The Association of Plasma Selenium and Selenoprotein P Levels with Depression Severity and Anxiety Symptoms Among Medical Students in Latvia
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Design and Sampling
2.3. Inclusion and Exclusion Criteria
2.4. Measurement Tools and Procedures
2.4.1. Initial Phase
2.4.2. Collection and Storage of Plasma Samples
2.4.3. Determination of Blood Plasma Selenium Concentration
2.4.4. Determination of Blood Plasma Selenoprotein P Concentration
2.5. Statistical Analysis
3. Results
3.1. Descriptive Statistics of the Study Population
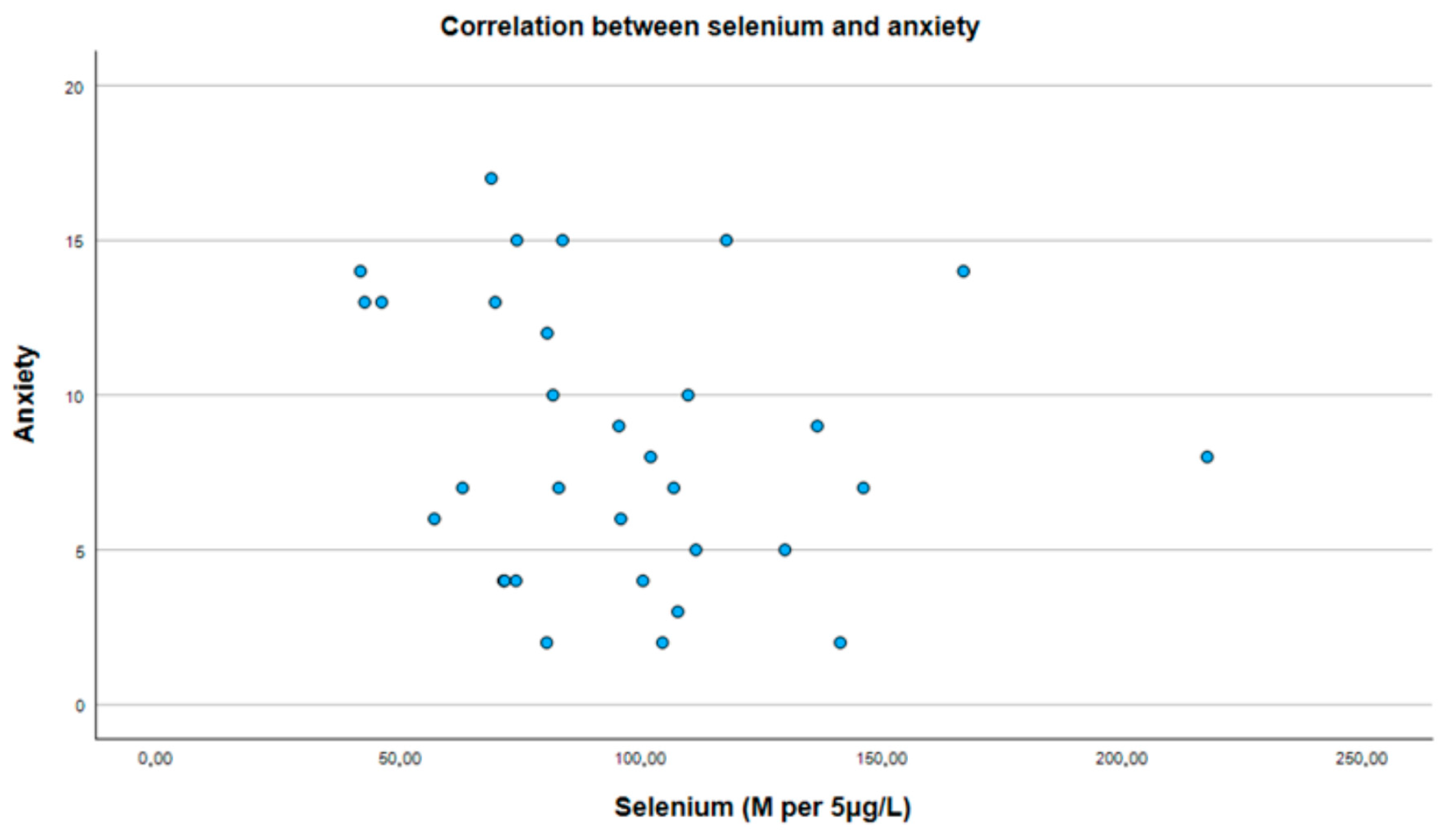
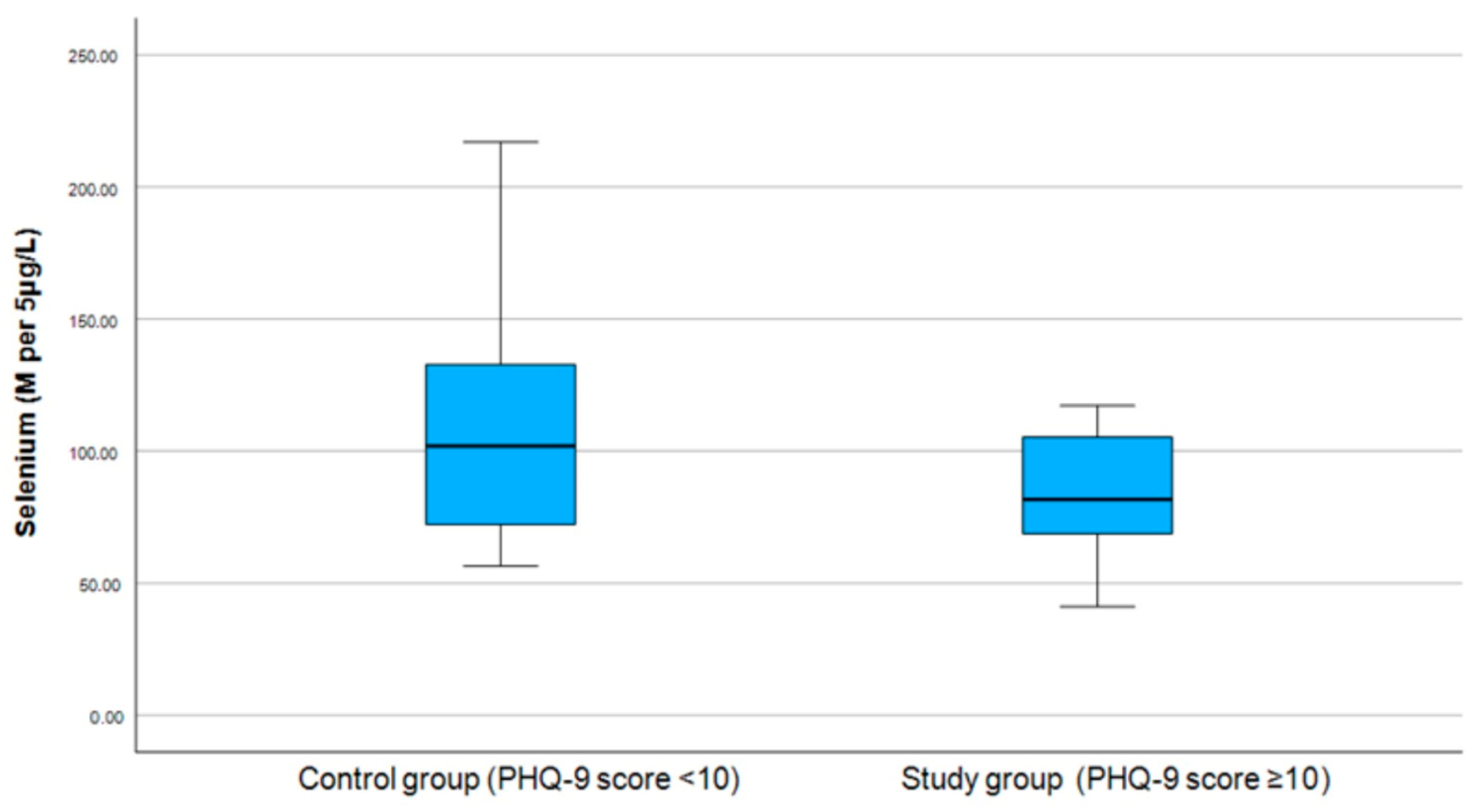
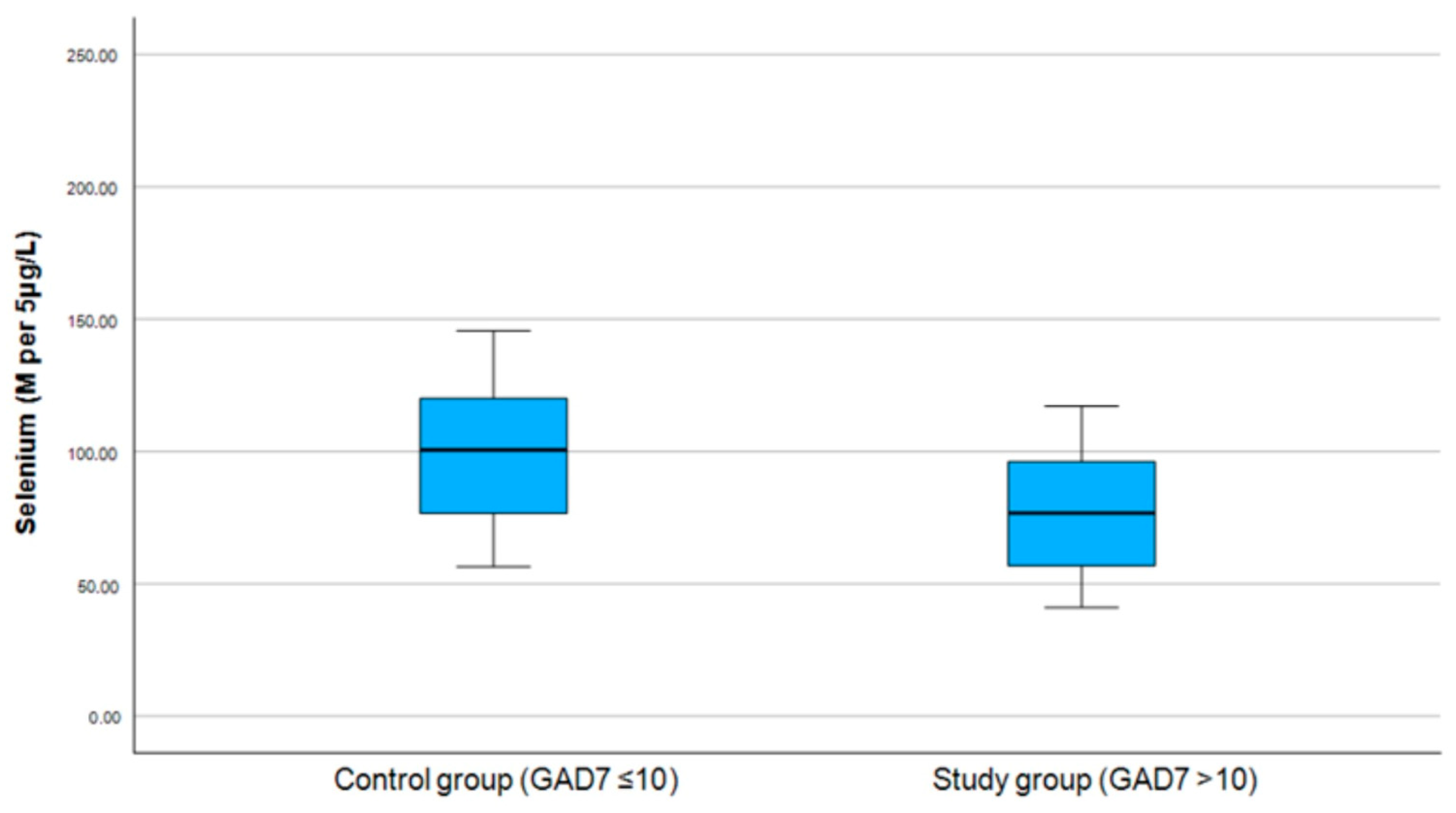
3.2. Correlation Between Selenium and PHQ-9 and GAD-7 Scores
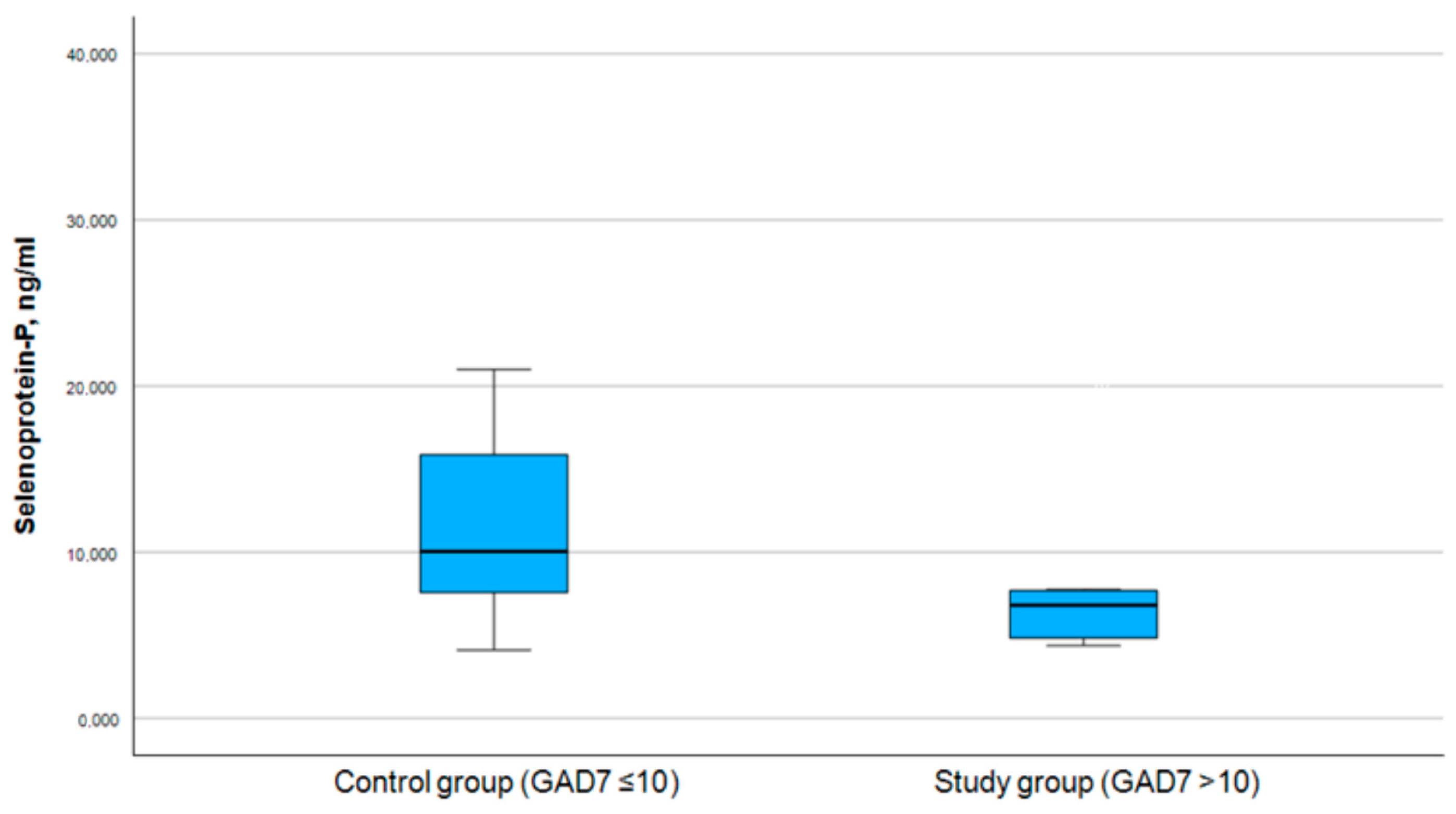
3.3. Correlation Between Selenoprotein P and PHQ-9 and GAD-7 Scores
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kupcova, I.; Danisovic, L.; Klein, M.; Harsanyi, S. Effects of the COVID-19 pandemic on mental health, anxiety, and depression. BMC Psychol. 2023, 11, 108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Fedoce, A.D.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxidative Med. Cell. Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zakeri, N.; Rezaei kelishadi, M.; Asbaghi, O.; Naeini, F.; Afsharfar, M.; Mirzadeh, E.; Naserizadeh, S.K. Selenium supplementation and oxidative stress: A review. PharmaNutrition 2021, 17, 100263. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Sies, H. Selenium homeostasis and antioxidant selenoproteins in brain: Implications for disorders in the central nervous system. Arch. Biochem. Biophys. 2013, 536, 152–157. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Selenium and Its Compounds in the Treatment of Anxiety and Related Disorders: A Scoping Review of Translational and Clinical Research. Futur. Pharmacol. 2022, 2, 608–624. [Google Scholar] [CrossRef]
- Siwek, M.; Sowa-Kućma, M.; Dudek, D.; Styczeń, K.; Szewczyk, B.; Kotarska, K.; Misztakk, P.; Pilc, A.; Wolak, M.; Nowak, G. Oxidative stress markers in affective disorders. Pharmacol. Rep. 2013, 65, 1558–1571. [Google Scholar] [CrossRef]
- Hasani, M.; Djalalinia, S.; Khazdooz, M.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Ansari, H.; Qorbani, M.; Heshmat, R. Effect of selenium supplementation on antioxidant markers: A systematic review and meta-analysis of randomized controlled trials. Hormones 2019, 18, 451–462, Erratum in: Hormones 2020, 19, 451. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sajjadi, S.S.; Foshati, S.; Haddadian-Khouzani, S.; Rouhani, M.H. The role of selenium in depression: A systematic review and meta-analysis of human observational and interventional studies. Sci. Rep. 2022, 12, 1045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solovyev, N.; Drobyshev, E.; Bjørklund, G.; Dubrovskii, Y.; Lysiuk, R.; Rayman, M.P. Selenium, selenoprotein P, and Alzheimer's disease: Is there a link? Free. Radic. Biol. Med. 2018, 127, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Hill, K.E. Selenoprotein P-expression, functions, and roles in mammals. Biochim. Biophys. Acta 2009, 1790, 1441–1447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fawzy, M.; Hamed, S.A. Prevalence of psychological stress, depression and anxiety among medical students in Egypt. Psychiatry Res. 2017, 255, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.K.; Kelly, S.J.; Adams, C.E.; Glazebrook, C. A systematic review of studies of depression prevalence in university students. J. Psychiatr. Res. 2013, 47, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Alvi, T.; Assad, F.; Ramzan, M.; Khan, F.A. Depression, anxiety and their associated factors among medical students. J. Coll. Physicians Surg. Pak. 2010, 20, 122–126. [Google Scholar] [PubMed]
- Bassols, A.M.; Okabayashi, L.S.; Silva, A.B.; Carneiro, B.B.; Feijó, F.; Guimarães, G.C.; Cortes, G.N.; Rohde, L.A.; Eizirik, C.L. First- and last-year medical students: Is there a difference in the prevalence and intensity of anxiety and depressive symptoms? Rev. Bras. Psiquiatr. 2014, 36, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, N.A.; Yaqoob, R.; Raza, A.; Shehzad, M.A.; Zeshan, S.C. Anxiety and depression among medical students: A cross-sectional study. J. Pak. Med. Assoc. 2010, 60, 699–702. [Google Scholar] [PubMed]
- Puthran, R.; Zhang, M.W.; Tam, W.W.; Ho, R.C. Prevalence of depression amongst medical students: A meta-analysis. Med. Educ. 2016, 50, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Hope, V.; Henderson, M. Medical student depression, anxiety and distress outside North America: A systematic review. Med. Educ. 2014, 48, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Banu, H.; Al-Fageer, R.; Al-Suwaidi, R. Cognitive emotions: Depression and anxiety in medical students and staff. J. Crit. Care 2009, 24, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, T.L.; Davis, L.; Wimsatt, L.A. Depression, stigma, and suicidal ideation in medical students. JAMA 2010, 304, 1181–1190. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, M.; Joneborg, N.; Runeson, B. Stress and depression among medical students: A cross-sectional study. Med. Educ. 2005, 39, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Kumano-Go, T.; Suganuma, N.; Adachi, H.; Yamamura, S.; Morishima, H.; Shigedo, Y.; Mikami, A.; Takeda, M.; Sugita, Y. Anxiety, neuroticism and oxidative stress. Psychiatry Clin. Neurosci. 2010, 64, 435–441. [Google Scholar] [CrossRef]
- Hapuarachchi, J.R.; Chalmers, A.H.; Winefield, A.H.; Blake-Mortimer, J.S. Changes in clinically relevant metabolites with psychological stress parameters. Behav. Med. 2003, 29, 52–59, Erratum in: Behav. Med. 2004, 29. [Google Scholar] [CrossRef] [PubMed]
- Vrublevska, J.; Trapencieris, M.; Rancans, E. Adaptation and validation of the Patient Health Questionnaire-9 to evaluate major depression in a primary care sample in Latvia. Nord. J. Psychiatry 2018, 72, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001, 16, 606–613. [Google Scholar] [CrossRef]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.; Löwe, B. A brief measure for assessing generalized anxiety disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef]
- Swinson, R.P. The GAD-7 scale was accurate for diagnosing generalised anxiety disorder. Evid. Based Med. 2006, 11, 184. [Google Scholar] [CrossRef]
- Vrublevska, J.; Renemane, L.; Kivite-Urtane, A.; Rancans, E. Validation of the generalized anxiety disorder scales (GAD-7 and GAD-2) in primary care settings in Latvia. Front. Psychiatry 2022, 13, 972628. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Batra, J. Oxidative stress and psychological functioning among medical students. Ind. Psychiatry J. 2014, 23, 127–133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sivonová, M.; Zitnanová, I.; Hlincíková, L.; Skodácek, I.; Trebatická, J.; Duracková, Z. Oxidative stress in university students during examinations. Stress 2004, 7, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Alfthan, G. A micromethod for the determination of selenium in tissues and biological fluids by single-test-tube fluorimetry. Anal. Chim. Acta 1984, 165, 187–194. [Google Scholar] [CrossRef]
- Human Selenoprotein P(SEPP1) ELISA Kit. Available online: https://www.cusabio.com/ELISA-Kit/Human-Selenoprotein-PSEPP1-ELISA-kit-102874.html (accessed on 19 August 2024).
- Ferreira de Almeida, T.L.; Petarli, G.B.; Cattafesta, M.; Zandonade, E.; Bezerra, O.M.P.A.; Tristão, K.G.; Salaroli, L.B. Association of Selenium Intake and Development of Depression in Brazilian Farmers. Front Nutr. 2021, 8, 671377, Erratum in: Front. Nutr. 2021, 8, 756637. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Portnoy, J.; Wang, J.; Wang, F.; Um, P.; Irving, S.Y.; Hackl, L.; Liu, J. Lower serum selenium concentration associated with anxiety in children. J. Pediatr. Nurs. 2022, 63, e121–e126. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shokati, S.; Kavian, Z.; Shahraki, M.; Afshari, M. The Relationship of Dietary Intake of Zinc, Selenium, and Magnesium and Anthropometric Profiles with Depression in Female Medical Students at Zahedan University of Medical Sciences. J. Nutr. Food Secur. 2021, 6, 232–238. [Google Scholar] [CrossRef]
- Conner, T.S.; Richardson, A.C.; Miller, J.C. Optimal serum selenium concentrations are associated with lower depressive symptoms and negative mood among young adults. J. Nutr. 2015, 145, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dogaru, C.B.; Muscurel, C.; Duță, C.; Stoian, I. “Alphabet” Selenoproteins: Their Characteristics and Physiological Roles. Int. J. Mol. Sci. 2023, 24, 15992. [Google Scholar] [CrossRef] [PubMed]
- Moghadaszadeh, B.; Beggs, A.H. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology 2006, 21, 307–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saito, Y. Selenium Transport Mechanism via Selenoprotein P-Its Physiological Role and Related Diseases. Front. Nutr. 2021, 8, 685517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amirkhizi, F.; Khalese-Ranjbar, B.; Mansouri, E.; Hamedi-Shahraki, S.; Asghari, S. Correlations of selenium and selenoprotein P with asymmetric dimethylarginine and lipid profile in patients with polycystic ovary syndrome. J. Trace Elements Med. Biol. 2023, 75, 127101. [Google Scholar] [CrossRef] [PubMed]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almadani, A.H.; Alsubaihi, A.A.; Alsqabi, H.A.; Alkathiri, M.A.; Alassaf, M.I.; Alagel, O.A.; Alshowihi, S.S.; Alolayan, M.A. Comparison of depression and anxiety in first- versus non-first generation Saudi medical students: A cross-sectional study. Medicine 2024, 103, e39115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nunes, J.K.V.R.S.; Figueiredo, V.M.e.S.d.; Santos, J.V.S.S.; Mendes, N.M.L.d.S.; Neto, J.A.d.F. Anxiety and depression in medical students: A cross-sectional study. Rev. Med. 2022, 101, e-195874. [Google Scholar] [CrossRef]
- Mallaram, G.K.; Shaik, S.; Kattula, D. Anxiety, Depression and Stress Among Female Medical Students During the Second Wave of the COVID-19 Pandemic and their Association with Family Functioning, Coping and Personality. Curr. Med Issues 2023, 21, 31–36. [Google Scholar] [CrossRef]




| Baseline Characteristic | n (%) |
|---|---|
| Male | 3 (9.4) |
| Female | 29 (90.6) |
| Previously diagnosed anxiety or depression | 13 (40.6) |
| Age (mean (SD)) Selenium (M per 5 μg/L) | 22.2 (0.7) 81.7 |
| Patient Health Questionnaire-9 (PHQ-9) | Generalized Anxiety Disorder (GAD-7) | |
|---|---|---|
| Study group | ||
| Median score | 15.0 | 13.0 |
| IQR | 10.3–19.8 | 9.3–14.8 |
| Control group | ||
| Median score | 4.5 | 4.5 |
| IQR | 3.0–7.8 | 3.3–7.0 |
| GAD-7 Score (Symptoms of Anxiety) | PHQ-9 Score (Symptoms of Depression) | |
|---|---|---|
| Selenium | p = 0.275, r = −0.199 | p = 0.267, r = −0.202 |
| Selenium Level | Below the Normal Range, n (% of This Group) | Within the Normal Range, n (% of This Group) | Above the Normal Range, n (% of This Group) | p-Value |
|---|---|---|---|---|
| Control group | 7 (53.85) | 8 (61.54) | 1 (16.67) | 0.174 |
| Study group | 6 (46.15) | 5 (38.46) | 5 (83.33) |
| Selenoprotein P, ng/mL (Mean) | Anxiety | Depression | |
|---|---|---|---|
| Selenoprotein P | 8.1 ng/mL | p = 0.12 | p = 0.006 |
| PHQ-9 Score (Symptoms of Depression) | GAD-7 Score (Symptoms of Anxiety) | |||||
|---|---|---|---|---|---|---|
| Selenoprotein P Level (Median) | Chi-Square | p-Value | Selenoprotein P Level (Median) | Chi-Square | p-Value | |
| Control group | 10.049 | 9.534 | ||||
| 17.052 | <0.001 | 7.888 | 0.005 | |||
| Study group | 6.770 | 6.770 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birģele, Z.; Vimba, P.M.; Ševčenko, A.; Šķesters, A.; Ancāne, G.; Valaine, L. The Association of Plasma Selenium and Selenoprotein P Levels with Depression Severity and Anxiety Symptoms Among Medical Students in Latvia. Medicina 2025, 61, 3. https://doi.org/10.3390/medicina61010003
Birģele Z, Vimba PM, Ševčenko A, Šķesters A, Ancāne G, Valaine L. The Association of Plasma Selenium and Selenoprotein P Levels with Depression Severity and Anxiety Symptoms Among Medical Students in Latvia. Medicina. 2025; 61(1):3. https://doi.org/10.3390/medicina61010003
Chicago/Turabian StyleBirģele, Zanda, Paula Marija Vimba, Anastasija Ševčenko, Andrejs Šķesters, Gunta Ancāne, and Laura Valaine. 2025. "The Association of Plasma Selenium and Selenoprotein P Levels with Depression Severity and Anxiety Symptoms Among Medical Students in Latvia" Medicina 61, no. 1: 3. https://doi.org/10.3390/medicina61010003
APA StyleBirģele, Z., Vimba, P. M., Ševčenko, A., Šķesters, A., Ancāne, G., & Valaine, L. (2025). The Association of Plasma Selenium and Selenoprotein P Levels with Depression Severity and Anxiety Symptoms Among Medical Students in Latvia. Medicina, 61(1), 3. https://doi.org/10.3390/medicina61010003






