Diagnostic Challenges in Follicular Cholangitis Mimicking Hilar Cholangiocarcinoma: A Case Report and Review of the Literature
Abstract
1. Introduction
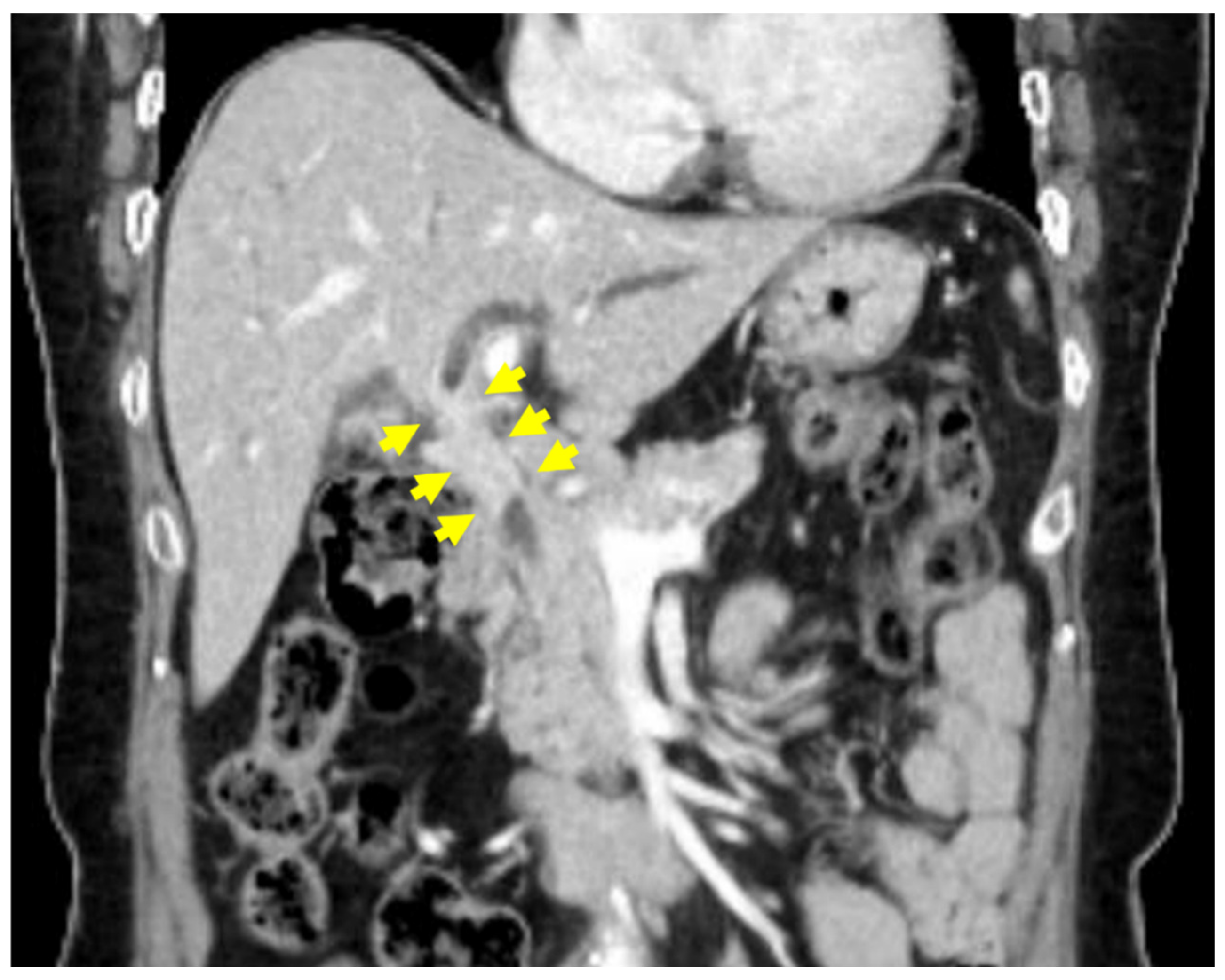
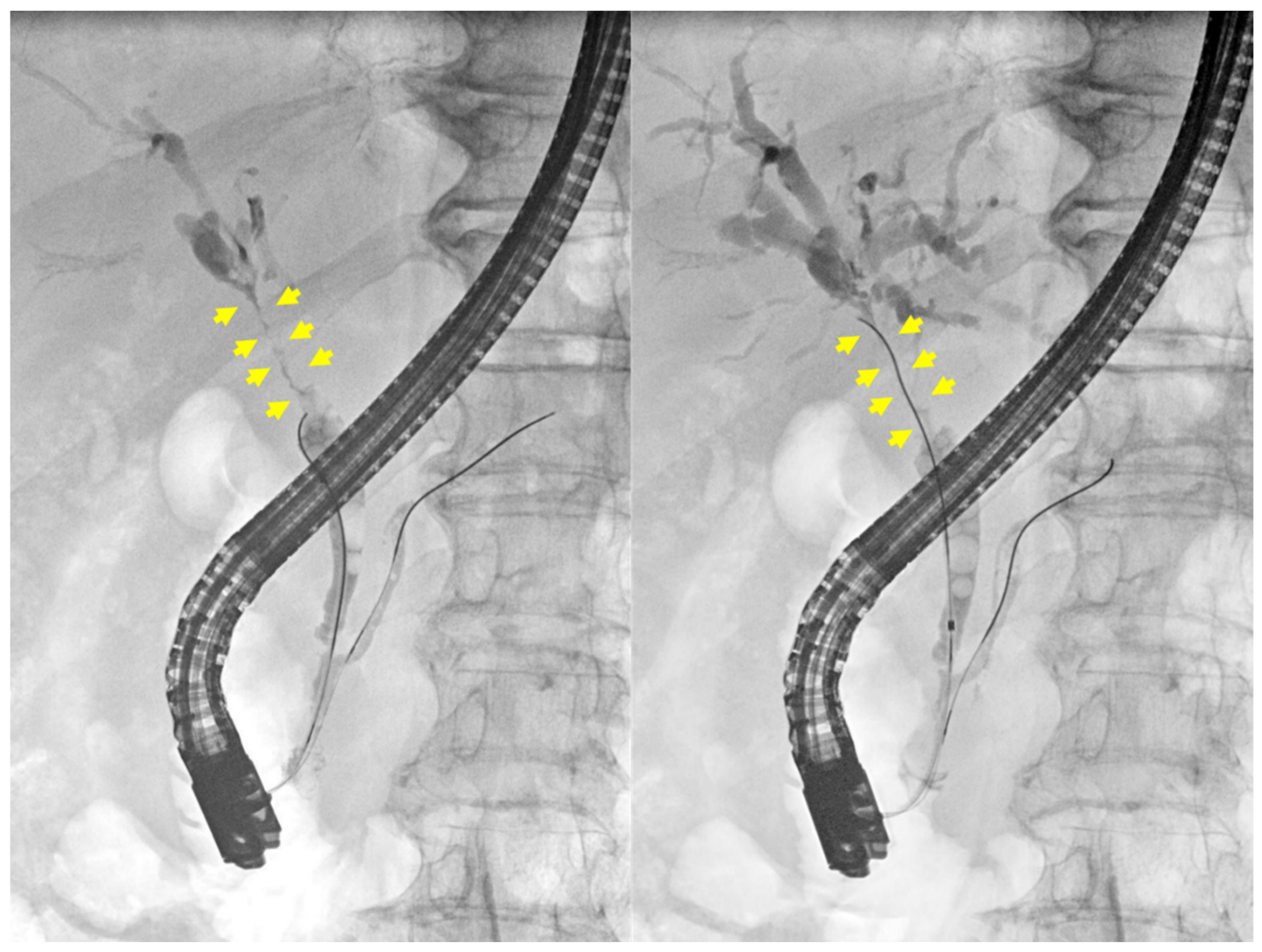
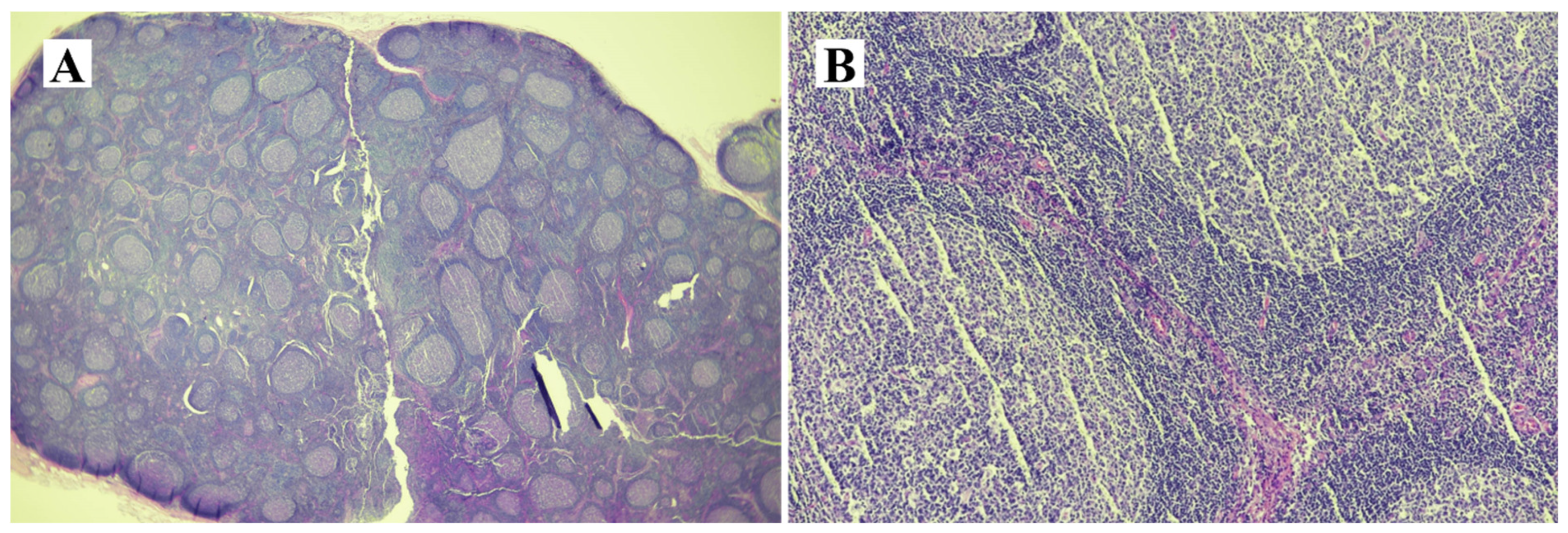
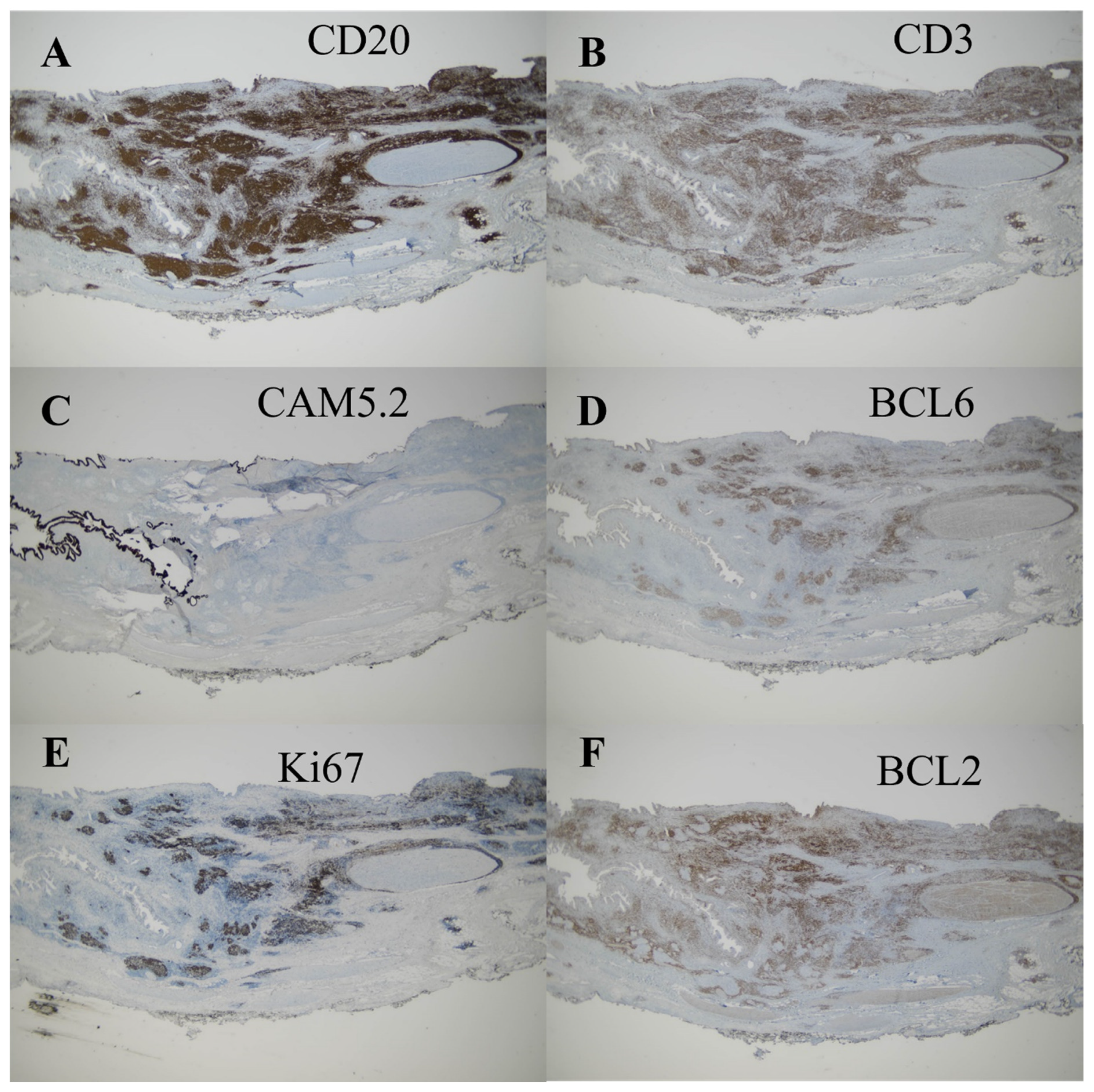
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Burnett, A.S.; Calvert, T.J.; Chokshi, R.J. Sensitivity of endoscopic retrograde cholangiopancreatography standard cytology: 10-y review of the literature. J. Surg. Res. 2013, 184, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Fujii-Lau, L.L.; Thosani, N.C.; Al-Haddad, M.; Acoba, J.; Wray, C.J.; Zvavanjanja, R.; Amateau, S.K.; Buxbaum, J.L.; Calderwood, A.H.; Chalhoub, J.M. ASGE Guideline on the role of endoscopy in the diagnosis of malignancy in biliary strictures of undetermined etiology: Summary and Recommendations. Gastrointest. Endosc. 2023, 98, 685–693. [Google Scholar] [CrossRef]
- Aoki, T.; Kubota, K.; Oka, T.; Hasegawa, K.; Hirai, I.; Makuuchi, M. Follicular cholangitis: Another cause of benign biliary stricture. Hepato-Gastroenterol. 2003, 50, 639–642. [Google Scholar]
- Gerhards, M.F.; Vos, P.; Van Gulik, T.M.; Rauws, E.A.J.; Bosma, A.; Gouma, D.J. Incidence of benign lesions in patients resected for suspicious hilar obstruction. Br. J. Surg. 2001, 88, 48–51. [Google Scholar] [CrossRef]
- Clayton, R.A.E.; Clarke, D.L.; Currie, E.J.; Madhavan, K.K.; Parks, R.W.; Garden, O.J. Incidence of benign pathology in patients undergoing hepatic resection for suspected malignancy. Surgeon 2003, 1, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, A.K.P.; Tirumani, S.H.; Prasad, S.R.; Fasih, N.; McInnes, M. Benign biliary strictures: A current comprehensive clinical and imaging review. AJR Am. J. Roentgenol. 2011, 197, W295-036. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lim, J.H.; Lim, H.K. Follicular cholangitis mimicking hilar cholangiocarcinoma. Abdom. Imaging 2005, 30, 744–747. [Google Scholar] [CrossRef]
- Fujita, T.; Kojima, M.; Kato, Y.; Gotohda, N.; Takahashi, S.; Konishi, M.; Kinoshita, T. Clinical and histopathological study of “follicular cholangitis”: Sclerosing cholangitis with prominent lymphocytic infiltration masquerading as hilar cholangiocarcinoma. Hepatol. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Zen, Y.; Ishikawa, A.; Ogiso, S.; Heaton, N.; Portmann, B. Follicular cholangitis and pancreatitis–clinicopathological features and differential diagnosis of an under-recognized entity. Histopathology 2012, 60, 261–269. [Google Scholar] [CrossRef]
- Fujii, M.; Shiode, J.; Niguma, T.; Ito, M.; Ishiyama, S.; Fujiwara, A.; Nose, S.; Yoshioka, M.; Mimura, T. A case of follicular cholangitis mimicking hilar cholangiocarcinoma. Clin. J. Gastroenterol. 2014, 7, 62–67. [Google Scholar] [CrossRef]
- Saito, R.; Fukuda, T.; Amano, H.; Nakahara, M.; Yoshida, M.; Yamaki, M.; Hanada, K.; Yonehara, S.; Noriyuki, T. Follicular cholangitis associated with focal biliary stricture treated with left hepatectomy after 8 years of follow-up: A rare case report. Mol. Clin. Oncol. 2016, 4, 114–118. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chang, L.S.; Tomimaru, Y.; Nishida, T.; Tamura, H.; Yoshioka, R.; Noguchi, K.; Noura, S.; Imamura, H.; Matsubara, T.; Adachi, S. Follicular cholangitis mimicking cholangiocarcinoma treated with right hepatectomy: A case report and review of published works. Hepatol. Res. 2019, 49, 1475–1480. [Google Scholar] [CrossRef] [PubMed]
- Kosone, T.; Takagi, H.; Takakusagi, S.; Hoshino, T.; Yokoyama, Y.; Kizawa, K.; Marubashi, K.; Watanabe, A.; Araki, K.; Harimoto, N. A Resected Case of Follicular Cholangitis That Was Positive on 18F-fluorodeoxyglucose-positron Emission Tomography. Intern. Med. 2020, 59, 2123–2128. [Google Scholar] [CrossRef]
- Koneri, K.; Goi, T.; Katayama, H.; Tagai, N.; Shimada, M.; Kurebayashi, H.; Sawai, K.; Morikawa, M.; Tamaki, M.; Hirono, Y. Follicular cholangitis mimicking a common bile duct cancer: A case report. Surg. Case Rep. 2023, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Draganov, P.V.; Chauhan, S.; Wagh, M.S.; Gupte, A.R.; Lin, T.; Hou, W.; Forsmark, C.E. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: A prospective, long-term follow-up study. Gastrointest. Endosc. 2012, 75, 347–353. [Google Scholar] [CrossRef]
- Gerges, C.; Beyna, T.; Tang, R.S.Y.; Bahin, F.; Lau, J.Y.W.; van Geenen, E.; Neuhaus, H.; Reddy, D.N.; Ramchandani, M. Digital single-operator peroral cholangioscopy-guided biopsy sampling versus ERCP-guided brushing for indeterminate biliary strictures: A prospective, randomized, multicenter trial (with video). Gastrointest. Endosc. 2020, 91, 1105–1113. [Google Scholar] [CrossRef]
- Fukuda, Y.; Tsuyuguchi, T.; Sakai, Y.; Tsuchiya, S.; Saisyo, H. Diagnostic utility of peroral cholangioscopy for various bile-duct lesions. Gastrointest. Endosc. 2005, 62, 374–382. [Google Scholar] [CrossRef]
- Chiang, A.; Theriault, M.; Salim, M.; James, P.D. The incremental benefit of EUS for the identification of malignancy in indeterminate extrahepatic biliary strictures: A systematic review and meta-analysis. Endosc. Ultrasound 2019, 8, 310. [Google Scholar]
- Heimbach, J.K.; Sanchez, W.; Rosen, C.B.; Gores, G.J. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB 2011, 13, 356–360. [Google Scholar] [CrossRef]
- Njei, B.; McCarty, T.R.; Mohan, B.P.; Fozo, L.; Navaneethan, U. Artificial intelligence in endoscopic imaging for detection of malignant biliary strictures and cholangiocarcinoma: A systematic review. Ann. Gastroenterol. 2023, 36, 223. [Google Scholar] [CrossRef]
- Marya, N.B.; Powers, P.D.; Petersen, B.T.; Law, R.; Storm, A.; Abusaleh, R.R.; Rau, P.; Stead, C.; Levy, M.J.; Martin, J. Identification of patients with malignant biliary strictures using a cholangioscopy-based deep learning artificial intelligence (with video). Gastrointest. Endosc. 2023, 97, 268–278. [Google Scholar] [CrossRef] [PubMed]



| Case | Author /Region | Year | Age/Sex | Increase in Bilirubin or Liver Enzyme | CA19-9 Elevation | Location | Preoperative Diagnosis | CD3 | CD20 | Treatment | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aoki [3] /Japan | 2003 | 57/F | + | − | Hilar | Hilar cholangiocarcinoma | + | + | Extended right hepatectomy | no data |
| 2 | Lee [7] /Korea | 2005 | 61/M | + | no data | Hilar, right and left hepatic duct, cystic duct, gallbladder neck | Hilar cholangiocarcinoma | no data | no data | Extrahepatic bile duct and part of the right hepatic duct resection | no data |
| 3, 4 | Fujita [8] /Japan | 2010 | 47/M | + | − | Hilar, right hepatic duct | Hilar cholangiocarcinoma | + | + | Extended right hepatectomy | 10 m alive |
| 44/F | − | − | Hilar | Hilar cholangiocarcinoma | + | + | Extended left hepatectomy | 24 m dead | |||
| 5, 6, 7 | Zen [9] /Japan | 2012 | 73/F | + | + | Right hepatic duct | Hilar cholangiocarcinoma | + | + | Right hepatectomy | no data |
| 70/M | + | + | Left perihilar duct | Hilar cholangiocarcinoma | + | + | Left hepatectomy | no data | |||
| 42/F | + | − | Hilar | Primary sclerosing cholangitis | + | + | Liver transplantation | no data | |||
| 8 | Fujii [10] /Japan | 2014 | 60/F | + | − | Hilar, right and left hepatic duct | Hilar cholangiocarcinoma | no data | no data | Left trisegmentectomy | 24 m alive |
| 9 | Saito [11] /Japan | 2016 | 69/F | − | − | B3 | Hepatolithiasis | no data | + | Left hepatectomy | 12 m alive |
| 10 | Chang [12] /Japan | 2019 | 60/M | + | − | Right hepatic duct posterior branch | Hilar cholangiocarcinoma | + | + | Right hepatectomy and caudate lobectomy | 18 m alive |
| 11 | Kosone [13] /Japan | 2020 | 70/M | + | − | Hilar | Hilar cholangiocarcinoma | no data | no data | Hilar cholangiocarcinoma | 30 m alive |
| 12 | Koneri [14] /Japan | 2023 | 77/F | + | − | Distal bile duct | Distal bile duct cancer | + | + | Subtotal stomach-preserving pancreaticoduodenectomy | 42 m alive |
| 13 | Lee (our case) /Korea | 2023 | 57/F | + | + | Hilar | Hilar cholangiocarcinoma | Subtotal stomach-preserving pancreaticoduodenectomy | 3 m alive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jeong, S.; Lee, D.H.; Choi, S.J.; Shin, W.Y. Diagnostic Challenges in Follicular Cholangitis Mimicking Hilar Cholangiocarcinoma: A Case Report and Review of the Literature. Medicina 2024, 60, 1513. https://doi.org/10.3390/medicina60091513
Lee J, Jeong S, Lee DH, Choi SJ, Shin WY. Diagnostic Challenges in Follicular Cholangitis Mimicking Hilar Cholangiocarcinoma: A Case Report and Review of the Literature. Medicina. 2024; 60(9):1513. https://doi.org/10.3390/medicina60091513
Chicago/Turabian StyleLee, Jungnam, Seok Jeong, Don Haeng Lee, Suk Jin Choi, and Woo Young Shin. 2024. "Diagnostic Challenges in Follicular Cholangitis Mimicking Hilar Cholangiocarcinoma: A Case Report and Review of the Literature" Medicina 60, no. 9: 1513. https://doi.org/10.3390/medicina60091513
APA StyleLee, J., Jeong, S., Lee, D. H., Choi, S. J., & Shin, W. Y. (2024). Diagnostic Challenges in Follicular Cholangitis Mimicking Hilar Cholangiocarcinoma: A Case Report and Review of the Literature. Medicina, 60(9), 1513. https://doi.org/10.3390/medicina60091513






