Introducing the Index of Response to Stimulation (IRES): A Novel Metric for Assessing Vagus Nerve Stimulation Outcomes in Drug-Resistant Epilepsy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objective
2.2. Study Design
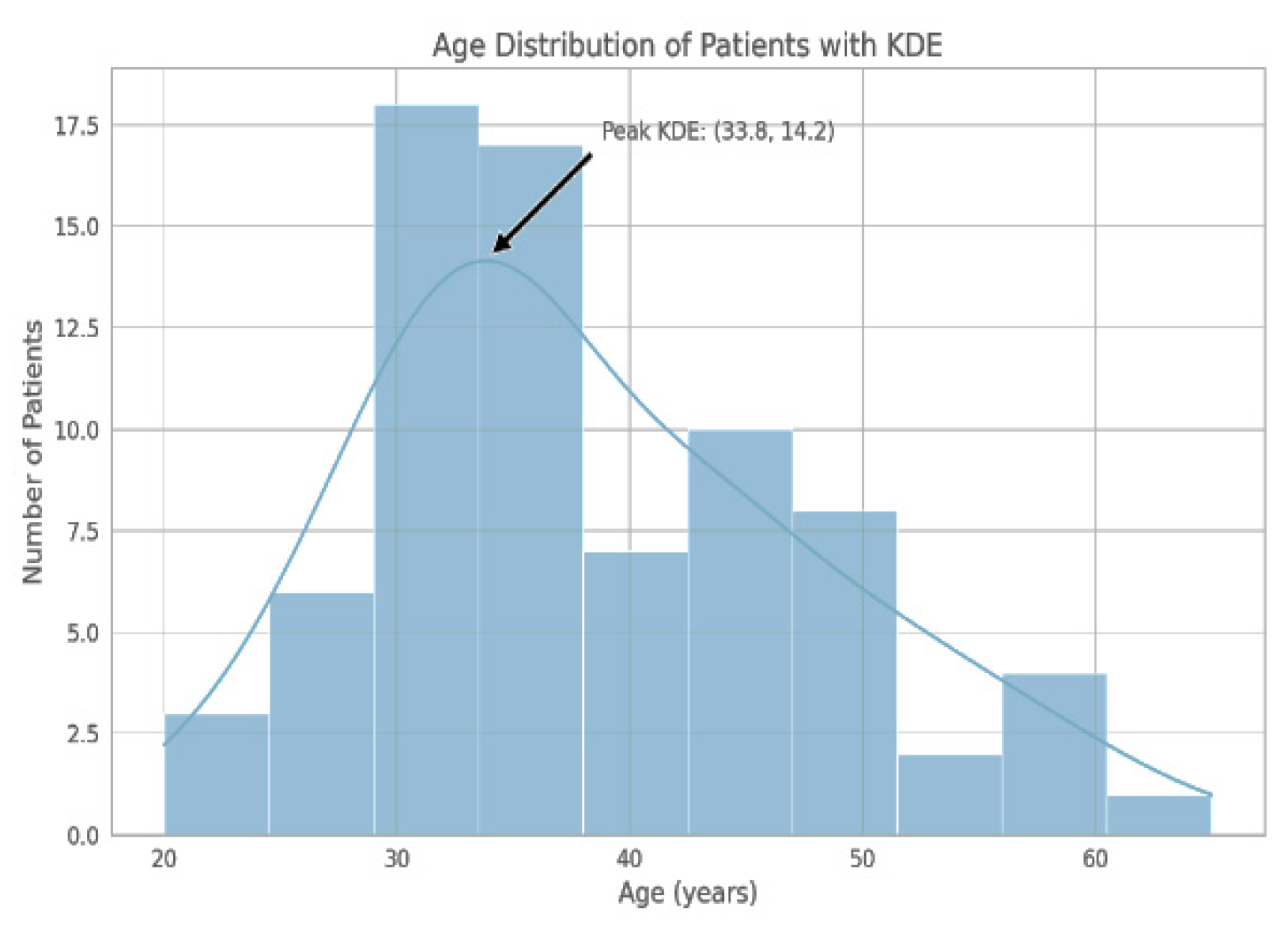
2.3. Participants
2.4. IRES Score Review
2.5. VNS Therapy Settings
2.6. Ethical Considerations
2.7. Results
2.7.1. Scoring Criteria
- 0 points means little or no change (0–25% improvement).
- 1 point means minimal to moderate improvement (25–50% improvement).
- 2 points are given to those with strong improvement, more than 50%.
- As each dimension is assessed within this range then the overall IRES score will be from 0 to 8 where 0 represents no observed improvement and 8 represents observed maximum improvement over all assessed aspects.
2.7.2. Interpretation of IRES Scores
- Scores between 0 and 2 indicate minimal or no improvement in the response to VNS therapy and, therefore, the treatment had little effect on the patient.
- Scores of 3–6 are indicative of partial response to treatment in some of the dimensions evaluated, with the improvement being moderate to modest. The range suggests that symptomatic improvement has taken place for the patient following therapy.
- Scores greater than 6 points manifest a highly significant response to treatment or remission, reflecting substantial therapeutic benefit that may be translated into improvement in the quality of life or control over symptoms.
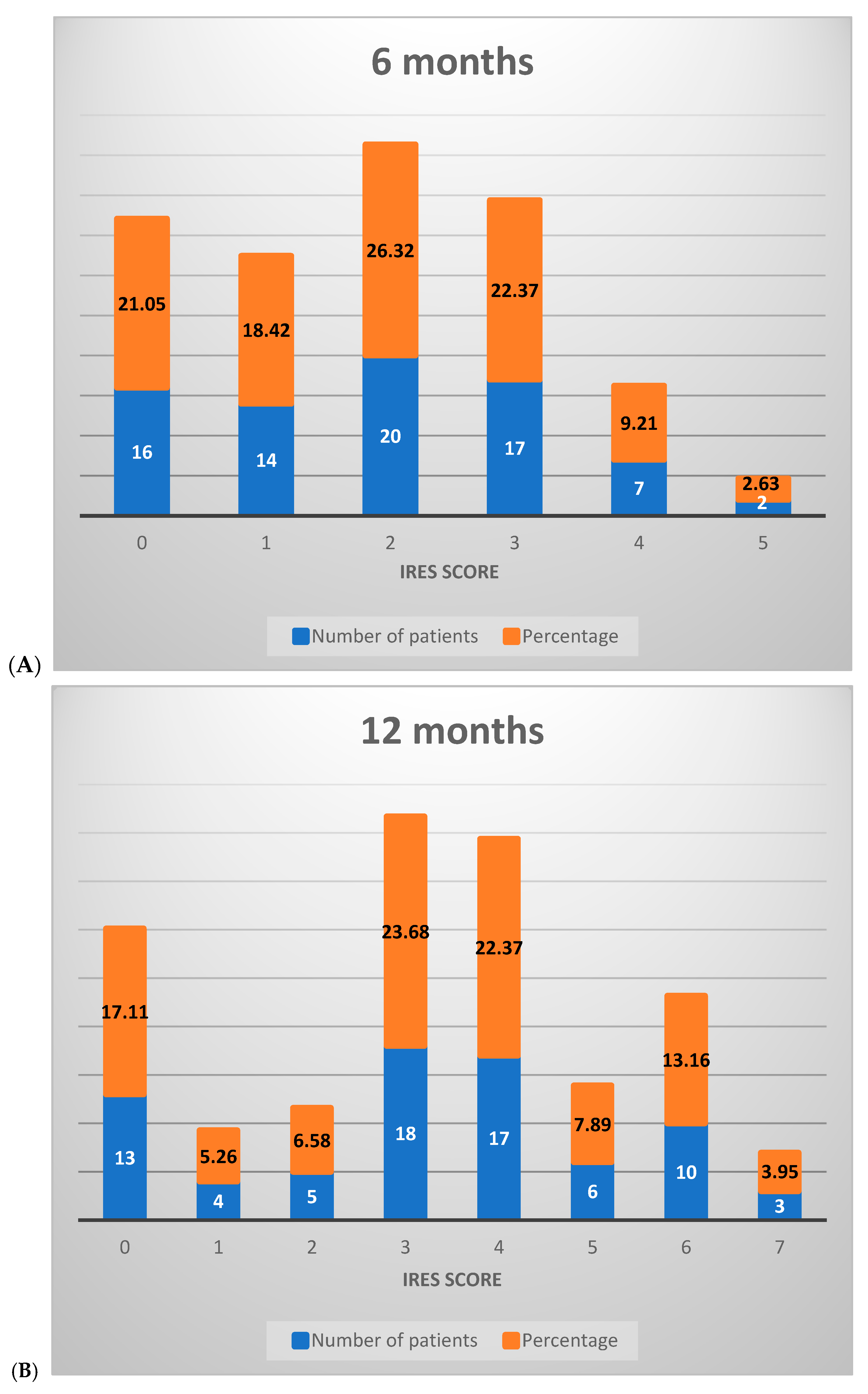
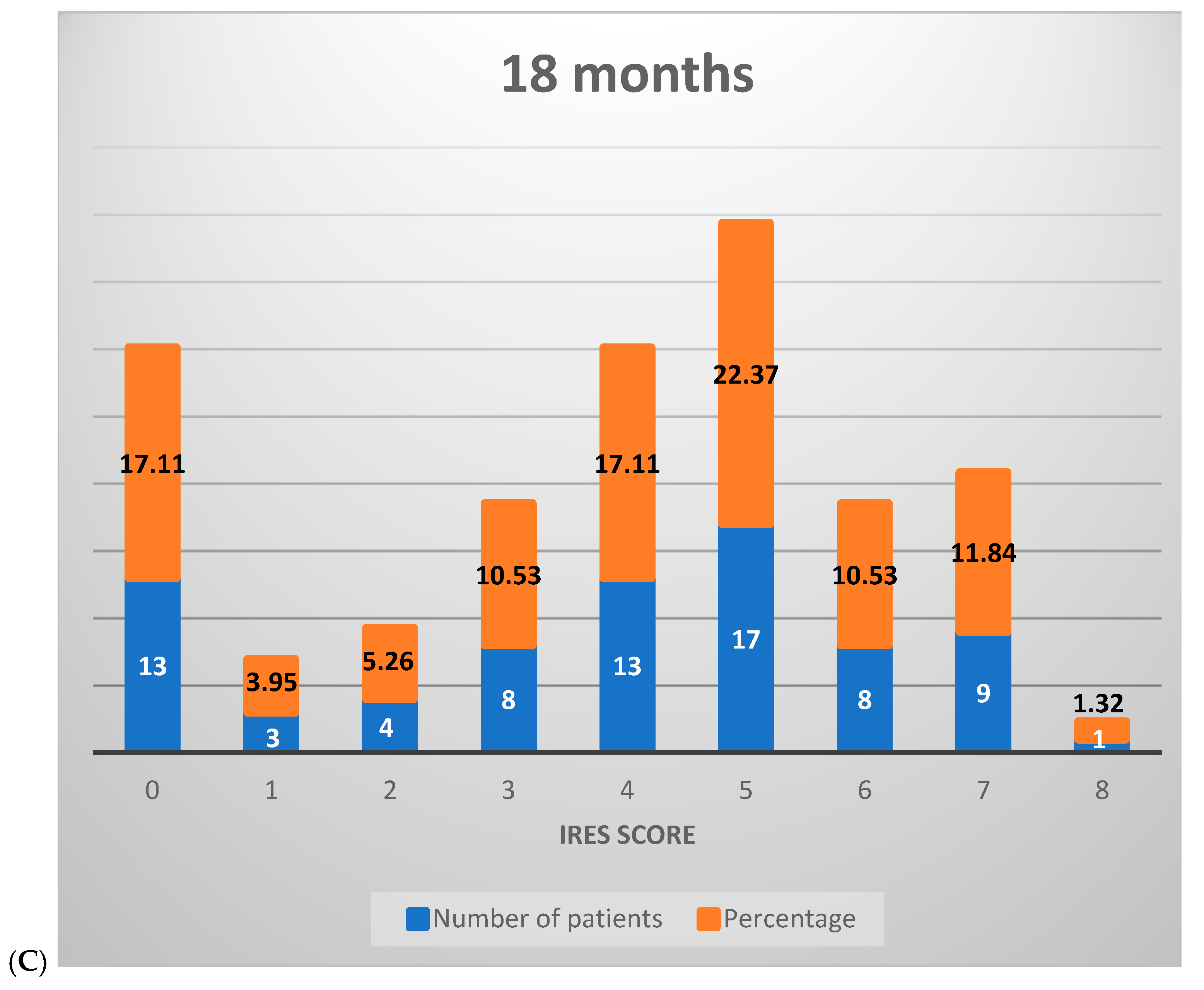
2.7.3. IRES Score Assessment
- -
- SDD: shows a gradual increase in mean scores from 0.51 at 6 months to 0.96 at 18 months, indicating a moderate improvement in seizure duration over time.
- -
- SID: the mean scores go up from 0.22 at 6 months to 0.55 at 18 months, with a suggestion of slight to moderate reduction in seizure intensity across the study period.
- -
- IQoF: marked improvement, with the mean score increasing from 0.74 at 6 months to 1.17 at 18 months.
- -
- SFD: the mean scores rise from 0.41 at 6 months to 1.14 at 18 months.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kawai, K.; Tanaka, T.; Baba, H.; Bunker, M.; Ikeda, A.; Inoue, Y.; Yamamoto, T. Outcome of vagus nerve stimulation for drug-resistant epilepsy: The first three years of a prospective Japanese registry. Epileptic Disord. 2017, 19, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Colicchio, G.; Montano, N.; Fuggetta, F.; Papacci, F.; Signorelli, F.; Meglio, M. Vagus nerve stimulation in drug-resistant epilepsies. Analysis of potential prognostic factors in a cohort of patients with long-term follow-up. Acta Neurochir. 2012, 154, 2237–2240. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Z.; Meng, F.; Huang, L.; Qu, W.; Hao, H.; Li, L. Chronic vagus nerve stimulation reverses heart rhythm complexity in patients with drug-resistant epilepsy: An assessment with multiscale entropy analysis. Epilepsy Behav. 2018, 83, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, G.; Zhu, L.; Chen, D.; Xu, D.; Chu, S.S.; Liu, L. Predictors of seizure reduction outcome after vagus nerve stimulation in drug-resistant epilepsy. Seizure 2019, 66, 53–60. [Google Scholar] [CrossRef]
- Elliott, R.E.; Morsi, A.; Kalhorn, S.P.; Marcus, J.; Sellin, J.; Kang, M.; Doyle, W.K. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: Long-term outcomes and predictors of response. Epilepsy Behav. 2011, 20, 57–63. [Google Scholar] [CrossRef]
- Rong, P.; Liu, A.; Zhang, J.; Wang, Y.; Yang, A.; Li, L.; Zhu, B. An alternative therapy for drug-resistant epilepsy: Transcutaneous auricular vagus nerve stimulation. Chin. Med. J. 2014, 127, 300-4. [Google Scholar] [CrossRef]
- Hajtovic, S.; LoPresti, M.; Zhang, L.; Katlowitz, K.A.; Kizek, D.J.; Lam, S. The role of vagus nerve stimulation in genetic etiologies of drug-resistant epilepsy: A meta-analysis. J. Neurosurg. Pediatr. 2022, 29, 667–680. [Google Scholar] [CrossRef]
- Constantinescu, V.; Matei, D.; Constantinescu, I.; Cuciureanu, D.I. Cardiac autonomic modulation in drug-resistant epilepsy patients after vagus nerve stimulation therapy. Neurol. Neurochir. Pol. 2020, 54, 329–336. [Google Scholar] [CrossRef]
- Sen, A.; Verner, R.; Valeriano, J.; Lee, R.; Zafar, M.; Thomas, R.; The, C.V. Vagus nerve stimulation therapy in people with drug-resistant epilepsy (CORE-VNS): Rationale and design of a real-world post-market comprehensive outcomes registry. BMJ Neurol. Open. 2021, 3, e000218. [Google Scholar] [CrossRef]
- Liu, H.Y.; Yang, Z.; Meng, F.; Guan, Y.G.; Ma, Y.S.; Liang, S.L.; Li, L.M. Preoperative heart rate variability as predictors of vagus nerve stimulation outcome in patients with drug-resistant epilepsy. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Bauer, S.; Baier, H.; Baumgartner, C.; Bohlmann, K.; Fauser, S.; Graf, W.; Hamer, H.M. Transcutaneous Vagus Nerve Stimulation (tVNS) for treatment of drug-resistant epilepsy: A randomized, double-blind clinical trial (cMPsE02). Brain Stimul. 2016, 9, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, R.; Fu, X.M. Efficacy analysis of vagus nerve stimulation for the treatment of drug-resistant epilepsy: A report of 110 cases. Chin. J. Neurosurg. 2019, 35, 1059–1063. [Google Scholar]
- Lagae, L.; Verstrepen, A.; Nada, A.; Van Loon, J.; Theys, T.; Ceulemans, B.; Jansen, K. Vagus nerve stimulation in children with drug-resistant epilepsy: Age at implantation and shorter duration of epilepsy as predictors of better efficacy? Epileptic Disord. 2015, 17, 308–314. [Google Scholar] [CrossRef]
- Benedetti-Isaac, J.C.; Torres-Zambrano, M.; Fandiño-Franky, J.; Polo-Verbel, L.M.; Bolaño-Esquirol, M.; Villa-Delgado, R.; Alcalá-Cerra, G. Vagus nerve stimulation therapy in patients with drug-resistant epilepsy and previous corpus callosotomy. Neurocirugia 2012, 23, 244–249. [Google Scholar] [CrossRef]
- Nalbantoğlu, M.; Ozkara, C.; Yeni, N.; Demirbilek, V.; Yalcinkaya, C.; Delil, Ş.; Uzan, M. Efficacy of Vagus Nerve Stimulation in Patients with Drug Resistant Epilepsy. J. Turk Epilepsi Soc. 2014, 20, 23–32. [Google Scholar] [CrossRef]
- Verrier, R.L.; Nearing, B.D.; Olin, B.; Boon, P. T-wave alternans levels and cardiac electrical stability response to vagus nerve stimulation: A heart rhythm study. Heart Rhythm. 2016, 13, 982–987. [Google Scholar]
- Shan, H.; Zhang, X.; Zhao, X.; Wang, M.; Qi, X.; Luan, G. Clinical outcome and safety of vagus nerve stimulation in drug resistant epilepsy: A single center experience. Front Neurol. 2022, 13, 716487. [Google Scholar]
- Bao, E.L.; Chao, L.Y.; Ni, P. Vagus Nerve Stimulation for Epilepsy: Long-term therapy outcomes in a Chinese population. Chin. Med. J. 2011, 124, 2336–2340. [Google Scholar]
- Tanaka, M.; Toda, K.; Hayashida, Y.; Shan, W.; Wang, Q. Efficacy and safety of transcutaneous vagus nerve stimulation in drug-resistant epilepsy: A randomized clinical trial. Clin. Neurophysiol. 2017, 128, 992–999. [Google Scholar]
- DeGiorgio, C.M.; Schachter, S.C.; Handforth, A.; Salinsky, M.; Thompson, J.; Uthman, B.; Reed, R.; Collins, S.; Tecoma, E.; Morris, G.L.; et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 2000, 41, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Chang, E.F.; Auguste, K.I. Vagus nerve stimulation for epilepsy: A meta-analysis of efficacy and predictors of response. J. Neurosurg. 2011, 115, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
| Component | 0 Points | 1 Point | 2 Points |
| Seizure Duration Decrease | 0–25% improvement | 25–50% improvement | >50% improvement |
| Seizure Intensity Decrease | 0–25% improvement | 25–50% improvement | >50% improvement |
| Improvement in Quality of Life | No changes in daily life No observable improvement in the patient’s life after VNS | Feels a little better Reflects a slight but noticeable enhancement in day-to-day well-being | Good improvement Marked reductions in depressive symptoms and an overall betterment of mood and functionality |
| Seizure Frequency Decrease | 0–25% improvement | 25–50% improvement | >50% improvement |
| Total IRES Points: | 0–2 points | 3–6 points | >6 points |
| Interpretation: | Minimal or no response | Partial or moderate improvement | Significant or complete response |
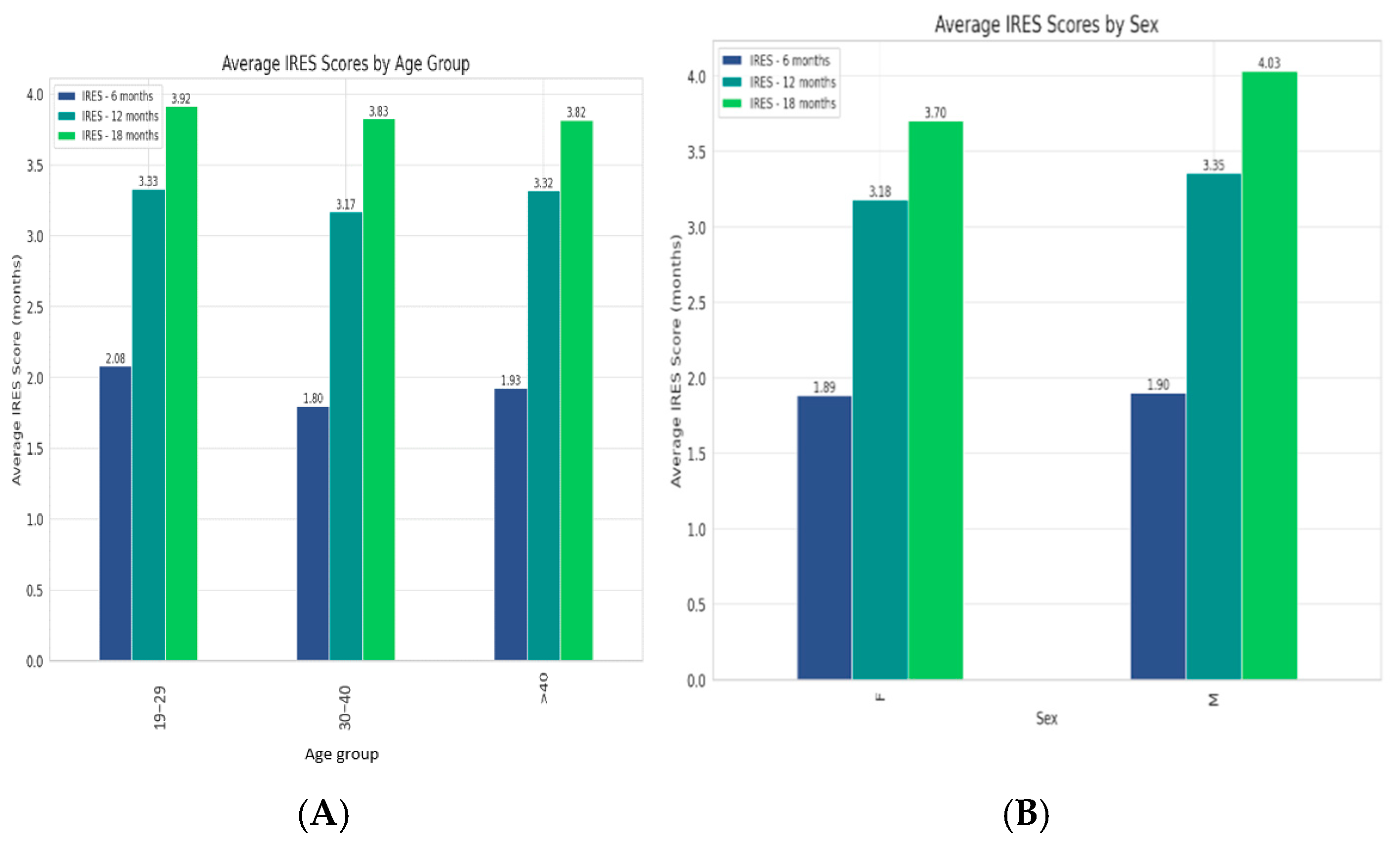
| Category | IRES 6 Months Mean | IRES 12 Months Mean | IRES 18 Months Mean | 6–12 Months t-Value | 6–12 Months p-Value | 12–18 Months t-Value | 12–18 Months p-Value |
|---|---|---|---|---|---|---|---|
| 19–29 | 2.08 | 3.33 | 3.92 | −5.23945 | <0.05 | −4.98573 | <0.05 |
| 30–40 | 1.80 | 3.17 | 3.83 | −7.69827 | <0.05 | −4.99831 | <0.05 |
| >40 | 1.93 | 3.32 | 3.82 | −7.43701 | <0.05 | −4.52522 | <0.05 |
| Sex F | 1.88 | 3.18 | 3.70 | −9.00464 | <0.05 | −5.87516 | <0.05 |
| Sex M | 1.90 | 3.35 | 4.03 | −7.21193 | <0.05 | −4.7678 | <0.05 |
| IRES Score | Pearson 6 Months | Significance p-Value 6 Months | Pearson 12 Months | Significance p-Value 12 Months | Pearson 18 Months | Significance p-Value 18 Months |
|---|---|---|---|---|---|---|
| ICV | 0.716 | <0.0001 | 0.863 | <0.0001 | 0.874 | <0.0001 |
| SDC | 0.779 | <0.0001 | 0.754 | <0.0001 | 0.816 | <0.0001 |
| SFD | 0.731 | <0.0001 | 0.819 | <0.0001 | 0.834 | <0.0001 |
| SIC | 0.303 | <0.01 | 0.54 | <0.001 | 0.454 | <0.0001 |
| Study Reference | Patient Cohort | Follow-Up Duration | Main Outcome Measures | Key Findings |
|---|---|---|---|---|
| Kawai et al., 2017 [1] | 385 patients | Up to 36 months | Seizure frequency reduction, quality of life (QoL) | Seizure control progressively improved during treatment, showing a significant median reduction of seizures at various time points. |
| Colicchio et al., 2012 [2] | 53 patients | Mean 55.96 months | Seizure frequency, responder rate, response time (RT) | Lesion etiology and an age at implant of <18 years are found to have a better response. |
| Liu et al., 2018 [3] | 32 patients | 1 year | Heart rhythm complexity HRV | VNS reversed the increased complexity in heart rhythm, which might be related to therapeutic effect. |
| Wang et al., 2019 [4] | Meta-analysis | Varies | Seizure frequency reduction >50% | A significant predictor of VNS outcomes was duration of epilepsy. |
| Elliott et al., 2011 [5] | 436 patients | Up to 11 years | Seizure frequency reduction, safety outcomes | Over 60% of these patients showed a decrease in seizure burden by at least 50%. |
| Rong et al., 2014 [6] | 50 patients | 24 weeks | Seizure frequency reduction | The effectiveness and safety of ta-VNS was confirmed with 38% seizure reduction in seizure frequency in patients up to 24 weeks. |
| Hajtovic et al., 2022 [7] | Meta-analysis on genetic etiologies | Varies | Seizure freedom rate, ≥ 50% seizure reduction | Patients with TSC had substantial seizure reduction; those with DS had less robust seizure reduction. |
| Constantinescu et al., 2020 [8] | 5 patients | 3 months | HRV parameters | VNS did not alter cardiac autonomic function after 3 months of neurostimulation. |
| Sen et al., 2021 [9] | International registry | 36–60 months | Seizure frequency, seizure severity, QoL | Aims to examine long-term safety and clinical outcomes of VNS in people with DRE. |
| Liu et al., 2018 [10] | 63 patients | 1 year | Preoperative HRV, VNS outcomes | Preoperative HRV analyses can help predict VNS outcomes in patients with DRE. |
| Bauer et al., 2016 [11] | Randomized trial | 20 weeks | Seizure frequency reduction | No superiority of 25 Hz tVNS over 1 Hz tVNS in reducing seizure frequency. |
| Zhang et al., 2019 [12] | 110 patients | Post-operative period | Seizure frequency and severity, McHugh seizure outcome | Significant reduction in seizure frequency observed; VNS effect improves over time. |
| Lagae et al., 2015 [13] | 70 patients | Varies | Responder rate, age at implantation effect | Younger age at VNS implantation might result in a better outcome. |
| Benedetti-Isaac et al., 2012 [14] | 4 patients | Post-VNS implant | Seizure frequency reduction | VNS was an alternative for seizure frequency reduction in patients with previous corpus callosotomy. |
| Nalbantoğlu et al., 2014 [15] | 35 patients | 26 ± 19.2 months | Seizure outcomes, responder rate | A total of 80% of the patients were responders, thus showing VNS as an alternative good treatment. |
| Verrier et al., 2016 [16] | 28 patients | Pre- and post-implantation | T-wave alternans (TWA) levels, cardiac electrical stability | TWA level was decreased by VNS treatment in 70% of the patients. |
| Shan et al., 2022 [17] | 45 patients | November 2016–August 2021 | Clinical outcome, safety | Confirmed efficacy and safety of VNS; no significant prognostic factors were identified. |
| Bao et al., 2011 [18] | 45 cases | Over 1 year | Seizure frequency, treatment duration | Critical for improved prognosis was a longer duration of VNS therapy. |
| Tanaka et al., 2017 [19] | Randomized, double-blind clinical trial | 20 weeks | Efficacy and safety of tVNS | tVNS was associated with high adherence to treatment and was well tolerated. The efficacy results justify further trials. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urian, F.-I.; Toader, C.; Covache Busuioc, R.-A.; Corlatescu, A.-D.; Costin, H.P.; Iacob, G.; Ciurea, A.V. Introducing the Index of Response to Stimulation (IRES): A Novel Metric for Assessing Vagus Nerve Stimulation Outcomes in Drug-Resistant Epilepsy. Medicina 2025, 61, 75. https://doi.org/10.3390/medicina61010075
Urian F-I, Toader C, Covache Busuioc R-A, Corlatescu A-D, Costin HP, Iacob G, Ciurea AV. Introducing the Index of Response to Stimulation (IRES): A Novel Metric for Assessing Vagus Nerve Stimulation Outcomes in Drug-Resistant Epilepsy. Medicina. 2025; 61(1):75. https://doi.org/10.3390/medicina61010075
Chicago/Turabian StyleUrian, Flavius-Iuliu, Corneliu Toader, Razvan-Adrian Covache Busuioc, Antonio-Daniel Corlatescu, Horia Petre Costin, Gabriel Iacob, and Alexadru Vlad Ciurea. 2025. "Introducing the Index of Response to Stimulation (IRES): A Novel Metric for Assessing Vagus Nerve Stimulation Outcomes in Drug-Resistant Epilepsy" Medicina 61, no. 1: 75. https://doi.org/10.3390/medicina61010075
APA StyleUrian, F.-I., Toader, C., Covache Busuioc, R.-A., Corlatescu, A.-D., Costin, H. P., Iacob, G., & Ciurea, A. V. (2025). Introducing the Index of Response to Stimulation (IRES): A Novel Metric for Assessing Vagus Nerve Stimulation Outcomes in Drug-Resistant Epilepsy. Medicina, 61(1), 75. https://doi.org/10.3390/medicina61010075






