Impact of Microbiota on Irritable Bowel Syndrome Pathogenesis and Management: A Narrative Review
Abstract
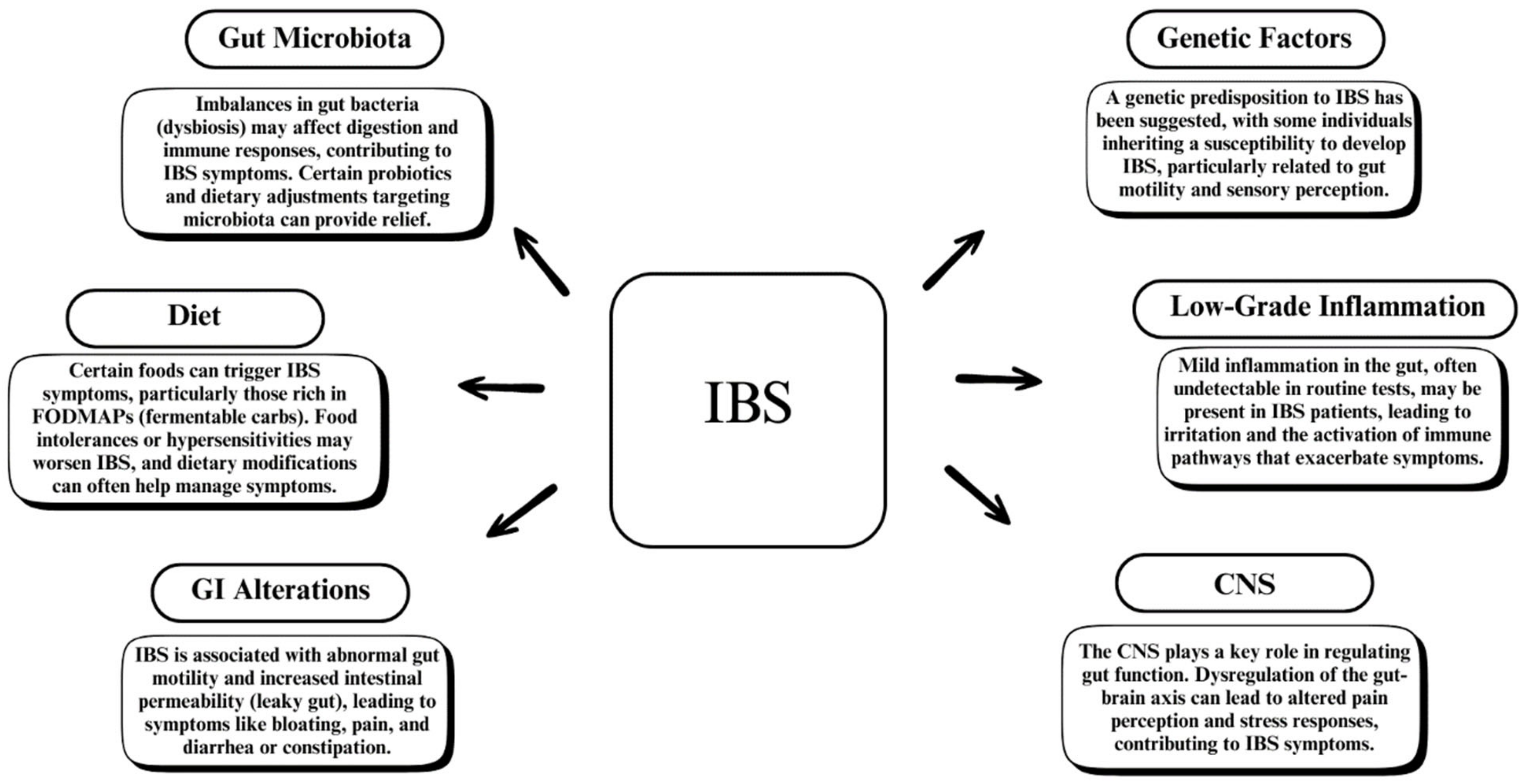
1. Introduction
2. The Gut Microbiome in Health and Disease
3. Alterations in Gut Microbiota in IBS Patients
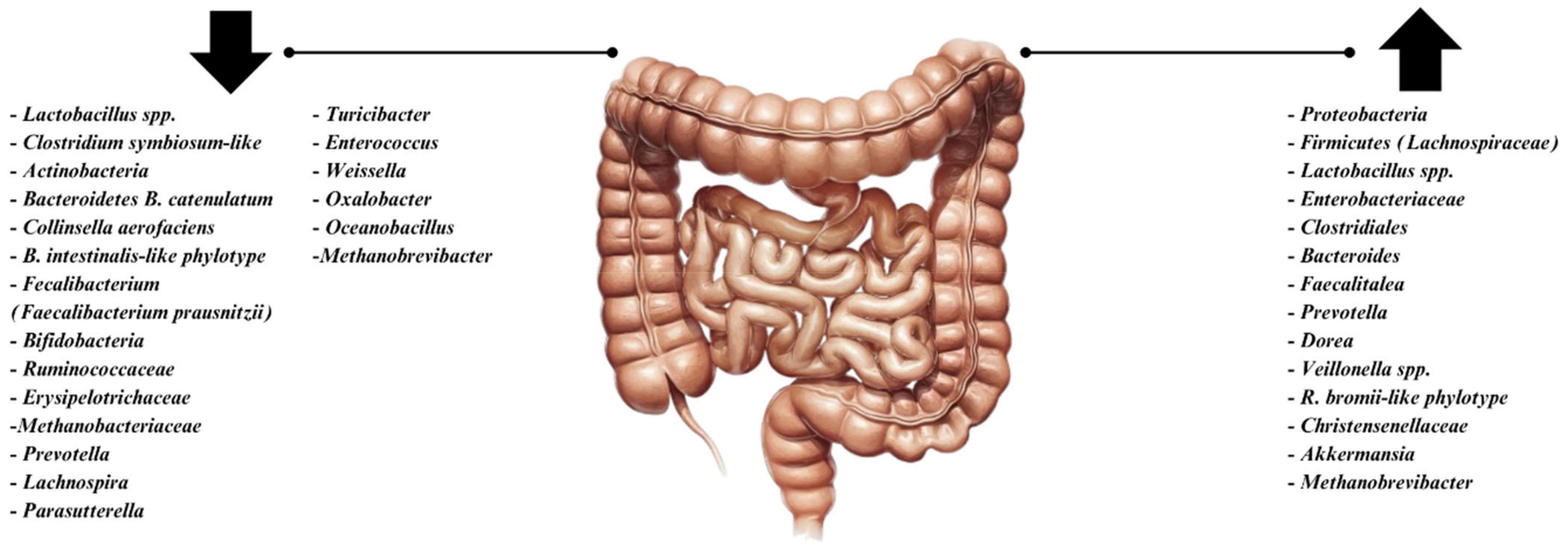
4. The Complex Relationship Between SIBO and IBS
5. Biofilms and IBS
6. Gut–Brain Axis and IBS Symptoms
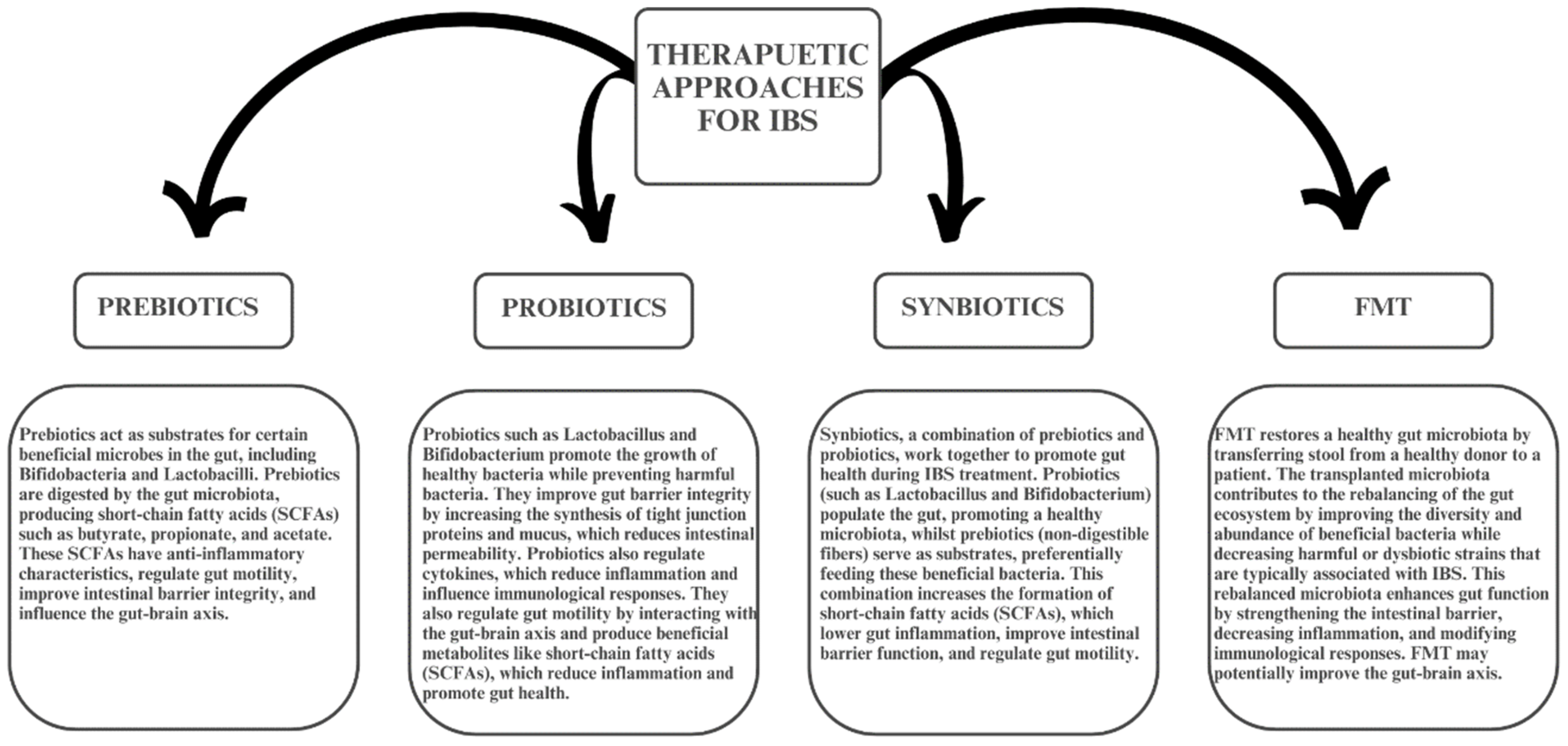
7. Microbiome Targeted Treatment in IBS
8. Prebiotics in IBS
9. Synbiotics and Postbiotics
10. Fecal Microbiota Transplantation (FMT) in IBS
11. Dietary Interventions for IBS
12. Gluten-Free Diet (GFD) and IBS
13. Personalized Approaches in IBS Management
14. Challenges and Future Research Directions in Microbiome-Targeted Treatments for IBS
15. Gaps in Current Knowledge
16. Potential for Personalized Treatments
17. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2020, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The microbiome and irritable bowel syndrome—A review on the pathophysiology, current research and future therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, P.; Shoaie, S.; Nielsen, L.K. Irritable bowel syndrome and microbiome: Switching from conventional diagnosis and therapies to personalized interventions. J. Transl. Med. 2022, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, C.; Maasfeh, L.; Öhman, L.; Simrén, M. Modulating the gut microenvironment as a treatment strategy for irritable bowel syndrome: A narrative review. Gut Microbiome 2022, 3, e7. [Google Scholar] [CrossRef]
- Huang, K.-Y.; Wang, F.-Y.; Lv, M.; Ma, X.-X.; Tang, X.-D.; Lv, L. Irritable bowel syndrome: Epidemiology, overlap disorders, pathophysiology and treatment. World J. Gastroenterol. 2023, 29, 4120–4135. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Ford, A.C. Antibiotics and probiotics for irritable bowel syndrome. Drugs 2023, 83, 687–699. [Google Scholar] [CrossRef]
- Mamieva, Z.; Poluektova, E.; Svistushkin, V.; Sobolev, V.; Shifrin, O.; Guarner, F.; Ivashkin, V. Antibiotics, gut microbiota, and irritable bowel syndrome: What are the relations? World J. Gastroenterol. 2022, 28, 1204–1219. [Google Scholar] [CrossRef]
- Dale, H.F.; Lied, G.A. Gut microbiota and therapeutic approaches for dysbiosis in irritable bowel syndrome: Recent developments and future perspectives. Turk. J. Med. Sci. 2020, 50, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Burcharth, J.; Pommergaard, H.C.; Rosenberg, J. Irritable bowel syndrome, the microbiota and the gut-brain axis. Gut Microbes. 2016, 7, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut dysbiosis in irritable bowel syndrome: A narrative review on correlation with disease subtypes and novel therapeutic implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef]
- Porcari, S.; Ingrosso, M.R.; Maida, M.; Eusebi, L.H.; Black, C.; Gasbarrini, A.; Cammarota, G.; Ford, A.C.; Ianiro, G. Prevalence of irritable bowel syndrome and functional dyspepsia after acute gastroenteritis: Systematic review and meta-analysis. Gut 2024, 73, 1431–1440. [Google Scholar] [CrossRef]
- Kraimi, N.; Ross, T.; Pujo, J.; De Palma, G. The gut microbiome in disorders of gut-brain interaction. Gut Microbes 2024, 16, 2360233. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Stanghellini, V.; Barbara, G. Gut microbiota signatures and modulation in irritable bowel syndrome. Microbiome Res. Rep. 2022, 1, 11. [Google Scholar] [CrossRef] [PubMed]
- Mazzawi, T. Gut microbiota manipulation in irritable bowel syndrome. Microorganisms 2022, 10, 1332. [Google Scholar] [CrossRef] [PubMed]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Bianco, F.; Stanghellini, V.; Barbara, G. Microbiota modulation in disorders of gut-brain interaction. Dig. Liver Dis. 2024, 56, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Xiao, L.; Luo, M.; Xie, P.; Xiong, L. Intestinal crosstalk between bile acids and microbiota in irritable bowel syndrome. J. Gastroenterol. Hepatol. 2023, 38, 1072–1082. [Google Scholar] [CrossRef] [PubMed]
- Agnello, M.; Carroll, L.N.; Imam, N.; Pino, R.; Palmer, C.; Varas, I.; Greene, C.; Hitschfeld, M.; Gupta, S.; Almonacid, D.E.; et al. Gut microbiome composition and risk factors in a large cross-sectional IBS cohort. BMJ Open Gastroenterol. 2020, 7, e000345. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, G.M.; Singh, P.K. Methods of determining irritable bowel syndrome and efficiency of probiotics in treatment: A review. Curr. Ther. Res. Clin. Exp. 2023, 99, 100721. [Google Scholar] [CrossRef]
- Shrestha, B.; Shrestha, B.; Patel, D.; Patel, D.; Shah, H.; Shah, H.; Hanna, K.S.; Hanna, K.S.; Kaur, H.; Kaur, H.; et al. The role of gut microbiota in the pathophysiology and therapy of irritable bowel syndrome: A systematic review. Cureus 2022, 14, e28064. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef] [PubMed]
- Mishima, Y.; Ishihara, S. Molecular mechanisms of microbiota-mediated pathology in irritable bowel syndrome. Int. J. Mol. Sci. 2020, 21, 8664. [Google Scholar] [CrossRef] [PubMed]
- Takakura, W.; Pimentel, M. Small intestinal bacterial overgrowth and irritable bowel syndrome—An update. Front. Psychiatry 2020, 11, 664. [Google Scholar] [CrossRef]
- Hillestad, E.M.R.; van der Meeren, A.; Nagaraja, B.H.; Bjørsvik, B.R.; Haleem, N.; Benitez-Paez, A.; Sanz, Y.; Hausken, T.; Lied, G.A.; Lundervold, A.; et al. Gut bless you: The microbiota-gut-brain axis in irritable bowel syndrome. World J. Gastroenterol. 2022, 28, 412–431. [Google Scholar] [CrossRef]
- Surdea-Blaga, T.; Ciobanu, L.; Ismaiel, A.; Dumitrascu, D.L. Microbiome in irritable bowel syndrome: Advances in the field—A scoping review. Microb. Health Dis. 2024, 6, e1017. [Google Scholar] [CrossRef]
- Spiller, R. Impact of diet on symptoms of the irritable bowel syndrome. Nutrients 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Algera, J.; Colomier, E.; Simrén, M. The dietary management of patients with irritable bowel syndrome: A narrative review of the existing and emerging evidence. Nutrients 2019, 11, 2162. [Google Scholar] [CrossRef] [PubMed]
- Rej, A.; Aziz, I.; Tornblom, H.; Sanders, D.S.; Simrén, M. The role of diet in irritable bowel syndrome: Implications for dietary advice. J. Intern. Med. 2019, 286, 490–502. [Google Scholar] [CrossRef]
- Kashyap, P.; Moayyedi, P.; Quigley, E.M.M.; Simren, M.; Vanner, S. Critical appraisal of the SIBO hypothesis and breath testing: A clinical practice update endorsed by the European society of neurogastroenterology and motility (ESNM) and the American neurogastroenterology and motility society (ANMS). Neurogastroenterol. Motil. 2024, 36, e14817. [Google Scholar] [CrossRef]
- Aziz, I.; Törnblom, H.; Simrén, M. Small intestinal bacterial overgrowth as a cause for irritable bowel syndrome. Curr. Opin. Gastroenterol. 2017, 33, 196–202. [Google Scholar] [CrossRef]
- Motta, J.P.; Wallace, J.L.; Buret, A.G.; Deraison, C.; Vergnolle, N. Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 314–334. [Google Scholar] [CrossRef]
- Jandl, B.; Dighe, S.; Baumgartner, M.; Makristathis, A.; Gasche, C.; Muttenthaler, M. Gastrointestinal biofilms—Endoscopic detection, disease relevance and therapeutic strategies. Gastroenterology 2024, 167, 1098–1112.e5. [Google Scholar] [CrossRef] [PubMed]
- Algera, J.P.; Törnblom, H.; Simrén, M. Treatments targeting the luminal gut microbiota in patients with irritable bowel syndrome. Curr. Opin. Pharmacol. 2022, 66, 102284. [Google Scholar] [CrossRef] [PubMed]
- Karakan, T.; Ozkul, C.; Küpeli Akkol, E.; Bilici, S.; Sobarzo-Sánchez, E.; Capasso, R. Gut-brain-Microbiota axis: Antibiotics and functional gastrointestinal disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef]
- Manos, J. The human microbiome in disease and pathology. APMIS 2022, 130, 690–705. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef]
- Herndon, C.C.; Wang, Y.-P.; Lu, C.-L. Targeting the gut microbiota for the treatment of irritable bowel syndrome. Kaohsiung J. Med. Sci. 2020, 36, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Luo, M.; Deng, X.; Fan, J.; Xiong, L. Outcome-specific efficacy of different probiotic strains and mixtures in irritable bowel syndrome: A systematic review and network meta-analysis. Nutrients 2023, 15, 3856. [Google Scholar] [CrossRef]
- Shang, X.; E, F.-F.; Guo, K.-L.; Li, Y.-F.; Zhao, H.-L.; Wang, Y.; Chen, N.; Nian, T.; Yang, C.-Q.; Yang, K.-H.; et al. Effectiveness and safety of probiotics for patients with constipation-predominant irritable bowel syndrome: A systematic review and meta-analysis of 10 randomized controlled trials. Nutrients 2022, 14, 2482. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Y.; Zheng, Q.; Li, L. The efficacy of probiotics, prebiotics, synbiotics, and fecal microbiota transplantation in irritable bowel syndrome: A systematic review and network meta-analysis. Nutrients 2024, 16, 2114. [Google Scholar] [CrossRef] [PubMed]
- Goodoory, V.C.; Mais Khasawneh Black, C.J.; Eamonn Martin Quigley Martin, P.; Ford, A.C. Efficacy of Probiotics in Irritable Bowel Syndrome: Systematic Review and Meta-analysis. Gastroenterology 2023, 165, 1206–1218. [Google Scholar] [CrossRef]
- Kajander, K.; Myllyluoma, E.; Rajilić-Stojanović, M.; Kyrönpalo, S.; Rasmussen, M.; Järvenpää, S.; Zoetendal, E.G.; DE Vos, W.M.; Vapaatalo, H.; Korpela, R. Clinical trial: Multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment. Pharmacol. Ther. 2007, 27, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Wollny, T.; Daniluk, T.; Piktel, E.; Wnorowska, U.; Bukłaha, A.; Głuszek, K.; Durnaś, B.; Bucki, R. Targeting the gut microbiota to relieve the symptoms of irritable bowel syndrome. Pathogens 2021, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2018, 48, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M. The use of probiotics, prebiotics and synbiotics in the management of irritable bowel syndrome. Eur. Gastroenterol. Hepatol. Rev. 2012, 7, 233–236. [Google Scholar]
- Hojo, M.; Nagahara, A. Current perspectives on irritable bowel syndrome: A narrative review. J. Int. Med. Res. 2022, 50, 3000605221126370. [Google Scholar] [CrossRef] [PubMed]
- Myneedu, K.; Deoker, A.; Schmulson, M.J.; Bashashati, M. Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2019, 7, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Jamshidi, P.; Farsi, Y.; Nariman, Z.; Hatamnejad, M.R.; Mohammadzadeh, B.; Akbarialiabad, H.; Nasiri, M.J.; Sechi, L.A. Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Int. J. Mol. Sci. 2023, 24, 14562. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Feng, S.; Li, Y.; Zhang, C.; Chao, G.; Zhang, S. Gut microbiota and intestinal immunity—A crosstalk in irritable bowel syndrome. Immunology 2024, 172, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; de Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal Microbiota and Diet in IBS: Causes, consequences, or epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Patcharatrakul, T.; Gonlachanvit, S. The role of diet in the pathophysiology and management of irritable bowel syndrome. Indian J. Gastroenterol. 2021, 40, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.; Zhang, J.; Sun, F.; Duan, L. Efficacy of probiotics for irritable bowel syndrome: A systematic review and network meta-analysis. Front. Cell. Infect. Microbiol. 2022, 12, 859967. [Google Scholar] [CrossRef]
- Umeano, L.; Iftikhar, S.; Alhaddad, S.F.; Paulsingh, C.N.; Riaz, M.F.; Garg, G.; Mohammed, L. Effectiveness of probiotic use in alleviating symptoms of irritable bowel syndrome: A systematic review. Cureus 2024, 16, e58306. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, C.; Escudero-López, B.; Fernández-Pachón, M.-S. Evaluation of the efficacy of probiotics as treatment in irritable bowel syndrome. Endocrinol. Diabetes Nutr. 2024, 71, 19–30. [Google Scholar] [CrossRef]
- Black, C.J.; Thakur, E.R.; Houghton, L.A.; Quigley, E.M.M.; Moayyedi, P.; Ford, A.C. Efficacy of psychological therapies for irritable bowel syndrome: Systematic review and network meta-analysis. Gut 2020, 69, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
| Reference | Methods | Results |
|---|---|---|
| Myneedu et al. 2019 [47] | Authors searched PubMed, Embase, Google Scholar, and abstract books from Digestive Disease Week and United European Gastroenterology Week (2010–2018) for studies on IBS, and they retrieved single-arm and RCTs on FMT for IBS, where the diagnosis was confirmed by a physician or based on ROME I-IV criteria. | Following the SATs, almost 60% of IBS patients reported considerable symptom relief. However, the RCTs revealed varied findings. Some studies indicated improvements in symptoms, while others found no significant difference between the FMT and control groups. |
| Zhang et al. 2022 [52] | Authors searched for RCTs on the efficacy of probiotics in treating IBS until August 25, 2021. The primary focus was on the rate of symptom reduction as well as changes in overall symptoms. Meta-regression was used to determine whether the length and dose of probiotic treatment had an impact on effectiveness. | B. coagulans was found to be the most effective probiotic species for relieving IBS symptoms. L. plantarum was shown to confer the highest quality of life (QOL). Meta-regression revealed that probiotic dose had no significant impact on outcomes, whereas treatment time did. Further subgroup analysis found that B. coagulans given for 8 weeks was the most effective in relieving these symptoms, outperforming probiotic combinations in the research. |
| Shang et al. 2022 [39] | Authors conducted a comprehensive search of PubMed, Embase, the Cochrane Library, Web of Science, and China Biology Medicine (CBM). Intervention parameters included probiotic strains, dose, duration, form, and placebo use as well as outcome measures such as symptom reports and scale use. | Three RCTs with 71 patients found that probiotics significantly improved stool consistency compared to placebo. An 8-week therapy period was effective, while 12 weeks provided no benefit. Two RCTs with 74 patients found that probiotics significantly improved fecal Bifidobacterium and Lactobacillus levels after four weeks. There was no effect after 8 weeks. |
| Xie et al. 2023 [38] | Reviewers independently gathered crucial information from eligible trials, such as RCT details, participant characteristics, and results. Intention-to-treat analyses were conducted. Transitivity was established by comparing major clinical variables between studies. The network’s consistency was verified by node splitting and loop-specific analysis. | The most effective probiotics were L. acidophilus (efficacy level A). Other strains, such as B. bifidum and C. butyricum, provided considerable benefits (efficacy level B). The multistrain group demonstrated the greatest improvement in quality of life. C. butyricum also showed significant improvements over placebo. B. coagulans MTCC 5856 and S. cerevisiae CNCM I-3856 were found to be most effective at improving stool consistency in IBS-D patients. There were no significant differences between probiotics and placebo for IBS-C in this network analysis. |
| Jamshidi et al. 2023 [48] | A complete search of the PubMed/Medline and Embase databases was performed to include all relevant publications up to 14 June 2023. To reduce heterogeneity, subgroup analyses were conducted based on FMT preparation, frequency of administration, and route of administration. | Single dosage of FMT administered via colonoscopy significantly decreased patient complaints.Using frozen FMT as an oral capsule significantly increased symptoms compared to the non-FMT placebo.Patients undergoing multiple-donor FMT showed considerable improvement compared to autologous. However, compared to the non-FMT placebo, it had a negative effect on IBS symptoms. |
| Wu et al. 2024 [40] | Databases were searched, including Medline, Embase and Embase Classic, Cochrane Central Register of Controlled Trials, and Web of Science. Authors also searched for unpublished trial data on ClinicalTrials.gov. Standardized mean differences (SMDs) were utilized to compare the effect sizes of the experimental and placebo groups. The study looked at the effects of several probiotic strains by dividing interventions into subgroups based on the strain. | Probiotics had the greatest treatment benefit over placebo. FMT also showed considerable improvement. Prebiotics did not differ significantly from placebo. Synbiotics also did not reveal significant differences from placebo. Probiotics performed much better than prebiotics and synbiotics. FMT performed much better than prebiotics and synbiotics. Probiotics helped to improve abdominal pain and bloating when compared to placebo. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almonajjed, M.B.; Wardeh, M.; Atlagh, A.; Ismaiel, A.; Popa, S.-L.; Rusu, F.; Dumitrascu, D.L. Impact of Microbiota on Irritable Bowel Syndrome Pathogenesis and Management: A Narrative Review. Medicina 2025, 61, 109. https://doi.org/10.3390/medicina61010109
Almonajjed MB, Wardeh M, Atlagh A, Ismaiel A, Popa S-L, Rusu F, Dumitrascu DL. Impact of Microbiota on Irritable Bowel Syndrome Pathogenesis and Management: A Narrative Review. Medicina. 2025; 61(1):109. https://doi.org/10.3390/medicina61010109
Chicago/Turabian StyleAlmonajjed, Mhd Bashir, Mahdi Wardeh, Abdallah Atlagh, Abdulrahman Ismaiel, Stefan-Lucian Popa, Flaviu Rusu, and Dan L. Dumitrascu. 2025. "Impact of Microbiota on Irritable Bowel Syndrome Pathogenesis and Management: A Narrative Review" Medicina 61, no. 1: 109. https://doi.org/10.3390/medicina61010109
APA StyleAlmonajjed, M. B., Wardeh, M., Atlagh, A., Ismaiel, A., Popa, S.-L., Rusu, F., & Dumitrascu, D. L. (2025). Impact of Microbiota on Irritable Bowel Syndrome Pathogenesis and Management: A Narrative Review. Medicina, 61(1), 109. https://doi.org/10.3390/medicina61010109







