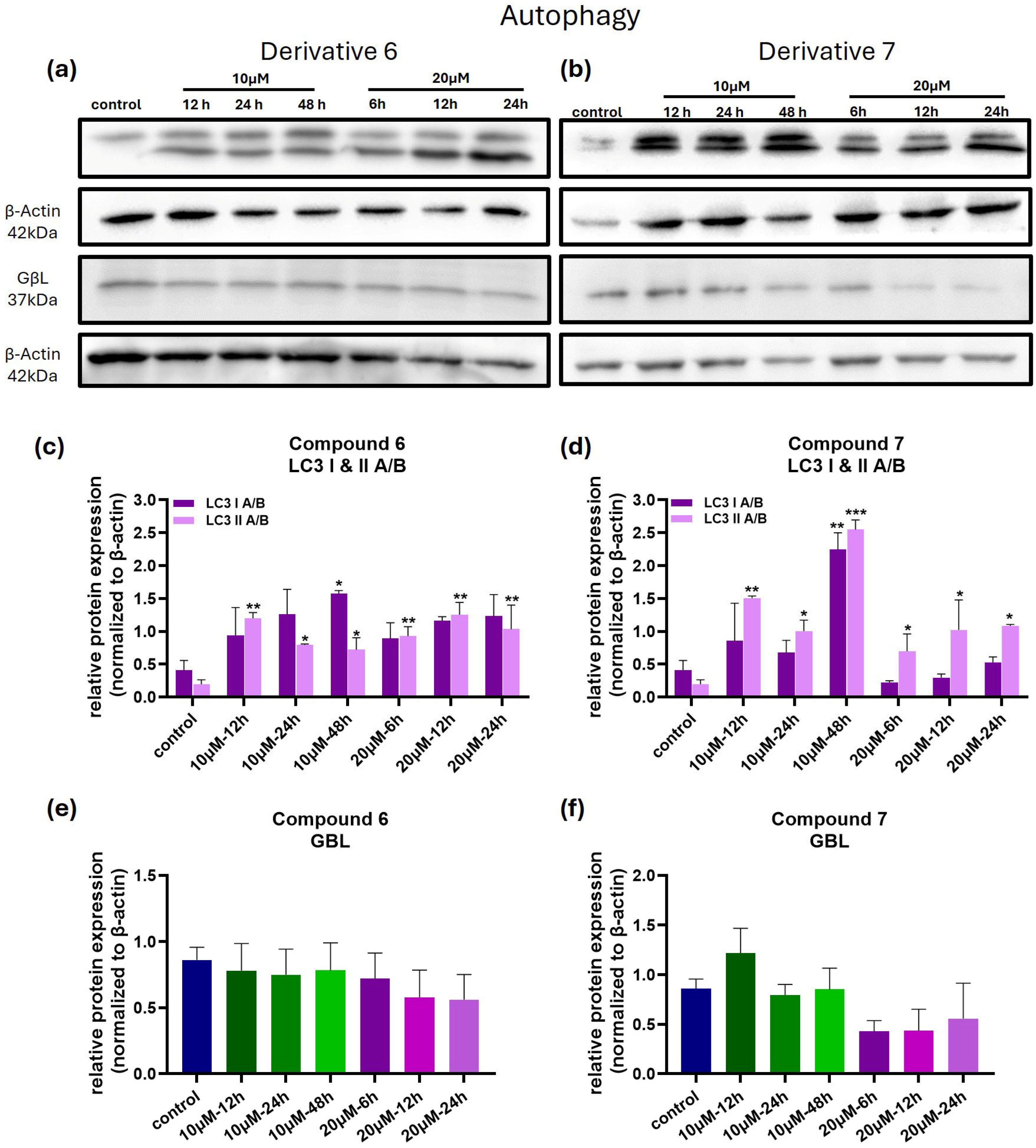
3.5. Compounds 6 and 7 Induce Cell Death in Melanoma Cells via Apoptosis and Autophagy-Related Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A.A | Acetic Acid |
| AI | Artificial Intelligence |
| CM | Cutaneous Melanoma |
| ECL | Enhanced Chemiluminescence |
| HRP | Horseradish Peroxidase |
| PGRMC1 | Progesterone Receptor Membrane Component 1 |
| PVDF | Polyvinylidene Difluoride |
| RT | Room Temperature |
| T0 | Time Zero |
| TCA | Trichloroacetic Acid |
| TLC | Thin-Layer Chromatography |
| TMEM97 | Transmembrane Protein 97 |
| WHO | World Health Organization |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Joint Research Centre. Cancer Cases and Deaths on the Rise in the EU. Available online: https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/cancer-cases-and-deaths-rise-eu-2023-10-02_en (accessed on 16 July 2025).
- American Cancer Society. What Is Melanoma Skin Cancer? Available online: https://www.cancer.org/cancer/types/melanoma-skin-cancer/about/what-is-melanoma.html (accessed on 23 May 2023).
- Dubey, V.K.; Kaushik, V.D. Epidermis lesion detection via optimized distributed capsule neural network. Comput. Biol. Med. 2024, 168, 107833. [Google Scholar] [CrossRef] [PubMed]
- Volkovova, K.; Bilanicova, D.; Bartonova, A.; Letaiová, S.; Dusinska, M. Associations between environmental factors and incidence of cutaneous melanoma. Environ. Heal. 2012, 11, S12. [Google Scholar] [CrossRef] [PubMed]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.-P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A Landscape of Driver Mutations in Melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef]
- Slominski, R.M.; Kim, T.-K.; Janjetovic, Z.; Brożyna, A.A.; Podgorska, E.; Dixon, K.M.; Mason, R.S.; Tuckey, R.C.; Sharma, R.; Crossman, D.K.; et al. Malignant Melanoma: An Overview, New Perspectives, and Vitamin D Signaling. Cancers 2024, 16, 2262. [Google Scholar] [CrossRef]
- Ferle-Vidovicl, A.; Kastelanl, M.; Petrovicl, D.; Hmanl, L.; Kaselj, M. Synthesis and biological activity of phencyclidine and its adamantylamine derivatives. Eur. J. Med. Chem. 1993, 28, 243–250. [Google Scholar] [CrossRef]
- Fytas, C.; Kolocouris, A.; Fytas, G.; Zoidis, G.; Valmas, C.; Basler, C.F. Influence of an additional amino group on the potency of aminoadamantanes against influenza virus A. II—Synthesis of spiropiperazines and in vitro activity against influenza A H3N2 virus. Bioorg Chem. 2010, 38, 247–251. [Google Scholar] [CrossRef]
- Kolocouris, A.; Dimas, K.; Pannecouque, C.; Witvrouw, M.; Foscolos, G.B.; Stamatiou, G.; Fytas, G.; Zoidis, G.; Kolocouris, N.; Andrei, G.; et al. New 2-(1-Adamantylcarbonyl)pyridine and 1-Acetyladamantane Thiosemicarbazones-Thiocarbonohydrazones: Cell Growth Inhibitory, Antiviral and Antimicrobial Activity Evaluation. Bioorganic Med. Chem. Lett. 2002, 12, 723–727. [Google Scholar] [CrossRef]
- Zoidis, G.; Tsotinis, A.; Tsatsaroni, A.; Taylor, M.C.; Kelly, J.M.; Efstathiou, A.; Smirlis, D.; Fytas, G. Lipophilic conformationally constrained spiro carbocyclic 2,6-diketopiperazine-1-acetohydroxamic acid analogues as trypanocidal and leishmanicidal agents: An extended SAR study. Chem. Biol. Drug Des. 2018, 91, 408–421. [Google Scholar] [CrossRef]
- Blanpied, T.A.; Clarke, R.J.; Johnson, J.W. Amantadine inhibits NMDA receptors by accelerating channel closure during channel block. J. Neurosci. 2005, 25, 3312–3322. [Google Scholar] [CrossRef]
- Maugh, T.H. Panel Urges Wide Use of Antiviral Drug. Science 1979, 206, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, R.F. Adamantanes for the treatment of neurodegenerative diseases in the presence of SARS-CoV-2. Front. Neurosci. 2023, 17, 1128157. [Google Scholar] [CrossRef] [PubMed]
- Riganas, S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Bourguignon, J.-J.; Serin, G.; Mirjolet, J.-F.; Dimas, K.; Kourafalos, V.N.; Eleutheriades, A.; et al. Synthesis, σ 1, σ 2-receptors binding affinity and antiproliferative action of new C1-substituted adamantanes. Bioorg Med. Chem. 2012, 20, 3323–3331. [Google Scholar] [CrossRef] [PubMed]
- Riganas, S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Dimas, K.; Kourafalos, V.N.; Eleutheriades, A.; Moutsos, V.I.; Khan, H.; Margarita, P.; et al. New Adamantane Derivatives with Sigma Affinity and Antiproliferative Activity. Med. Chem. 2012, 8, 569–586. [Google Scholar] [CrossRef]
- Riganas, S.; Papanastasiou, I.; Foscolos, G.B.; Tsotinis, A.; Serin, G.; Mirjolet, J.-F.; Dimas, K.; Kourafalos, V.N.; Eleutheriades, A.; Moutsos, V.I.; et al. New adamantane phenylalkylamines with σ-receptor binding affinity and anticancer activity, associated with putative antagonism of neuropathic pain. J. Med. Chem. 2012, 55, 10241–10261. [Google Scholar] [CrossRef]
- Fytas, C.; Zoidis, G.; Tsotinis, A.; Fytas, G.; Khan, M.A.; Akhtar, S.; Rahman, K.M.; Thurston, D.E. Novel 1-(2-aryl-2-adamantyl)piperazine derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 93, 281–290. [Google Scholar] [CrossRef]
- Kunkel, M.W.; Coussens, N.P.; Morris, J.; Taylor, R.C.; Dexheimer, T.S.; Jones, E.M.; Doroshow, J.H.; Teicher, B.A. HTS384 NCI60: The Next Phase of the NCI60 Screen. Cancer Res. 2024, 84, 2403–2416. [Google Scholar] [CrossRef]
- Sereti, E.; Tsimplouli, C.; Kalaitsidou, E.; Sakellaridis, N.; Dimas, K. Study of the Relationship between Sigma Receptor Expression Levels and Some Common Sigma Ligand Activity in Cancer Using Human Cancer Cell Lines of the NCI-60 Cell Line Panel. Biomedicines 2021, 9, 38. [Google Scholar] [CrossRef]
- Liu, K.-T.; Sheu, H.-C. Solvolysis of 2-Aryl-2-Chloroadamantanes. A New Y Scale for Benzylic. 1991. Available online: https://pubs.acs.org/sharingguidelines (accessed on 16 July 2025).
- Chang, Y.F.; Lin, Y.Y.; Wang, H.E.; Liu, R.S.; Pang, F.; Hwang, J.J. Monitoring of tumor growth and metastasis potential in MDA-MB-435s/tk-luc human breast cancer xenografts. Nucl. Instrum. Methods Phys. Res. A 2007, 571, 155–159. [Google Scholar] [CrossRef]
- Lopez, L.; Shuster-Hyman, H.; Marco, E.; Khan, H.; Gasner, A.; Uzelac, A.; Wyse, B.; Mander, P.; Sangaralingam, M.; Fish, J.; et al. Human Umbilical Cord Perivascular Cells Prevent Tumor Growth in a Melanoma Tumor-Bearing Mouse Model and Modulate Breast Cancer and Melanoma Cells in a Cell Line-Dependent Manner In Vitro. Stem Cells Int. 2023, 2023, 1–20. [Google Scholar] [CrossRef]
- Lee, A.C.; Shedden, K.; Rosania, G.R.; Crippen, G.M. Data mining the NCI60 to predict generalized cytotoxicity. J. Chem. Inf. Model. 2008, 48, 1379–1388. [Google Scholar] [CrossRef]
- Wang, P.; Song, L.; Yi, H.; Zhang, M.; Zhu, S.; Deng, H.; Shao, M. Convenient one-pot synthesis of fluorinated DHPs derivatives and their further transformations. Tetrahedron Lett. 2010, 51, 3975–3977. [Google Scholar] [CrossRef]
- Schinor, B.; Hruschka, S.; Daniliuc, C.G.; Schepmann, D.; Wünsch, B.; Haufe, G. Fluorinated 2-Arylcyclopropan-1-amines—A new class of sigma receptor ligands. Bioorganic Med. Chem. 2020, 28, 115726. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Sabatini, D.M. Defining the Role of mTOR in Cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Jacinto, E.; Loewith, R.; Schmidt, A.; Lin, S.; Rüegg, M.A.; Hall, A.; Hall, M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004, 6, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Sarbassov, D.D.; Ali, S.M.; Latek, R.R.; Guntur, K.V.; Erdjument-Bromage, H.; Tempst, P.; Sabatini, D.M. GbetaL, a Positive Regulator of the Rapamycin-Sensitive Pathway Required for the Nutrient-Sensitive Interaction Between Raptor and mTOR. Mol. Cell 2003, 11, 895–904. [Google Scholar] [CrossRef]
- Korpis, K.; Weber, F.; Brune, S.; Wünsch, B.; Bednarski, P.J. Involvement of apoptosis and autophagy in the death of RPMI 8226 multiple myeloma cells by two enantiomeric sigma receptor ligands. Bioorg Med. Chem. 2014, 22, 221–233. [Google Scholar] [CrossRef]
- Su, T.P.; Hayashi, T.; Maurice, T.; Buch, S.; Ruoho, A.E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010, 31, 557–566. [Google Scholar] [CrossRef]
- Mavlyutov, T.A.; Nickells, R.W.; Guo, L.-W. Accelerated Retinal Ganglion Cell Death in Mice Deficient in the Sigma-1 Receptor. Mol. Vis. 2011, 17, 1034–1043. [Google Scholar]
- Meunier, J.; Hayashi, T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor κB. J. Pharmacol. Exp. Ther. 2010, 332, 388–397. [Google Scholar] [CrossRef]
- Mach, R.H.; Zeng, C.; Hawkins, W.G. The σ2 receptor: A novel protein for the imaging and treatment of cancer. J. Med. Chem. 2013, 56, 7137–7160. [Google Scholar] [CrossRef]
- Bowen, W.D.; Vilner, B.J.; Williams, W.; Bertha, C.M.; Kuehne, M.E.; Jacobson, A.E. Ibogaine and its congeners are tr: Receptor-selective ligands with moderate affinity. Eur. J. Pharmacol. 1995, 179, R1–R3. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, M.S.; Fehrenbacher, N.; Hoyer-Hansen, M.; Thomsen, C.; Farkas, T.; Jäättelä, M. Effective tumor cell death by σ-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 2005, 65, 8975–8983. [Google Scholar] [CrossRef]
- Crawford, K.W.; Coop, A.; Bowen, W.D. Sigma2 Receptors Regulate Changes in Sphingolipid Levels in Breast Tumor Cells. Eur. J. Pharmacol. 2002, 443, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Rothfuss, J.; Zhang, J.; Chu, W.; Vangveravong, S.; Tu, Z.; Pan, F.; Chang, K.C.; Hotchkiss, R.; Mach, R.H. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br. J. Cancer 2012, 106, 693–701. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Høyer-Hansen, M.; Bastholm, L.; Fehrenbacher, N.; Olsen, O.D.; Groth-Pedersen, L.; Puustinen, P.; Kirkegaard-Sørensen, T.; Nylandsted, J.; Farkas, T.; et al. Anti-cancer agent siramesine is a lysosomotropic detergent that induces cytoprotective autophagosome accumulation. Autophagy 2008, 4, 487–4992008. [Google Scholar] [CrossRef]
- Cassano, G.; Gasparre, G.; Niso, M.; Contino, M.; Scalera, V.; Colabufo, N.A. F281, synthetic agonist of the sigma-2 receptor, induces Ca2+ efflux from the endoplasmic reticulum and mitochondria in SK-N-SH cells. Cell Calcium 2009, 45, 340–345. [Google Scholar] [CrossRef]
- Crawford, K.W.; Bowen, W.D. Sigma-2 Receptor Agonists Activate a Novel Apoptotic Pathway and Potentiate Antineoplastic Drugs in Breast Tumor Cell Lines. Cancer Res. 2002, 62, 313–322. [Google Scholar]
- Korch, C.; Hall, E.M.; Dirks, W.G.; Ewing, M.; Faries, M.; Varella-Garcia, M.; Robinson, S.; Storts, D.; Turner, J.A.; Wang, Y.; et al. Authentication of M14 melanoma cell line proves misidentification of MDA-MB-435 breast cancer cell line. Int. J. Cancer 2018, 142, 561–572. [Google Scholar] [CrossRef]
- Ross, D.T.; Scherf, U.; Eisen, M.B.; Perou, C.M.; Rees, C.; Spellman, P.; Iyer, V.; Jeffrey, S.S.; Van de Rijn, M.; Waltham, M.; et al. Systematic Variation in Gene Expression Patterns in Human Cancer Cell Lines. Nat. Genet. 2000, 24, 227–235. [Google Scholar] [CrossRef]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
| Cancer Type | Cell Lines |
|---|---|
| Pancreas | AsPC-1, BxPC-3 |
| Breast | MCF7, MDA-MB-231 |
| Melanoma | SK-MEL-28, MDA-MB-435 |
| Colorectal | HCT116, HCT15 |
| Prostate | DU145, PC3 |
| Renal | Caki-1, SN12C |
| Lung | NCI-H23, NCI-H460 |
| GI50 (μΜ) | TGI (μΜ) | LC50 (μΜ) | |
|---|---|---|---|
| AsPC-1 | 4.6 | 8.6 | 72.1 |
| BxPC-3 | 6.6 | 28.7 | 89.6 |
| MCF7 | 5.6 | 19.1 | 83.8 |
| MDA-MB-231 | 3.3 | 6.9 | 43.2 |
| SK-MEL-28 | 4.0 | 7.0 | 10.1 |
| MDA-MB-435 | 4.0 | 6.8 | 9.5 |
| HCT116 | 5.8 | 25.4 | 86.2 |
| HCT-15 | 7.4 | 38.6 | 83.3 |
| DU145 | 7.4 | 36.2 | 77.5 |
| PC-3 | 6.9 | 34.6 | 78.8 |
| Caki-1 | 23.6 | 62.3 | 100.9 |
| SN12C | 11.9 | 45.9 | 85.0 |
| NCI-H23 | 24.6 | 60.8 | 97.0 |
| NCI-H460 | 40.5 | 68.5 | 96.6 |
| GI50 (μΜ) | TGI (μΜ) | LC50 (μΜ) | |
|---|---|---|---|
| AsPC-1 | 3.7 | 7.5 | 42.4 |
| BxPC-3 | 3.7 | 6.7 | 14.6 |
| MCF7 | 4.6 | 7.9 | 67.4 |
| MDA-MB-231 | 3.5 | 6.4 | 17.2 |
| SK-MEL-28 | 3.9 | 6.9 | 9.8 |
| MDA-MB-435 | 3.1 | 6.0 | 8.9 |
| HCT116 | 4.7 | 9.0 | 71.6 |
| HCT-15 | 4.9 | 8.7 | 64.2 |
| DU145 | 6.0 | 27.9 | 78.0 |
| PC-3 | 7.1 | 35.3 | 86.3 |
| Caki-1 | 9.1 | 42.0 | 88.1 |
| SN12C | 4.9 | 8.6 | 78.6 |
| NCI-H23 | 6.9 | 38.1 | 85.9 |
| NCI-H460 | 5.7 | 14.5 | 83.9 |
| Caki-1 | 3.7 | 7.5 | 42.4 |
| SN12C | 3.7 | 6.7 | 14.6 |
| NCI-H23 | 4.6 | 7.9 | 67.4 |
| NCI-H460 | 3.5 | 6.4 | 17.2 |
| CLogP | pKa | |
|---|---|---|
| Compound 6 | 4.434 | 8.956 |
| Compound 7 | 4.577 | 8.950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papapostolou, I.; Sereti, E.; Chatira, S.; Sakellaridis, N.; Fytas, G.; Zoidis, G.; Dimas, K. Novel 1-(2-Aryl-2-adamantyl)piperazine Derivatives Exhibit In Vitro Anticancer Activity Across Various Human Cancer Cell Lines, with Selective Efficacy Against Melanoma. Medicina 2025, 61, 1731. https://doi.org/10.3390/medicina61101731
Papapostolou I, Sereti E, Chatira S, Sakellaridis N, Fytas G, Zoidis G, Dimas K. Novel 1-(2-Aryl-2-adamantyl)piperazine Derivatives Exhibit In Vitro Anticancer Activity Across Various Human Cancer Cell Lines, with Selective Efficacy Against Melanoma. Medicina. 2025; 61(10):1731. https://doi.org/10.3390/medicina61101731
Chicago/Turabian StylePapapostolou, Irida, Evangelia Sereti, Stavroula Chatira, Nikos Sakellaridis, George Fytas, Grigoris Zoidis, and Konstantinos Dimas. 2025. "Novel 1-(2-Aryl-2-adamantyl)piperazine Derivatives Exhibit In Vitro Anticancer Activity Across Various Human Cancer Cell Lines, with Selective Efficacy Against Melanoma" Medicina 61, no. 10: 1731. https://doi.org/10.3390/medicina61101731
APA StylePapapostolou, I., Sereti, E., Chatira, S., Sakellaridis, N., Fytas, G., Zoidis, G., & Dimas, K. (2025). Novel 1-(2-Aryl-2-adamantyl)piperazine Derivatives Exhibit In Vitro Anticancer Activity Across Various Human Cancer Cell Lines, with Selective Efficacy Against Melanoma. Medicina, 61(10), 1731. https://doi.org/10.3390/medicina61101731









