Hemoadsorption in Multiorgan Failure Due to Viscerocutaneous Loxoscelism
Abstract
1. Introduction
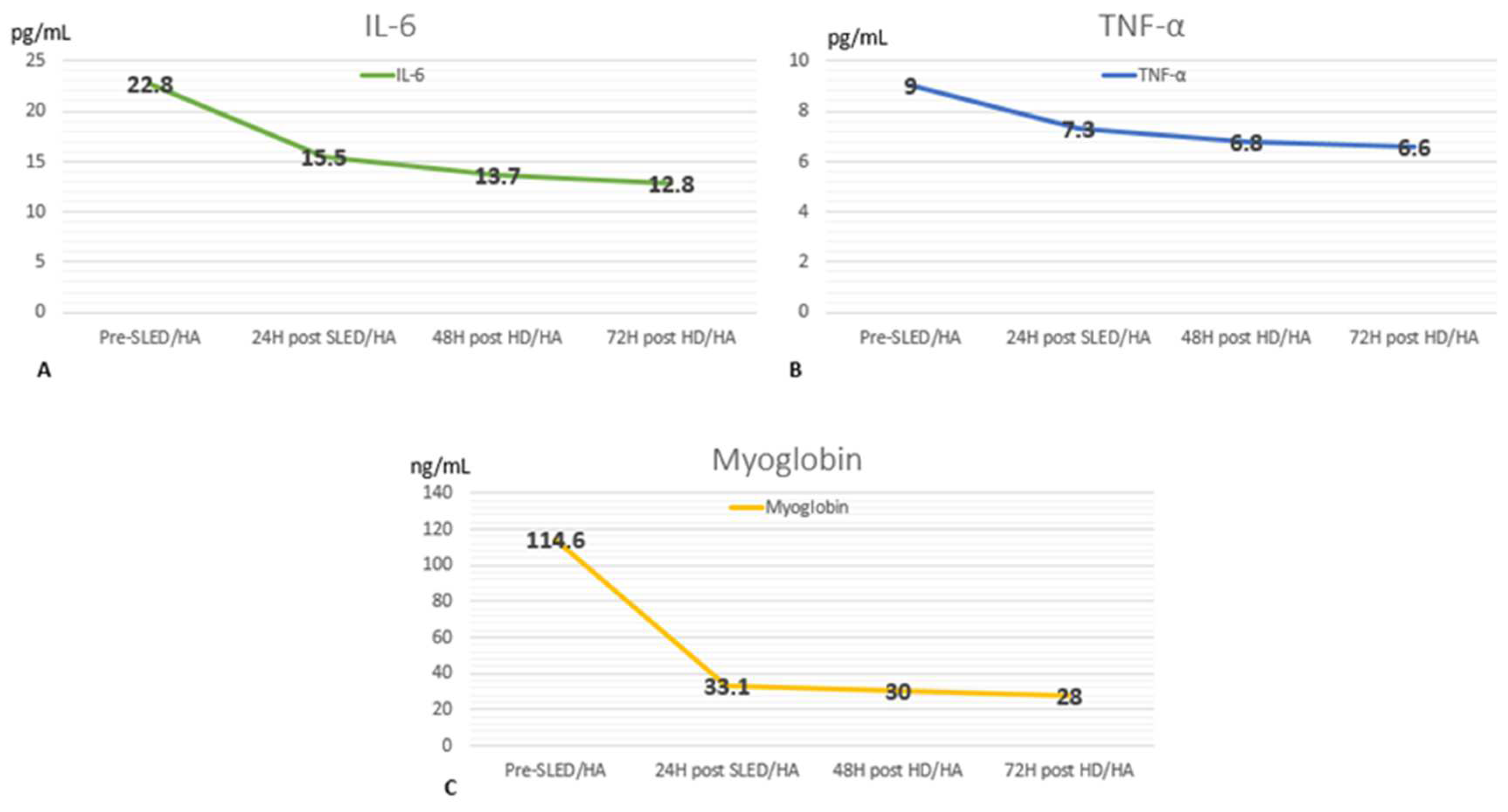
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Severino, F.B.; Vivanco, P.; Mix, A. Loxoscelismo: Revisión de la literatura a propósito de un caso: Loxoscelism: Literary review about a case. ARS MEDICA Rev. Cienc. Méd. 2022, 47, 29–35. [Google Scholar] [CrossRef]
- Ríos, J.C.; Pérez, M.; Sánchez, P.; Bettini, M.; Mieres, J.J.; Paris, E. Caracterización clínico-epidemiológica telefónica de la mordedura por araña de rincón, en un centro de información toxicológica de Chile, durante el año 2005. Rev. Méd. Chile 2007, 135, 1160–1165. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marques-da-Silva, E.; Souza-Santos, R.; Fischer, M.L.; Rubio, G.B.G. Loxosceles spider bites in the state of Paraná, Brazil: 1993–2000. J. Venom. Anim. Toxins Incl. Trop. Dis. 2006, 12, 110–123. [Google Scholar] [CrossRef]
- Alcides Zambrano, F.; Jorge González, C.; Guillermo Callejas, G. Desenlace fatal por loxoscelismo cutáneo visceral. Rev. Méd. Chile 2005, 133, 219–223. [Google Scholar]
- Gremski, L.H.; da Justa, H.C.; Polli, N.L.C.; Schluga, P.H.d.C.; Theodoro, J.L.; Wille, A.C.M.; Senff-Ribeiro, A.; Veiga, S.S. Systemic Loxoscelism, Less Frequent but More Deadly: The Involvement of Phospholipases D in the Pathophysiology of Envenomation. Toxins 2023, 15, 17. [Google Scholar] [CrossRef]
- Albuquerque, P.L.M.M.; Tessarolo, L.D.; Menezes, F.H.; Lima, T.B.D.; Paiva, J.H.H.G.L.; Silva, G.B.D.; Martins, A.M.C.; Daher, E.D.F. Acute kidney injury due to systemic Loxoscelism: A cross-sectional study in Northeast Brazil. Rev. Soc. Bras. Med. Trop. 2018, 51, 695–699. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, R.B.; dos Santos Filho, J.F.; Mangili, O.C.; Veiga, S.S.; Gremski, W.; Nader, H.B.; von Dietrich, C.P. Identification of proteases in the extract of venom glands from brown spiders. Toxicon 2002, 40, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Gremski, L.H.; da Justa, H.C.; da Silva, T.P.; Polli, N.L.C.; Antunes, B.C.; Minozzo, J.C.; Wille, A.C.M.; Senff-Ribeiro, A.; Arni, R.K.; Veiga, S.S. Forty Years of the Description of Brown Spider Venom Phospholipases-D. Toxins 2020, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Malaque, C.M.; Ori, M.; Santos, S.A.; Andrade, D.R. Production of TNF-alpha by primary cultures of human keratinocytes challenged with loxosceles gaucho venom. Rev. Inst. Med. Trop. Sao Paulo 1999, 41, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Tambourgi, D.V.; Petricevich, V.L.; Magnoli, F.C.; Assaf, S.L.; Jancar, S.; Dias Da Silva, W. Endotoxemic-like shock induced by Loxosceles spider venoms: Pathological changes and putative cytokine mediators. Toxicon 1998, 36, 391–403. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.L.; Malaque, C.M.; Sztajnbok, J.; Romano, C.C.; Duarte, A.J.; Seguro, A.C. Loxosceles venom-induced cytokine activation, hemolysis, and acute kidney injury. Toxicon 2008, 51, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.; Torosyan, R.; Ginsberg, C.; Liapis, H.; Morrison, A.R. Clinicopathological course of acute kidney injury following brown recluse (Loxoscles reclusa) envenomation. Clin. Kidney J. 2013, 6, 609–612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucía, C. Loxoscelismo cutáneo. Dermatol. Argent. 2019, 25, 64–68, ISSN 1515-8411. [Google Scholar]
- Pauli, I.; Puka, J.; Gubert, I.C.; Minozzo, J.C. The efficacy of antivenom in loxoscelism treatment. Toxicon 2006, 48, 123–137. [Google Scholar] [CrossRef]
- Mehr, J.; Kim, J. The use of therapeutic plasma exchange in systemic loxoscelism induced treatment resistant hemolytic anemia: A case report. Transfus. Apher. Sci. 2024, 63, 103960. [Google Scholar] [CrossRef] [PubMed]
- Harry, S.; Brugioni, E.; Madhusudhana, S. Acute hemolytic anemia caused by loxoscelism treated with plasmapheresis: A case report. J. Med. Cases 2022, 13, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, B.; Stahl, K.; David, S. Extracorporeal techniques for blood purification in sepsis: An update. Internist 2020, 61, 1010–1016. [Google Scholar] [CrossRef]
- Saldaña-Gastulo, J.J.C.; Llamas-Barbarán, M.D.R.; Coronel-Chucos, L.G.; Hurtado-Roca, Y. Cytokine hemoadsorption with CytoSorb® in patients with sepsis: A systematic review and meta-analysis. Crit. Care Sci. 2023, 35, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Bellomo, R. Hemoperfusion: Technical aspects and state of the art. Crit. Care 2022, 26, 1–12. [Google Scholar] [CrossRef]
- KDIGO. KDIGO Clinical Practice Guideline for Acute Kidney Injury [Internet]. 2012. Available online: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-AKI-Guideline-English.pdf (accessed on 22 March 2012).
- Droppelmann, K.; Majluf-Cáceres, P.; Sabatini-Ugarte, N.; Valle, E.; Herrera, H.; Acuña, D. Caracterización clínica y epidemiológica de 200 pacientes con loxoscelismo cutáneo y cutáneo visceral [Loxoscelism. Experience in 200 patients]. Rev. Med. Chil. 2021, 149, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Malaque, C.M.S.; Novaes, C.T.G.; Piorelli, R.O.; Risk, J.Y.; Murad, J.C.; Lara, A.N.; Virgulino, C.C.; Miyaji, K.T.; Santoro, M.L. Impact of antivenom administration on the evolution of cutaneous lesions in loxoscelism: A prospective observational study. PLoS Negl. Trop. Dis. 2022, 16, e0010842. [Google Scholar] [CrossRef] [PubMed]
- Tolwani, A. Continuous Renal-Replacement Therapy for Acute Kidney Injury. N. Engl. J. Med. 2012, 367, 2505–2514. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.; Santos, R.; Coelho, S. The role of sustained low-efficiency dialysis (SLED) in the Intensive Care Unit, Portuguese. J. Nephrol. Hypertens. 2022, 36, 138–147. [Google Scholar] [CrossRef]
- Cruz Llanos, L.E.; Valenzuela Córdova, C.R.; León Rabanal, C.P.; Cieza Zevallos, J.A. Hemoperfusión en pacientes con enfermedades críticas e injuria renal aguda. Reporte de tres casos. Rev. Nefrol. Dial. Traspl. 2021, 41, 48–54. [Google Scholar]
- Kellum, J.A.; Ronco, C. The role of endotoxin in septic shock. Crit. Care 2023, 27, 400. [Google Scholar] [CrossRef]
- Ricci, Z.; Romagnoli, S.; Reis, T.; Bellomo, R.; Ronco, C. Hemoperfusion in the intensive care unit. Intensive Care Med. 2022, 48, 1397–1408. [Google Scholar] [CrossRef]
- Asgharpour, M.; Mehdinezhad, H.; Bayani, M.; Zavareh, M.S.H.; Hamidi, S.H.; Akbari, R.; Ghadimi, R.; Bijani, A.; Mouodi, S. Effectiveness of extracorporeal blood purification (hemoadsorption) in patients with severe coronavirus disease 2019 (COVID-19). BMC Nephrol. 2020, 20, 21. [Google Scholar] [CrossRef]
- Darazam, I.A.; Kazempour, M.; Pourhoseingholi, M.A.; Hatami, F.; Rabiei, M.M.; Gharehbagh, F.J.; Amirdosara, M.; Hajiesmaeili, M.; Shabani, M.; Shokouhi, S.; et al. Efficacy of Hemoperfusion in Severe and Critical Cases of COVID-19. Blood Purif. 2022, 52, 1–9. [Google Scholar]
- Sazonov, V.; Abylkassov, R.; Tobylbayeva, Z.; Saparov, A.; Mironova, O.; Poddighe, D. Case Series: Efficacy and Safety of Hemoadsorption With HA-330 Adsorber in Septic Pediatric Patients with Cancer. Front. Pediatr. 2021, 9, 672260. [Google Scholar] [CrossRef]
- Honoré, P.M.; De Bels, D.; Gutierrez, L.B.; Spapen, H.D. Hemoadsorption therapy in the critically ill: Solid base but clinical haze. Ann. Intensive Care 2019, 9, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Onuk, S.; Akin, A.K.; Sari, A.; Gundogan, K.; Baskol, G.; Dogru, K.; Sungur, M. The Clinical and Laboratory Efficacy of HA 330 Treatment Combined with Continuous Renal Replacement Therapy in Septic Shock Patients: A Case Series. Blood Purif. 2023, 52, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Biological, N. TNF-alpha Antibody (52B83) [Internet]. Novus Biologicals. 2014. Available online: https://www.novusbio.com/products/tnf-alpha-antibody-52b83_nb600-1422 (accessed on 3 November 2014).
- Reis, T.; Ronco, C.; Soranno, D.E.; Clark, W.; De Rosa, S.; Forni, L.G.; Lorenzin, A.; Ricci, Z.; Villa, G.; Kellum, J.A.; et al. Standardization of Nomenclature for the Mechanisms and Materials Utilized for Extracorporeal Blood Purification. Blood Purif. 2023, 1–14, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Heymann, M.; Schorer, R.; Putzu, A. Mortality and adverse events of hemoadsorption with CytoSorb® in critically ill patients: A systematic review and meta-analysis of randomized controlled trials. Acta Anaesthesiol. Scand. 2022, 66, 1037–1050. [Google Scholar] [CrossRef]
- Abraham, M.; Tilzer, L.; Sarah Hoehn, K.; Thornton, S.L. Therapeutic Plasma Exchange for Refractory Hemolysis After Brown Recluse Spider (Loxosceles reclusa) Envenomation. J. Med. Toxicol. 2015, 11, 364–367. [Google Scholar] [CrossRef] [PubMed]
| Day 1 | Day 2 | Day 3 | Day 6 | Day 10 | Day 18 | Normal Values | |
|---|---|---|---|---|---|---|---|
| Hematocrit (%) | 42 | 35 | 32 | 28 | 26 | 27 | 36–44 |
| White blood count (/mm3) | 10,700 | 15,290 | 10,990 | 10,390 | 8090 | 6920 | 4000–12,000 |
| Platelets (103/mm3) | 180 | 173 | 160 | 196 | 258 | 321 | 150–400 |
| Serum glucose (mg/dL) | 134 | 173 | 178 | 120 | 76 | 80 | 70–125 |
| Serum urea (mg/dL) | 125 | 232 | 150 | 97 | 63 | 62 | 5–20 |
| Serum creatinine (mg/dL) | 1.82 | 5.51 | 5.08 | 4.45 | 2.35 | 1.5 | 0.6–1.1 |
| Serum sodium (mEq/L) | 132 | 135 | 136 | 137 | 138 | 141 | 135–145 |
| Serum potassium (mEq/L) | 4.8 | 4.38 | 4.9 | 4.5 | 4.3 | 4.38 | 3.5–5.5 |
| Serum chloride (mEq/L) | 100 | 101 | 101 | 100 | 97 | 104 | 96–106 |
| Serum Ionic calcium (mmol/L) | - | 0.95 | 1.05 | 1.2 | 1.18 | 1.1 | 1.15–1.29 |
| Alanine transaminase (U/L) | 158 | 29 | 44 | 32 | 24 | 22 | 4–36 |
| Aspartate transferase(U/L) | 195 | 32 | 45 | 41 | 38 | 24 | 8–33 |
| Alkaline phosphatase (U/L) | 80 | 64 | 59 | 72 | 130 | 117 | 44–147 |
| Prothrombin time (seconds) | - | 30 | 14.7 | 14.3 | 13.7 | 16,5 | 11–13.5 |
| PTTa (seconds) | - | 38 | 37.4 | 29 | 30 | 34 | 25–35 |
| INR | - | 2.93 | 1.05 | 1.02 | 1 | 1.1 | 0.8–1.1 |
| Total bilirubin (mg/dL) | 21.61 | 19.4 | 8.9 | 5.1 | 1.3 | 1 | 0.1–1.2 |
| Indirect bilirubin (mg/dL) | 16.81 | 17.4 | 7.5 | 4 | 1.1 | 0.5 | 0.2–0.8 |
| Serum Albumin (g/dL) | - | 2.8 | 2.4 | 2.5 | - | 2.9 | 3.4–5.4 |
| LDH (UI/L) | - | 2151 | 1593 | 523 | 320 | 290 | 140–280 |
| Ph | - | 7.25 | 7.3 | 7.46 | - | 7.48 | 7.37–7.42 |
| HCO3 (mmol/L) | - | 20.3 | 17 | 22 | - | 27 | 22–29 |
| pCO2 (mmHg) | - | 14 | 27 | 29 | - | 36 | 35–45 |
| pO2 (mmHg) | - | 115 | 100 | 89 | - | 90 | 75–100 |
| Oxygen saturation (%) | - | 93 | 95 | 94 | - | 97 | 95–100 |
| Fraction inspired O2 (%). | - | 21 | 30 | 21 | - | 21 | 21 |
| Lactate (mmol/L) | - | 0.6 | 0.8 | - | - | 1.2 | <1.6 |
| Urine pH | - | 4 | 6 | - | - | 5 | 4–7 |
| Urine density | - | 1025 | 1015 | - | - | 1015 | 1005–1030 |
| Urine hemoglobin | - | +3 | +3 | - | - | Neg. | Neg. |
| Urine bilirubin | - | Neg. | +2 | - | - | Neg. | Neg. |
| Albuminuria | - | +2 | +3 | - | - | Neg. | Neg. |
| Urine leucocytes per HPF | - | 6–10 | >100 | - | - | 2–4 | <5 |
| Urine red blood cells per HPF | - | 8–10 | 2–4 | - | - | 1–2 | <2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenzuela Córdova, R.; Rivera Estrella, D.; Bernardo, J.F.; Jiménez, D.; Rodríguez Tudero, C.; Elías, R.; De La Flor, J.C. Hemoadsorption in Multiorgan Failure Due to Viscerocutaneous Loxoscelism. Medicina 2025, 61, 143. https://doi.org/10.3390/medicina61010143
Valenzuela Córdova R, Rivera Estrella D, Bernardo JF, Jiménez D, Rodríguez Tudero C, Elías R, De La Flor JC. Hemoadsorption in Multiorgan Failure Due to Viscerocutaneous Loxoscelism. Medicina. 2025; 61(1):143. https://doi.org/10.3390/medicina61010143
Chicago/Turabian StyleValenzuela Córdova, Raúl, David Rivera Estrella, José F. Bernardo, Darío Jiménez, Celia Rodríguez Tudero, Raúl Elías, and José C. De La Flor. 2025. "Hemoadsorption in Multiorgan Failure Due to Viscerocutaneous Loxoscelism" Medicina 61, no. 1: 143. https://doi.org/10.3390/medicina61010143
APA StyleValenzuela Córdova, R., Rivera Estrella, D., Bernardo, J. F., Jiménez, D., Rodríguez Tudero, C., Elías, R., & De La Flor, J. C. (2025). Hemoadsorption in Multiorgan Failure Due to Viscerocutaneous Loxoscelism. Medicina, 61(1), 143. https://doi.org/10.3390/medicina61010143






