Evaluation of Maternal Ischemia-Modified Albumin Levels during Pregnancy and Their Effect on Fetal Birth Weight
Abstract
1. Introduction
2. Materials and Methods
2.1. Working Method
2.2. Ischemia-Modified Albumin
2.3. Statistical Evaluation
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, D.; Quiles, J.; Gaze, D.C.; Collinson, P.; Kaski, J.C.; Baxter, G.F. Role of reactive oxygen species on the formation of the novel diagnostic marker ischaemia modified albumin. Heart 2006, 92, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Montagnana, M.; Guidi, G.C. Albumin cobalt binding and ischemia modified albumin generation: An endogenous response to ischemia? Int. J Cardiol. 2006, 108, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Gulbis, B.; Burton, G.J. The human first trimester gestational sac limits rather than facilitates oxygen transfer to the foetus-a review. Placenta 2003, 24 (Suppl. A), S86–S93. [Google Scholar] [CrossRef]
- Jauniaux, E.; Watson, A.; Burton, G. Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7- and 16-weeks’ gestation. Am. J. Obs. Gynecol. 2001, 184, 998–1003. [Google Scholar] [CrossRef]
- Turedi, S.; Gunduz, A.; Mentese, A.; Topbas, M.; Karahan, S.C.; Yeniocak, S.; Turan, I.; Eroglu, O.; Ucar, U.; Karaca, Y.; et al. The value of ischemia-modified albümin compared with d-dimer in the diagnosis of pulmonary embolism. Respir. Res. 2008, 9, 49–61. [Google Scholar] [CrossRef]
- Turedi, S.; Gunduz, A.; Mentese, A.; Karahan, S.C.; Yilmaz, S.E.; Eroglu, O.; Nuhoglu, I.; Turan, I.; Topbas, M. Value of ischemia-modified albumin in the diagnosis of pulmonery embolism. Am. J. Emerg. Med. 2007, 25, 770–773. [Google Scholar] [CrossRef]
- Piwowar, A.; Knapik-Kordecka, M.; Warwas, M. Ischemia-modified albumin level in type 2 diabetus mellitus-preliminary report. Dis. Markers 2008, 24, 311–317. [Google Scholar] [CrossRef]
- Şeker, R.; Oğuz, A.K.; Özdemir, S.; Demirtaş, S.; Aylı, M.; Mergen, K. The evulation of IMA as a cardiac ischemia marker in the cases of hypohemoglobinemia and hypoxemia due to blood loss. Turk. J. Biochem. 2014, 39, 221–225. [Google Scholar] [CrossRef]
- Jaiswar, S.P.; Verma, S.; Agrawal, M.; Deo, S.; Goel, M.; Mahdi, A.A. Association of Maternal Serum Ischemia Modified Albumin (IMA) with Placental Histopathological Changes and Fetomaternal Outcome: A Prospective Case Control Study in Normotensive and Pre-eclamptic Women. J. Obs. Gynaecol. India 2022, 72 (Suppl. 1), 166–173. [Google Scholar] [CrossRef]
- Andıç, E.; Karaman, E.; Kolusarı, A.; Çokluk, E. Association of cord blood ischemia-modified albumin level with abnormal foetal Doppler parameters in intrauterine growth-restricted foetuses. J. Matern. Fetal Neonatal Med. 2021, 34, 1–6. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidants and human disease: A general introduction. Nutr. Rev. 1997, 55 Pt 2, S44–S49, discussion S49–S52. [Google Scholar] [CrossRef]
- Kharb, S. Lipid peroxidation in pregnancy with preeclampsia and diabetes. Gynecol. Obs. Investig. 2000, 50, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, M.; Ustüner, I.; Cengiz, B.; Söylemez, F.; Cavdar, A.O. Effects of nutrition on zinc, folic acid, and vitamin B12 levels during pregnancy. Biol. Trace Elem. Res. 2006, 109, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pekcan, G. Community Nutrition. Dietary Handbook, 3rd ed.; Hatipoğlu Printing and Publishing: Ankara, Turkey, 2004; pp. 63–65. [Google Scholar]
- Ministry of Health; General Directorate of Primary Health Care; Hacettepe University Department of Nutrition and Dietetics. Nutrition Guide Specific to Turkey; Ministry of Health Publication: Ankara, Turkey, 2004; pp. 58–60. [Google Scholar]
- Bar-Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia-a preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Bar–Or, D.; Lau, E.; Rao, N.; Bampos, N.; Winkler, J.V.; Curtis, C.G. Reduction in the cobalt binding capacity of human albumin with myocardial ischemia. Ann. Emerg. Med. 1999, 34 (Suppl. 4), 56. [Google Scholar] [CrossRef]
- Dusek, J.; St’asek, J.; Tichy, M.; Bis, J.; Gregor, J.; Vojacek, J.; Masin, V.; Polansky, P.; Brtko, M.; Cernohorsky, D. Prognostic significance of ischemia modified albümin after percutaneous coronary intervention. Clin. Chim. Acta 2006, 367, 77–80. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Lai, E.M.; Rios, P.A.; Yang, J.; Ortega-Lopez, A.M.; Shinoda, H. Evaluation of human serum albümin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarction. Clin. Chem. 2003, 49, 581–585. [Google Scholar] [CrossRef]
- Guven, S.; Alver, A.; Mentese, A.; Ilhan, F.C.; Calapoglu, M.; Unsal, M.A. The novel ischemia marker ‘ischemia-modified albumin’ is increased in normal pregnancies. Acta Obs. Gynecol. Scand. 2009, 88, 479–482. [Google Scholar] [CrossRef]
- Raijmakers, M.T.; Roes, E.M.; Poston, L.; Steegers, E.A.; Peters, W.H. The transient increase of oxidative stress during normal pregnancy is higher and persists after delivery in women with pre-eclampsia. Eur. J. Obs. Gynecol. Reprod. Biol. 2008, 138, 39–44. [Google Scholar] [CrossRef]
- Prefumo, F.; Gaze, D.C.; Papageorghiou, A.T.; Collinson, P.O.; Thilaganathan, B. First trimester maternal serum ischaemiamodified albumin: A marker of hypoxia- ischaemia-driven early trophoblast development. Hum. Reprod. 2007, 22, 2029–2032. [Google Scholar] [CrossRef]
- Papageorghiou, A.T.; Prefumo, F.; Leslie, K.; Gaze, D.C.; Collinson, P.O.; Thilaganathan, B. Defective endovascular trophoblast invasion in the first trimester is associated with increased maternal serum ischemia-modified albumin. Hum. Reprod. 2008, 23, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Gafsou, B.; Lefèvre, G.; Hennache, B.; Houfflin, D.V.; Ducloy-Bouthors, A.S. Maternal serum ischemia-modified albumin: A biomarker to distinguish between normal pregnancy and preeclampsia. Hypertens Pregnancy 2010, 29, 101–111. [Google Scholar] [CrossRef]
- Lacovidou, N.; Briana, D.D.; Boutsikou, M.; Liosi, S.; Baka, S.; Boutsikou, T.; Hassiakos, D.; Malamitsi-Puchner, A. Cord blood ischemia-modified albümin levels in normal and intrauterine growth restricted pregnancies. Mediat. Inflamm. 2008, 2008, 523081. [Google Scholar]
- Kumral, A.; Okyay, E.; Guclu, S.; Gencpinar, P.; Islekel, G.H.; Oguz, S.S.; Kant, M.; Demirel, G.; Duman, N.; Ozkan, H. Cord blood ischemia-modified albumin is it associated with abnormal Doppler findings in complicated pregnancies and predictive of perinatal asphyxia. J. Obs. Gynaecol. Res. 2013, 39, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Gölbaşı, C.; Gölbaşı, H.; Kocahakimoğlu Gültekin, C.; Gülseren, V.; Zeytinli Akşit, M.; Bayraktar, B.; Çolak, A.; Taner, C.E. Ischemia modified albumin levels in intrauterine growth restriction: Levels are increased in fetal cord blood but not in maternal blood. Ginekol. Pol. 2022, 93, 993–998. [Google Scholar] [CrossRef]
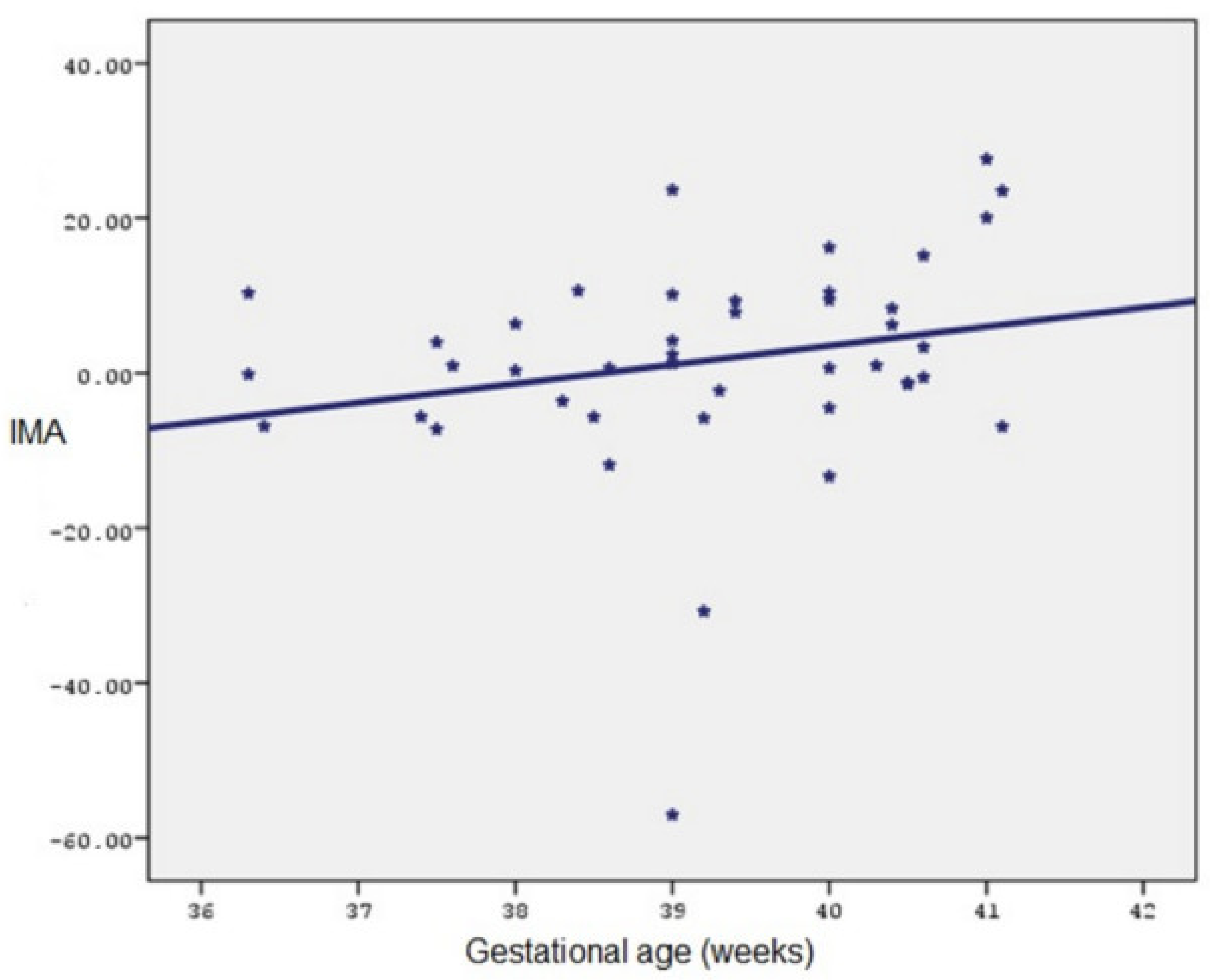
| (Median) Min–Max/N | Mean ± SD/% | ||
|---|---|---|---|
| Age (year) | 31 (20–41) | ||
| Body height (cm) | 162 (151–172) | ||
| Gravida | 4 (1–7) | ||
| Parity | 3 (0–5) | ||
| Abortus | 2 (0–3) | ||
| Live birth | 3 (0–5) | 1.09 ± 1.17 | |
| Duration of pregnancy (week) | 37 (36–41) | 39.21 ± 1.32 | |
| Pre-pregnancy weight (kilogram) | 73 (48–100) | 65.16 ± 11.19 | |
| Maternal weight at birth (kilogram) | 85 (62–115) | 81.35 ± 12.48 | |
| Fetal birth weight (gram) | 3450 (2800–4600) | 3391.40 ± 359.34 | |
| Complication in pregnancy | 6 (14%) | ||
| Intrauterine growth restriction (IUGR) | 3 (7%) | ||
| Preterm birth | 3 (7%) | ||
| Preeclampsia | 2 (4.7%) | ||
| Gestational diabetes mellitus (GDM) | 6 (14%) | ||
| Smoking in pregnancy | 6 (14%) | ||
| Vitamin supplementation in pregnancy | 41 (95.3%) | ||
| Nutritional level in pregnancy | 42 (97.7%) | ||
| Type of birth | Vaginal | 24 (55.8%) | |
| Cesarean | 19 (44.2%) | ||
| Min–Max | Mean ± SD | |
|---|---|---|
| I. Trimester IMA results | 0.408–0.656 | 0.53 ± 0.06 |
| II. Trimester IMA results | 0.519–1.289 | 0.64 ± 0.11 |
| III. Trimester IMA results | 0.466–0.769 | 0.64 ± 0.06 |
| I. Trimester maternal weight | 49–102 | 66.58 ± 10.77 |
| II. Trimester maternal weight | 52–105 | 71.16 ± 10.94 |
| III. Trimester maternal weight | 56–110 | 76.21 ± 11.62 |
| 75 gr OGTT ˣ at 0 Min. | 77–100 | 86.05 ± 6.10 |
| 75 gr OGTT at 60 Min. | 82–261 | 131.67 ± 35.38 |
| 75 gr OGTT at 120 Min. | 49–178 | 111.35 ± 25.28 |
| IMA | |||
|---|---|---|---|
| Mean | SD | a/bp | |
| I. Trimester | 0.53 | 0.06 | 0.001 ** |
| II. Trimester | 0.64 | 0.11 | |
| III. Trimester | 0.64 | 0.06 | |
| Maternal Weight | |||
| I. Trimester | 66.58 | 10.77 | |
| II. Trimester | 71.16 | 10.94 | 0.001 ** |
| III. Trimester | 76.21 | 11.62 | |
| OGTT | |||
| OGTT at 0 Min. | 86.05 | 6.09 | |
| OGTT at 60 Min. | 131.67 | 35.37 | 0.001 ** |
| OGTT at 120 Min. | 111.35 | 25.28 | |
| Age-IMA % Changes | |
|---|---|
| R | |
| I.—II. Trimester IMA % changes | 0.129 |
| I.—III. Trimester IMA % changes | −0.074 |
| II.—III. Trimester IMA % changes | −0.173 |
| Gestational age-IMA % changes | |
| I.—II. Trimester IMA % changes | −0.148 |
| I.—III. Trimester IMA % changes | 0.176 |
| II.—III. Trimester IMA % changes | 0.306 |
| Pre-pregnancy weight-IMA % Changes | |
| I.—II. Trimester IMA % changes | 0.062 |
| I.—III. Trimester IMA % changes | 0.102 |
| II.—III. Trimester IMA % changes | −0.070 |
| Maternal weight at birth-IMA % changes | |
| I.—II. Trimester IMA % changes | 0.014 |
| I.—III. Trimester IMA % changes | −0.015 |
| II.—III. Trimester IMA % changes | −0.121 |
| Fetal birth weight-IMA % changes | |
| I.—II. Trimester IMA % changes | −0.091 |
| I.—III. Trimester IMA % changes | 0.021 |
| II.—III. Trimester IMA % changes | 0.150 |
| I. Tri.—II. Tri. IMA % Changes | I. Tri.—III. Tri. IMA % Changes | II. Tri.—III. Tri. IMA % Changes | ||
|---|---|---|---|---|
| Mean ± SD (Med.) | Mean ± SD (Med.) | Mean ± SD (Med.) | ||
| Gravida | 1 (n = 10) | 27.65 ± 42.51 (17.37) | 18.65 ± 16.08 (10.36) | −1.36 ± 20.61 (4.32) |
| >2 (n = 33) | 19.31 ± 14.36 (17.69) | 21.72 ± 16.18 (21.19) | 2.51 ± 11.72 (0.96) | |
| c p | 0.908 | 0.314 | 0.818 | |
| Smoking in pregnancy | Yok (n = 37) | 21.57 ± 24.58 (17.86) | 20.27 ± 15.75 (19.22) | 0.98 ± 14.84 (0.96) |
| Var (n = 6) | 19.29 ± 18.01 (16.15) | 25.55 ± 18.42 (25.99) | 5.47 ± 7.80 (5.92) | |
| c p | 0.674 | 0.649 | 0.344 | |
| Socioeconomic status | Good (n = 35) | 22.32 ± 25.29 (20.97) | 21.93 ± 16.10 (21.19) | 1.88 ± 15.28 (2.41) |
| Poor (n = 8) | 16.57 ± 14.34 (15.72) | 16.94 ± 16.01 (12.24) | 0.39 ± 7.39 (−0.10) | |
| c p | 0.349 | 0.261 | 0.303 | |
| Vitamin supplementation in pregnancy | Yok (n = 2) | 22.49 ± 0.54 (22.49) | 15.80 ± 10.63 (15.80) | −5.44 ± 9.10 (−5.44) |
| Var (n = 41) | 21.19 ± 24.18 (16.88) | 21.26 ± 16.28 (19.22) | 1.95 ± 14.27 (1.25) | |
| p | - | - | - | |
| Nutrition level in pregnancy | Good (n = 42) | 21.62 ± 23.76 (17.78) | 21.21 ± 16.15 (19.46) | 1.49 ± 14.24 (0.98) |
| Poor (n = 1) | 5.51 | 12.22 | 6.36 | |
| p | - | - | - | |
| Maternal education level | ≥13 Years (High) (n = 23) | 23.81 ± 28.39 (17.86) | 20.00 ± 18.14 (18.12) | −0.59 ± 16.49 (2.41) |
| 9–12 Years (Medium) (n = 6) | 12.98 ± 10.27 (13.86) | 16.42 ± 9.14 (14.99) | 3.62 ± 11.18 (−1.33) | |
| 0–9 Years (Low) (n = 14) | 20.58 ± 18.75 (19.40) | 24.62 ± 14.64 (22.03) | 4.36 ± 10.67 (0.83) | |
| d p | 0.523 | 0.408 | 0.957 | |
| II. Tri.—I. Tri. IMA % Changes | III. Tri.—I. Tri. IMA % Changes | III. Tri.—II. Tri. IMA % Changes | ||
|---|---|---|---|---|
| Mean ± SD (Med.) | Mean ± SD (Med.) | Mean ± SD (Med.) | ||
| Complication in pregnancy | No (n = 37) | 20.57 ± 24.18 (16.69) | 20.84 ± 16.18 (18.12) | 2.16 ± 14.72 (2.41) |
| Yes (n = 6) | 25.45 ± 21.11 (24.14) | 22.04 ± 16.37 (20.21) | −1.77 ± 9.56 (−2.53) | |
| c p | 0.262 | 1.000 | 0.139 | |
| Intrauterine growth restriction (IUGR) | No (n = 40) | 22.05 ± 23.95 (17.29) | 20.89 ± 16.46 (18.91) | 0.81 ± 13.91 (0.98) |
| Yes (n = 3) | 10.54 ± 18.08 (20.97) | 22.50 ± 10.12 (19.22) | 12.27 ± 14.60 (10.63) | |
| p | - | - | - | |
| Preterm birth | No (n = 40) | 21.06 ± 23.72 (17.29) | 20.75 ± 15.64 (19.46) | 1.65 ± 14.49 (1.12) |
| Yes (n = 3) | 23.71 ± 26.78 (17.86) | 24.38 ± 24.52 (10.69) | 1.09 ± 8.66 (−0.16) | |
| p | - | - | - | |
| Preeclampsia | No (n = 41) | 20.48 ± 23.65 (16.88) | 20.27 ± 15.61 (18.12) | 1.72 ± 14.42 (1.25) |
| Yes (n = 2) | 36.95 ± 22.60 (36.95) | 35.96 ± 23.67 (35.96) | −0.80 ± 0.91 (−0.80) | |
| p | - | - | - | |
| Gestational diabetes mellitus (GDM) | No (n = 37) | 22.20 ± 24.40 (17.69) | 22.29 ± 16.73 (19.69) | 1.96 ± 14.63 (1.25) |
| Yes (n = 6) | 15.35 ± 18.56 (19.30) | 13.06 ± 7.15 (12.24) | −0.56 ± 10.92 (−4.06) | |
| c p | 0.700 | 0.132 | 0.309 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Çobanoğlu, U.; Birge, Ö.; Çetin, M.; Güven, E.S.G. Evaluation of Maternal Ischemia-Modified Albumin Levels during Pregnancy and Their Effect on Fetal Birth Weight. Medicina 2024, 60, 1530. https://doi.org/10.3390/medicina60091530
Çobanoğlu U, Birge Ö, Çetin M, Güven ESG. Evaluation of Maternal Ischemia-Modified Albumin Levels during Pregnancy and Their Effect on Fetal Birth Weight. Medicina. 2024; 60(9):1530. https://doi.org/10.3390/medicina60091530
Chicago/Turabian StyleÇobanoğlu, Uğur, Özer Birge, Mustafa Çetin, and Emine Seda Güvendağ Güven. 2024. "Evaluation of Maternal Ischemia-Modified Albumin Levels during Pregnancy and Their Effect on Fetal Birth Weight" Medicina 60, no. 9: 1530. https://doi.org/10.3390/medicina60091530
APA StyleÇobanoğlu, U., Birge, Ö., Çetin, M., & Güven, E. S. G. (2024). Evaluation of Maternal Ischemia-Modified Albumin Levels during Pregnancy and Their Effect on Fetal Birth Weight. Medicina, 60(9), 1530. https://doi.org/10.3390/medicina60091530






