Chitosan’s Ability to Remove the Smear Layer—A Systematic Review of Ex Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Selection Process and Data Collection
2.4. Risk of Bias Assessment
3. Results
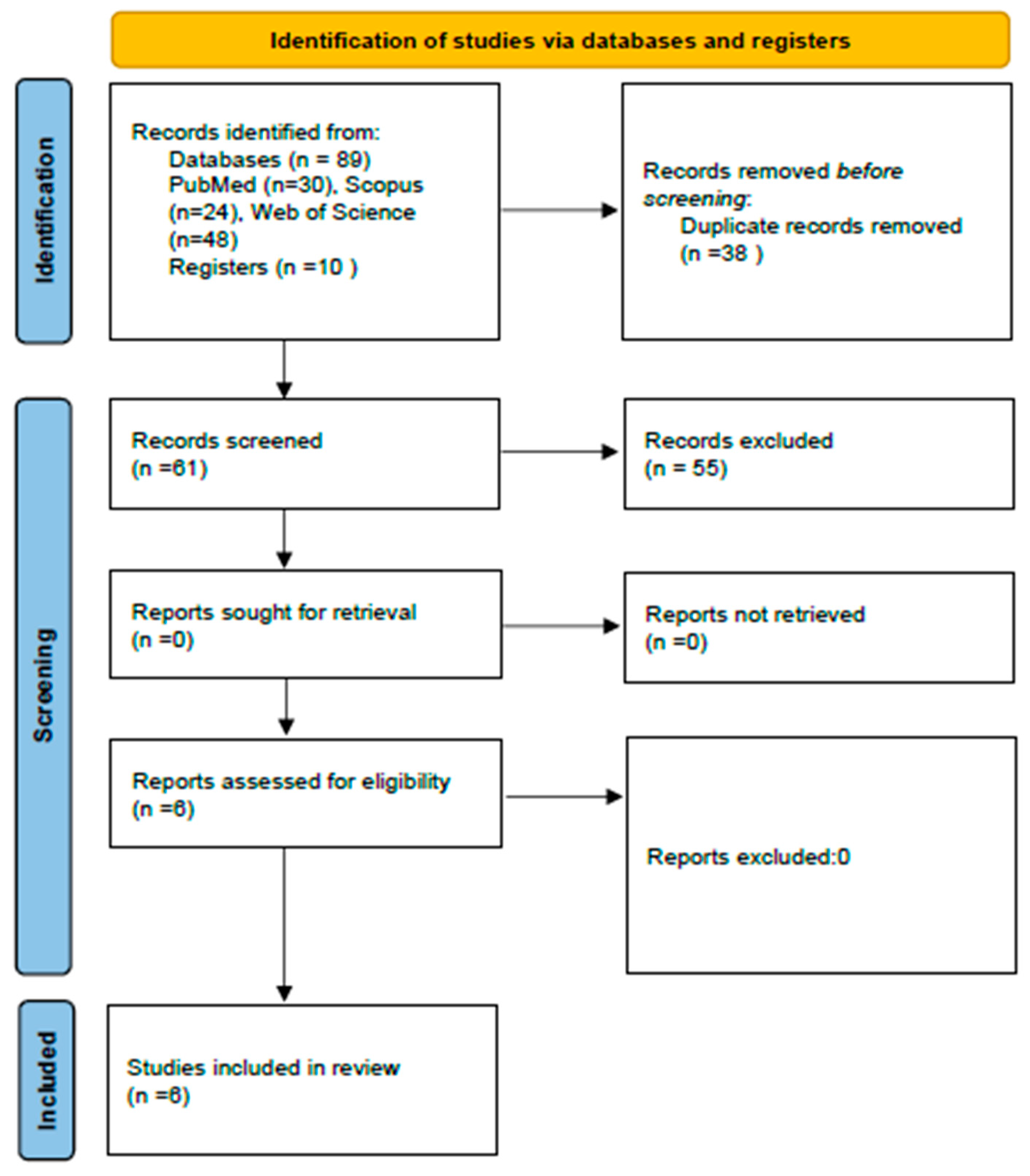
3.1. Selection of the Studies
3.2. Data Collection
3.2.1. Type of Teeth and Sample Size
3.2.2. Irrigation Protocols
3.2.3. Type of Chitosan Used
3.2.4. Volume and Time of Irrigation
3.2.5. Size of Apical Preparation
3.2.6. Selection of Irrigation Needle and Activation
3.2.7. Method of Smear Layer Assessment
3.2.8. Comparison of the Chitosan with Other Irrigants
3.3. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pimenta, J.A.; Zaparolli, D.; Pécora, J.D.; Cruz-Filho, A.M. Chitosan: Effect of a new chelating agent on the microhardness of root dentin. Braz. Dent. J. 2012, 23, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Vineetha, C.; Ravi, V.; Sampathkumar, S.J.; Prasad, S.A.; Soundappan, S.P.; Chittrarasu, M. Scanning electron microscopic comparative analysis of smear layer removal using ethylenediaminetetraacetic acid and chitosan activated by ultrasonics and diode laser: An In vitro study. Indian J. Dent. Sci. 2022, 14, 74–78. [Google Scholar] [CrossRef]
- Likhitkar, M.S.; Kulkarni, S.V.; Burande, A.; Solanke, V.; Kumar, C.S.; Kamble, B. To evaluate the influence of smear layer with different instruments and obturation methods on microleakage of root canal filled teeth: In vitro study. J. Int. Soc. Prev. Community Dent. 2016, 6, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Jyotsnanjali, T.; Ranjini, M.A.; Kumar, G.R.K.; Swapna, D.V.; Joshi, S.N.; Nadig, R.R. Evaluation of chelating effect of chitosan as intracanal lubricant and an irrigant on smear layer removal—An in-vitro scanning electron microscope study. Endodontology 2023, 35, 254–261. [Google Scholar] [CrossRef]
- Gómez-Delgado, M.; Camps-Font, O.; Luz, L.; Sanz, D.; Mercade, M. Update on citric acid use in endodontic treatment: A systematic review. Odontology 2023, 111, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, S.; Mallya, P.L.; Ballal, V.; Shenoy, R. To evaluate and compare the effect of 17% EDTA, 10% citric acid, 7% maleic acid on the dentinal tubule penetration depth of bio ceramic root canal sealer using confocal laser scanning microscopy: An in vitro study. F1000Res 2023, 11, 1561. [Google Scholar] [CrossRef]
- Meyappan, N.; Mahadevan, M.; Manimaran, N.D.; Paulaian, B.; Gopal, R.; Kumar, N. Scanning Electron Microscopy Analysis of Smear Layer Removal Ability of Conventional Endodontic Irrigation Regimen, MTAD, and QMix™ Versus a Mixture of Azadirachta indica and Citrus limon: An In Vitro Study. Cureus 2023, 15, e42877. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Vaswani, S.D.; Najan, H.B.; Mehta, D.L.; Kamble, A.B.; Chaudhari, S.D. Scanning electron microscopic evaluation of smear layer removal at the apical third of root canals using diode laser, endoActivator, and ultrasonics with chitosan: An in vitro study. J. Conserv. Dent. 2019, 22, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Nikhil, V.; Jaiswal, S.; Bansal, P.; Arora, R.; Raj, S.; Malhotra, P. Effect of phytic acid, ethylenediaminetetraacetic acid, and chitosan solutions on microhardness of the human radicular dentin. J. Conserv. Dent. 2016, 19, 179–183. [Google Scholar] [CrossRef]
- Rajachar, P.B.; Vidhya, M.S.; Karale, R.; Govindaraju, V.K.; Shetty, N.K. Evaluation of free available chlorine of sodium hypochlorite when admixed with 0.2% Chitosan: A preliminary study. J. Contemp. Dent. Pr. 2021, 22, 1171–1174. [Google Scholar]
- Chia, M.S.Y.; Parolia, A.; Lim, B.S.H.; Jayaraman, J.; Porto, I.C.C.M. Effect of QMix irrigant in removal of smear layer in root canal system: A systematic review of in vitro studies. Restor. Dent. Endod. 2020, 45, e28. [Google Scholar] [CrossRef]
- Saha, S.G.; Sharma, V.; Bharadwaj, A.; Shrivastava, P.; Saha, M.K.; Dubey, S.; Kala, S.; Gupta, S. Effectiveness of various endodontic irrigants on the micro-hardness of the root canal dentin: An in vitro study. J. Clin. Diagn. Res. 2017, 11, ZC01–ZC04. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz-Filho, A.M.; Bordin, A.R.D.V.; Souza-Flamini, L.E.; Guedes, D.F.D.C.; Saquy, P.C.; Silva, R.G.; Pécora, J.D. Analysis of the shelf life of chitosan stored in different types of packaging, using colorimetry and dentin microhardness. Restor. Dent. Endod. 2017, 42, 87–94. [Google Scholar] [CrossRef]
- Mankeliya, S.; Jaiswal, N.; Singhal, R.K.; Gupta, A.; Pathak, V.K.; Kushwah, A. A comparative evaluation of smear layer removal by using four different irrigation solutions like root canal irrigants: An in vitro SEM study. J. Contemp. Dent. Pr. 2021, 22, 527–531. [Google Scholar] [CrossRef]
- da Silva Mira, P.C.; Souza-Flamini, L.E.; da Costa Guedes, D.F.; Da Cruz-Filho, A.M.D. Evaluation of the chelating effect of chitosan solubilized in different acids. J. Conserv. Dent. 2017, 20, 297–301. [Google Scholar] [CrossRef]
- Rao, P.D.; Sandeep, A.H.; Madhubala, M.M.; Mahalaxmi, S. Comparative evaluation of effect of nisin-incorporated ethylenediamine tetraacetic acid and MTAD on endodontic biofilm eradication, smear layer removal, and depth of sealer penetration. Clin. Oral. Investig. 2023, 27, 7247–7259. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, M.; Pillai, R.; Varghese, N.O.; Salim, A.A.; Murali, N.; Nair, S.S. An in vitro study of comparative evaluation of efficacy of electrochemically activated water as a root canal irrigant in smear layer removal. J. Conserv. Dent. 2020, 23, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, M.; Pighinelli, L.; Tedesco, M.F.; Silva, M.M.; Reis, V. Chitosan-Properties and Applications in Dentistry. Adv. Tissue Eng. Regen. Med. Open Access 2017, 2, 205–211. [Google Scholar]
- İlhan, H.; Cakici, E.B.; Cakici, F. The comparative of chitosan and chitosan nanoparticle versus ethylenediaminetetraacetic acid on the smear layer removal: A systematic review and meta-analysis of in vitro study. Microsc. Res. Tech. 2024, 87, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Anbalagan, K.; Jena, A.; Mohanty, S.; Mallick, R.; Shashirekha, G.; Sarangi, P. Smear layer removal and antimicrobial efficacy of chitosan as a root canal irrigant: A systematic review of in-vitro studies. Odontology 2025, 113, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ferreira, I.; Lopes, C.; Ferreira, A.; Vaz, F.; Vaz, I.; Martín-Biedma, B. Effect of Plasma Treatment on Root Canal Sealers’ Adhesion to Intraradicular Dentin—A Systematic Review. Appl. Sci. 2023, 13, 8655. [Google Scholar] [CrossRef]
- Ferreira, I.; Braga, A.C.; Pina-Vaz, I. Effect of Gutta-percha Solvents on the Bond Strength of Sealers to Intraradicular Dentin: A Systematic Review. Iran. Endod. J. 2021, 16, 17–25. [Google Scholar]
- Abdelkafy, H.; Elsheikh, H.M.; Kataia, M.M.; Marzouk, R.M.; Abdeltwab, E.; Atta, A.; Taher, F.A.E.-R. Efficacy of using chitosan and chitosan nanoparticles as final irrigating solutions on smear layer removal and mineral content of intraradicular dentin. J. Indian Soc. Pedod. Prev. Dent. 2023, 41, 170–177. [Google Scholar] [CrossRef]
- Kamble, A.B.; Abraham, S.; Kakde, D.D.; Shashidhar, C.; Mehta, D.L. Scanning electron microscopic evaluation of efficacy of 17% ethylenediaminetetraacetic acid and chitosan for smear layer removal with ultrasonics: An in vitro study. Contemp. Clin. Dent. 2017, 8, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Ratih, D.N.; Enggardipta, R.A.; Kartikaningtyas, A.T. The effect of chitosan nanoparticle as a final irrigation solution on the smear layer removal, micro-hardness and surface roughness of root canal dentin. Open Dent. J. 2020, 14, 19–26. [Google Scholar] [CrossRef]
- Sehitoglu, G.; Cakici, F.; Soylemez, S.; Dengiz, C. Evaluation of the effect of graphene oxide-based nanocomposites on smear layer by a scanning electron microscope: Laboratory investigation. Aust. Endod. J. 2023, 50, 3–14. [Google Scholar] [CrossRef]
- Silva, P.V.; Guedes, D.F.C.; Nakadi, F.V.; Pécora, J.D.; Cruz-Filho, A.M. Chitosan: A new solution for removal of smear layer after root canal instrumentation. Int. Endod. J. 2013, 46, 332–338. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Q.; Wei, L.; Huang, S.; Zhao, S. A comparative scanning electron microscopy evaluation of smear layer removal with chitosan and MTAD. Niger. J. Clin. Pr. 2018, 21, 76–80. [Google Scholar]
- Ferreira, I.; Braga, A.C.; Lopes, M.A.; Pina-Vaz, I. Adjunctive procedure with solvent mixtures in non-surgical endodontic retreatment: Does it affect root dentin hardness? Odontology 2021, 109, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Veeraiyan, M.; Kumar, Y.P.; Chandhar, C.Y.; Priyanka, Y.; Jaiswal, M.; Kemasaram, D. Evaluation of smear layer removal and micro hardness alteration of radicular dentin after using various chelating agents—An atomic force microscopic study. J. Pharm. Bioall Sci. 2023, 15, S582–S587. [Google Scholar] [CrossRef]
- Darrag, A.M. Effectiveness of different final irrigation solutions on smear layer removal in intraradicular dentin. Tanta Dent. J. 2014, 11, 93–99. [Google Scholar] [CrossRef]
- Penumaka, R.; Konagala, R.; Shaik, J.; Ram Sunil, C.; Reddy, P.; Kiran Naik, M. Scanning electron microscopy evaluation of chitosan and carboxymethyl chitosan as retrograde smear layer removing agents. J. Conserv. Dent. 2019, 22, 573–577. [Google Scholar]
- Mathew, S.P.; Pai, V.S.; Usha, G.; Nadig, R.R. Comparative evaluation of smear layer removal by chitosan and ethylenediaminetetraacetic acid when used as irrigant and its effect on root dentine: An in vitro atomic force microscopic and energy-dispersive X-ray analysis. J. Conserv. Dent. 2017, 20, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Teja, K.V.; Janani, K.; Alqahtani, A.A.; Robaian, A.; Alhalabi, F.; Merdad, K.A.; Alam, M.K.; Shrivastava, D.; Jose, J.; Srivastava, K.C. Herbal agents versus Ethylene diamine tetra acetic acid on removal of the smear layer-A systematic review of in vitro studies. Int. J. Envrion. Res. Public. Health 2022, 19, 6870. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, M.; Mathai, V.; Sadasiva, K.; Santakumari, R.M.; Girish, S.; Shailajakumari, A.K. The evaluation of dentin microhardness after use of 17% EDTA, 17% EGTA, 10% citric acid, MTAD used as chelating agents combined with 2.5% sodium hypochlorite after rotary instrumentation: An in vitro SEM study. J. Pharm. Bioall Sci. 2019, 11, S156–S163. [Google Scholar] [CrossRef]
- Nanda, Z.; Singh, R.; Kamble, P.P.; Deshmukh, G.; Patil, N.; Patil, A.B.; Banerjee, S. Efficacy of Different Root Canal Irrigating Solutions in Removing Smear Layer: A Scanning Electron Microscopic Study. Cureus 2023, 15, e44618. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wan, J.; Yang, Y.; Yao, Y.; Yang, R.; Xie, W. Evolution of the combined effect of different irrigation solutions and activation techniques on the removal of smear layer and dentin microhardness in oval-shaped root canal: An in-vitro study. Biomol. Biomed. 2023, 23, 126–136. [Google Scholar] [CrossRef]
- Nirmal, G.D.; Sai Sankar, A.J.; Sridevi, E.; Sridhar, M.; Sankar, K.S.; Satish, P.R. Comparative evaluation of chelating efficacy of nano-chitosan, pomegranate extract, and ethylenediaminetetraacetic acid on primary radicular dentin: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2022, 40, 201–207. [Google Scholar]
- Del Carpio-Perochena, A.; Bramante, C.M.; Duarte, M.A.H.; de Moura, M.R.; Aouada, F.A.; Kishen, A. Chelating and antibacterial properties of chitosan nanoparticles on dentin. Restor. Dent. Endod. 2015, 40, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Karuppiah, G.; Ahamed, A.S.; Sreekrishnapillai, B.; Rajaraman, G.; Ravishankar, P.; Chittrarasu, M.; Sherwood, A. Comparison of efficacy of 17% ethylenediaminetetraacetic acid, 0.2% chitosan nanoparticles, and QMIX2in1 in smear layer removal at apical third of root canal, using endovac system irrigation system—An in vitro scanning electron microscope study. Endodontology 2021, 33, 206–211. [Google Scholar] [CrossRef]
- Hussein, E.R.; Shukri, B.M.S.; Ibrahim, R.H. The effect of chitosan nanoparticle, citric acid, and ethylenediaminetetraacetic acid on dentin smear layer using two different irrigation needles: A scanning electron microscope study. J. Conserv. Dent. 2022, 25, 431–435. [Google Scholar] [CrossRef]
- Praveen, M.; Aarthi, G.; Meenapriya, P.K.; Kumar, S.S.; Mohan, N.S.K.; Karunakaran, J.V. A comparative evaluation of intraradicular smear removal efficacy of 2% chitosan (low molecular weight), 4% Chitosan Citrate, and 10% Citric Acid when used as final rinse in irrigation protocols: A Field Emission Scanning Electron Microscopic Study. J. Pharm. Bioall Sci. 2017, 9, S73–S78. [Google Scholar] [CrossRef] [PubMed]
- Bastawy, H.A.E.N.; Ezzat, R. Impact of chitosan as chelating agent on microhardness and mineral content of intraradicular dentin. ADJ-Girls 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Virdee, S.S.; Seymour, D.W.; Farnell, D.; Bhamra, G.; Bhakta, S. Efficacy of irrigant activation techniques in removing intracanal smear layer and debris from mature permanent teeth: A systematic review and meta-analysis. Int. Endod. J. 2018, 51, 605–621. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Ex vivo studies | Reviews |
| Non-endodontically treated extracted human teeth | Letters |
| Permanent teeth with fully formed apex | Opinion articles |
| Sodium hypochlorite as main irrigant | Conference abstracts |
| Chitosan as final irrigant | Book chapters |
| SEM to observe smear layer removal | Articles with no control group |
| Database | Search Strategy | Findings |
|---|---|---|
| PubMed | #1 ((chitosan[Title/Abstract]) OR (chitosan[MeSH Terms]) | |
| #2 ((smear layer[Title/Abstract])) OR (smear layer removal[Title/Abstract]) OR (chelator[Title/Abstract]) OR (chelators[Title/Abstract]) OR (final irrigation[Title/Abstract]) OR (final irrigant[Title/Abstract]) OR (final irrigants[Title/Abstract]) OR (smear layer[MeSH Terms]) OR (chelator[MeSH Terms]) | ||
| #3 ((endodontic[Title/Abstract]) OR (endodontics[Title/Abstract]) OR (root canal therapy[Title/Abstract]) OR (root canal treatment[Title/Abstract]) OR (endodontic therapy[Title/Abstract]) OR (endodontic treatment[Title/Abstract]) OR (root canal treatment[MeSH Terms]) OR (endodontics[MeSH Terms]) | ||
| #1 and #2 and #3 | 30 | |
| Scopus | #1 TITLE-ABS-KEY(“chitosan”) | |
| #2 TITLE-ABS-KEY(“smear layer” or “smear layer removal” or “chelator” or “chelators” or “final irrigation” or “final irrigant” or “final irrigants”) | ||
| #3 TITLE-ABS-KEY(“endodontic” or “endodontics” or “root canal therapy” or “root canal treatment” or “endodontic therapy” or “endodontic treatment”) | ||
| #1 and #2 and #3 | 24 | |
| Web of Science | #1 TS=((chitosan)) | |
| #2 TS=((smear layer) OR (smear layer removal) OR (chelator) OR (chelators) OR (final irrigation) OR (final irrigant) OR (final irrigants)) | ||
| #3 TS=((endodontic) OR (endodontics) OR (root canal therapy) OR (root canal treatment) OR (endodontic therapy) OR (endodontic treatment)) | ||
| #1 and #2 and #3 | 48 |
| Main Results | “0.2% CH and CNP have comparable chelating effects to 17% EDTA and induce remineralization of the root canal dentin” | “0.2% CH removes SL 14 with greater efficiency than 17% EDTA in a third of root canals” | “0.2% CH had same effect on SL removal compared to 17% EDTA” | “There was no significant difference between EDTA and CH solutions in all three regions of the tooth” | “The 0.2% CH solution was able to remove SL and provide statistically similar results to 15% EDTA and 10% CA” | “CH was more effective in SL removal than MTAD” |
| Analysis Method | SEM 10 ×2000 1 half. C 11, M 12, A 13. 2 blinded observers | SEM ×1000 ×3000. Both halves. A. Blinded examiner | SEM ×1000 ×2000 both halves. A. 2 blinded examiners | SEM ×1000 Both halves. C, M, A. 2 blinded researchers | SEM ×350 1 half. Half M and A. 3 endodontic observers | SEM ×1000, ×2000. 1 half. C, M, A. 3 investigators |
| Activation | NM 9 | Ultrasonic | NM | NM | NM | NM |
| Time FI | 3′ | 5′ | 3′ | G1: NM G2, G4, G5, G6: 3′ G3: 1′ | 3’ | 3’ |
| Volume FI and Needle | 5 mL 31-gauge needle | 5 mL Needle NM | 5 mL sterile 30 G needle | 5 mL Needle NM | 5 mL 0.45 × 13.0 mm needle | 5 mL Needle NM |
| Type of Chitosan | Chitosan powder (90% deacetylation) | NM | Chitosan powder (>75% deacetylation) | Chitosan (>90% deacetylation) | Chitosan (90% deacetylation) | NM |
| Groups FI 1 | 2 CG: (GIA: saline, GIB: NP 4 + NT 5). GII: 0.2% CH 6; GIII: 0.2% CNP 7, GIV: 17% EDTA 8 | G1: 17% EDTA, G2: CG NT; G3: 0.2% CH; G4: NT | G1: 17% EDTA, G2: 0.2% CH, G3: CG 2.5% NaOCl | G1 (CG): DW 15; G2: GO 16; G3: 17% EDTA; G4: 0.2% CH; G5: GO-EDTA; G6: GO-CH | G1: 15% EDTA; G2: 0.2% CH; G3: 10% CA 17; G4: 1% AA 18; G5: CG (no FI). | G1: (CG) saline; G2: 0.2% CH; G3: MTAD 19 |
| Main Irrigant | 2.6% NaOCl 3 | 3% NaOCl | 2.5% NaOCl | 5.25% NaOCl | 1% NaOCl | 5.25% NaOCl |
| Apical Diameter | X3—30 | 35 | 30 (F3) | NM | NM | NM |
| Sample Size per Group | 12 CG 2 n = 6 | 10 | 8 | 10 | 5 | 10 |
| Type of Teeth | Straight single-rooted Vertucci’s type I | Single-rooted | Single-rooted mandibular premolars | Incisor | Maxillary canines | Single-rooted premolars |
| Author and Year | Abdelkafy, 2023 [24] | Kamble, 2017 [25] | Ratih, 2020 [26] | Sehitoglu, 2023 [27] | Silva, 2013 [28] | Zhou, 2018 [29] |
| Standardization of Samples Selection (Type of Teeth) | Sample Size Calculation | Randomization | Blinding | Standardized Preparation (One Single Operator) | Reporting of Data | Result of Risk of Bias Evaluation | |
|---|---|---|---|---|---|---|---|
| Abdelkafy, 2023 [24] | + | + | + | − | − | + | medium |
| Kamble, 2017 [25] | + | − | + | − | − | + | medium |
| Ratih, 2020 [26] | + | − | − | − | − | + | high |
| Sehitoglu, 2023 [27] | + | + | + | − | − | + | medium |
| Silva, 2012 [28] | + | − | + | − | − | + | medium |
| Zhou, 2018 [29] | + | − | − | − | − | + | high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira-Reguera, A.; Ferreira, I.; Pina-Vaz, I.; Martín-Biedma, B.; Martín-Cruces, J. Chitosan’s Ability to Remove the Smear Layer—A Systematic Review of Ex Vivo Studies. Medicina 2025, 61, 114. https://doi.org/10.3390/medicina61010114
Ferreira-Reguera A, Ferreira I, Pina-Vaz I, Martín-Biedma B, Martín-Cruces J. Chitosan’s Ability to Remove the Smear Layer—A Systematic Review of Ex Vivo Studies. Medicina. 2025; 61(1):114. https://doi.org/10.3390/medicina61010114
Chicago/Turabian StyleFerreira-Reguera, Ana, Inês Ferreira, Irene Pina-Vaz, Benjamín Martín-Biedma, and José Martín-Cruces. 2025. "Chitosan’s Ability to Remove the Smear Layer—A Systematic Review of Ex Vivo Studies" Medicina 61, no. 1: 114. https://doi.org/10.3390/medicina61010114
APA StyleFerreira-Reguera, A., Ferreira, I., Pina-Vaz, I., Martín-Biedma, B., & Martín-Cruces, J. (2025). Chitosan’s Ability to Remove the Smear Layer—A Systematic Review of Ex Vivo Studies. Medicina, 61(1), 114. https://doi.org/10.3390/medicina61010114







