Clinical Management of Endometriosis in Menopause: A Narrative Review
Abstract
1. Introduction
2. Diagnosis
2.1. Evaluation of Postmenopausal Pelvic Pain
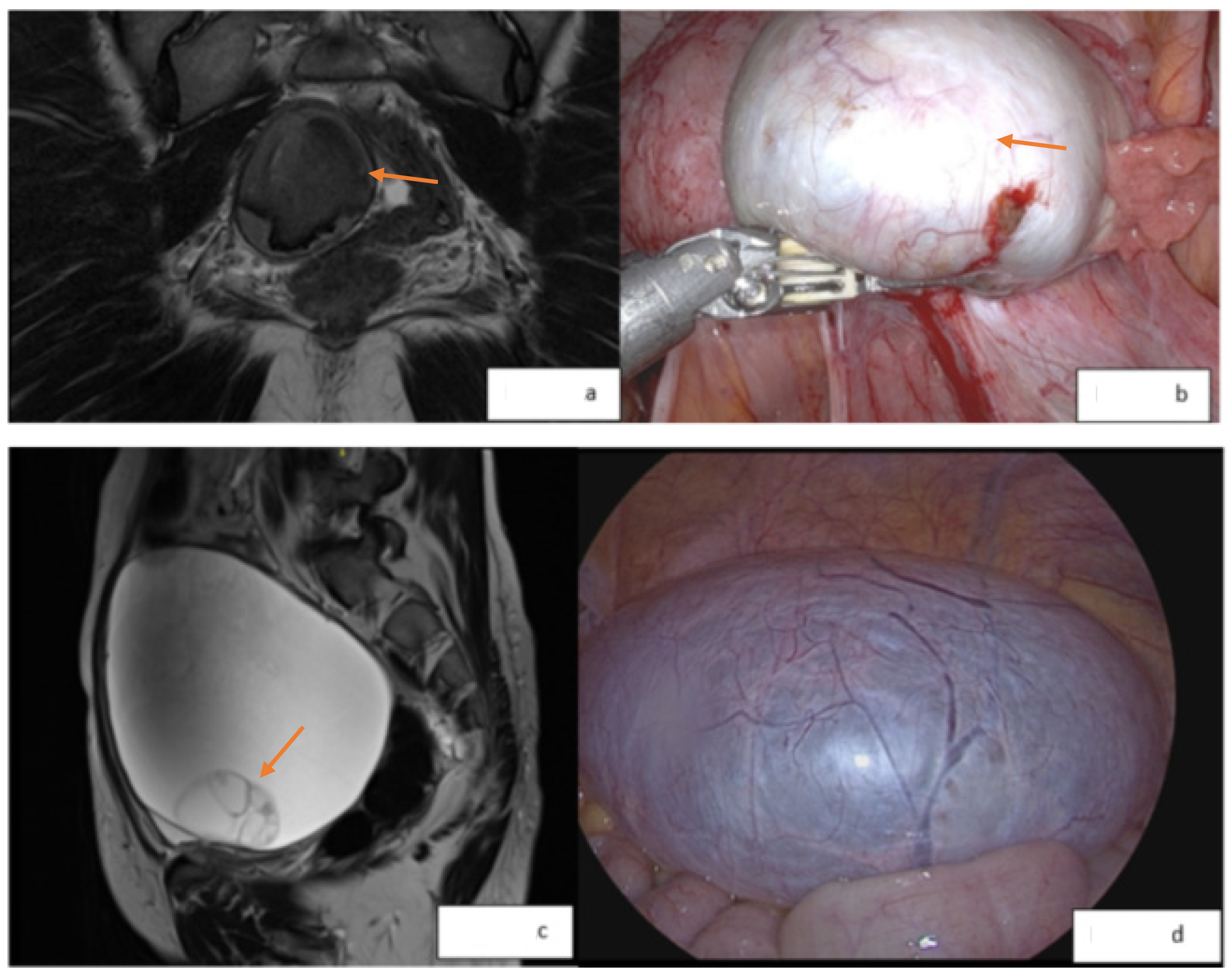
2.2. Imaging of Endometriosis
2.3. Laborotory Assessment: Biomarkers
3. Indications for Surgery
4. Hormone Replacement Therapy
5. De Novo Endometriosis Development
6. Malignant Transformative Potential
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Falcone, T.; Flyckt, R. Clinical Management of Endometriosis. Obstet. Gynecol. 2018, 131, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, S.; Zheng, Y.; Yi, X.; Qiu, J.; Zhang, X.; Zhang, Y.; Hua, K. Reproductive and postsurgical outcomes of infertile women with deep infiltrating endometriosis. BMC Women’s Health 2022, 22, 83. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, L.; Di Filippo, C.; Gabrielli, O.; Reppuccia, S.; La Rosa, V.L.; Ragusa, R.; Fichera, M.; Commodari, E.; Bifulco, G.; Giampaolino, P. The Burden of Endometriosis on Women’s Lifespan: A Narrative Overview on Quality of Life and Psychosocial Wellbeing. Int. J. Environ. Res. Public Health 2020, 17, 4683. [Google Scholar] [CrossRef]
- Hauser, W. Endometriosis and chronic overlapping pain conditions. Schmerz 2021, 35, 179–182. [Google Scholar]
- Chiuve, S.E.; Kilpatrick, R.D.; Hornstein, M.D.; Petruski-Ivleva, N.; Wegrzyn, L.R.; Dabrowski, E.C.; Velentgas, P.; Snabes, M.C.; Bateman, B.T. Chronic opioid use and complication risks in women with endometriosis: A cohort study in US administrative claims. Pharmacoepidemiol. Drug Saf. 2021, 30, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; A Flores, V. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Pascoal, E.; Wessels, J.M.; Aas-Eng, M.K.; Abrao, M.S.; Condous, G.; Jurkovic, D.; Espada, M.; Exacoustos, C.; Ferrero, S.; Guerriero, S.; et al. Strengths and limitations of diagnostic tools for endometriosis and relevance in diagnostic test accuracy research. Ultrasound Obstet. Gynecol. 2022, 60, 309–327. [Google Scholar] [CrossRef]
- Dunselman, G.A.J.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, A.; Delpero, E.; McKeown, S.; Tomlinson, G.; Bougie, O.; Murji, A. Endometriosis recurrence following post-operative hormonal suppression: A systematic review and meta-analysis. Hum. Reprod. Updat. 2021, 27, 96–107. [Google Scholar] [CrossRef]
- Abou-Setta, A.M.; Houston, B.; Al-Inany, H.G.; Farquhar, C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 2013, 12, CD005072.pub3. [Google Scholar] [CrossRef]
- Gibbons, T.; Georgiou, E.X.; Cheong, Y.C.; Wise, M.R. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 2021, 12, CD005072.pub4. [Google Scholar]
- Tang, H.; Lin, T.; Wu, M.; Tsai, S. Progesterone resistance in endometriosis: A pathophysiological perspective and potential treatment alternatives. Reprod. Med. Biol. 2024, 23, e12588. [Google Scholar] [CrossRef]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum. Reprod. Updat. 2020, 26, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-W. Recurrence of endometriosis and its control. Hum. Reprod. Updat. 2009, 15, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Veth, V.B.; van de Kar, M.M.; Duffy, J.M.; van Wely, M.; Mijatovic, V.; Maas, J.W. Gonadotropin-releasing hormone analogues for endometriosis. Cochrane Database Syst. Rev. 2023, 2023, CD014788. [Google Scholar] [CrossRef]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, CD008475. [Google Scholar] [PubMed]
- Secosan, C.; Balulescu, L.; Brasoveanu, S.; Balint, O.; Pirtea, P.; Dorin, G.; Pirtea, L. Endometriosis in Menopause—Renewed Attention on a Controversial Disease. Diagnostics 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.D.; Haj Hamoud, B.; Amza, M.; Gorecki, G.P.; Sima, R.M.; Gică, N.; Pleș, L. Endometriosis in Menopausal Women-A New Age Is Coming? Literature Review. Life 2024, 14, 485. [Google Scholar] [CrossRef]
- Kulkarni, M.T.; Shafrir, A.; Farland, L.V.; Terry, K.L.; Whitcomb, B.W.; Eliassen, A.H.; Bertone-Johnson, E.R.; Missmer, S.A. Association Between Laparoscopically Confirmed Endometriosis and Risk of Early Natural Menopause. JAMA Netw. Open 2022, 5, e2144391. [Google Scholar] [CrossRef]
- Steege, J.F.; Siedhoff, M.T. Chronic pelvic pain. Obstet. Gynecol. 2014, 124, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Fuentes, L.; Almoguera, B.; Chaves, P.; Vaquero, G.; Perez-Medina, T. Understanding the Female Physical Examination in Patients with Chronic Pelvic and Perineal Pain. J. Clin. Med. 2022, 11, 7490. [Google Scholar] [CrossRef]
- Chandler, J.; Wagner, E.; Riley, K. Evaluation of Female Pelvic Pain. Semin. Reprod. Med. 2018, 36, 099–106. [Google Scholar] [CrossRef]
- Siedhoff, M.T.; Carey, E.T.; Findley, A.D.; Hobbs, K.A.; Moulder, J.K.; Steege, J.F. Post-hysterectomy Dyspareunia. J. Minim. Invasive Gynecol. 2014, 21, 567–575. [Google Scholar] [CrossRef]
- Trehan, A.K.; Sanaullah, F. Laparoscopic Posthysterectomy Vaginal Vault Excision for Chronic Pelvic Pain and Deep Dyspareunia. J. Minim. Invasive Gynecol. 2009, 16, 326–332. [Google Scholar] [CrossRef]
- Abdelmonem, A.M. Vaginal length and incidence of dyspareunia after total abdominal versus vaginal hysterectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 190–192. [Google Scholar] [CrossRef] [PubMed]
- Kho, R.M.; Abrao, M.S. Ovarian remnant syndrome: Etiology, diagnosis, treatment and impact of endometriosis. Curr. Opin. Obstet. Gynecol. 2012, 24, 210–214. [Google Scholar] [CrossRef]
- Magtibay, P.M.; Magrina, J.F. Ovarian remnant syndrome. Clin. Obstet. Gynecol. 2006, 49, 526–534. [Google Scholar] [CrossRef]
- Leonardi, M.; Martins, W.P.; Espada, M.; Georgousopoulou, E.; Condous, G. Prevalence of negative sliding sign representing pouch of Douglas obliteration during pelvic transvaginal ultrasound for any indication. Ultrasound Obstet. Gynecol. 2020, 56, 928–933. [Google Scholar] [CrossRef]
- Reid, S.; Lu, C.; Casikar, I.; Reid, G.; Abbott, J.; Cario, G.; Chou, D.; Kowalski, D.; Cooper, M.; Condous, G. Prediction of pouch of Douglas obliteration in women with suspected endometriosis using a new real-time dynamic transvaginal ultrasound technique: The sliding sign. Ultrasound Obstet. Gynecol. 2013, 41, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.G.; VanBuren, W.M.; Sheedy, S.P. Endometriosis in the postmenopausal female: Clinical presentation, imaging features, and management. Abdom. Radiol. 2020, 45, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.A.; Menias, C.O.; Chen, L.; Schiappacasse, G.; Shaaban, A.M.; Caserta, M.P.; Elsayes, K.M.; VanBuren, W.M.; Bolan, C.W. Understanding malignant transformation of endometriosis: Imaging features with pathologic correlation. Abdom. Radiol. 2020, 45, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Tanase, Y.; Kawaguchi, R.; Takahama, J.; Kobayashi, H. Factors that Differentiate between Endometriosis-associated Ovarian Cancer and Benign Ovarian Endometriosis with Mural Nodules. Magn. Reson. Med. Sci. 2018, 17, 231–237. [Google Scholar] [CrossRef]
- Sadowski, E.A.; Thomassin-Naggara, I.; Rockall, A.; Maturen, K.E.; Forstner, R.; Jha, P.; Nougaret, S.; Siegelman, E.S.; Reinhold, C. O-RADS MRI Risk Stratification System: Guide for Assessing Adnexal Lesions from the ACR O-RADS Committee. Radiology 2022, 303, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.A.; Metwally, M.I.; Gamil, S.A.; Khater, H.M.; Aly, S.A.; El Sammak, A.A.; Zaitoun, M.M.A.; Khattab, E.M.; Azmy, T.M.; Alayouty, N.A.; et al. Comparison of O-RADS, GI-RADS, and IOTA simple rules regarding malignancy rate, validity, and reliability for diagnosis of adnexal masses. Eur. Radiol. 2021, 31, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, S.; Cozzi, A.; Dolciami, M.; Del Grande, F.; Scarano, A.L.; Papadia, A.; Gui, B.; Gandolfo, N.; Catalano, C.; Manganaro, L. O-RADS MRI: A Systematic Review and Meta-Analysis of Diagnostic Performance and Category-wise Malignancy Rates. Radiology 2023, 307, e220795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dai, X.; Li, W. Systematic Review and Meta-Analysis of O-RADS Ultrasound and O-RADS MRI for Risk Assessment of Ovarian and Adnexal Lesions. Am. J. Roentgenol. 2023, 221, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Radiology, A.C.O. O-RADS™ US v2022—Assessment Categories. 2022. Available online: https://www.acr.org/-/media/ACR/Files/RADS/O-RADS/US-v2022/O-RADS--US-v2022-Assessment-Categories.pdf (accessed on 6 June 2024).
- Radiology, A.C.O. O-RADS™ MRI Risk Score Governing Concepts. 2024. Available online: https://www.acr.org/-/media/ACR/Files/RADS/O-RADS/O-RADS-MRI-Risk-Score_v1_2020_May-2024.pdf (accessed on 6 June 2024).
- Abrão, M.; Podgaec, S.; Pinotti, J.; de Oliveira, R. Tumor markers in endometriosis. Int. J. Gynecol. Obstet. 1999, 66, 19–22. [Google Scholar] [CrossRef]
- Williams, T.I.; Toups, K.L.; Saggese, D.A.; Kalli, K.R.; Cliby, W.A.; Muddiman, D.C. Epithelial Ovarian Cancer: Disease Etiology, Treatment, Detection, and Investigational Gene, Metabolite, and Protein Biomarkers. J. Proteome Res. 2007, 6, 2936–2962. [Google Scholar] [CrossRef]
- Li, K.; Pei, Y.; Wu, Y.; Guo, Y.; Cui, W. Performance of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) in diagnosis of ovarian cancer: A systematic review and meta-analysis. J. Ovarian Res. 2020, 13, 6. [Google Scholar] [CrossRef]
- Moore, R.G.; Miller, M.C.; Steinhoff, M.M.; Skates, S.J.; Lu, K.H.; Lambert-Messerlian, G.; Bast, R.C. Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders. Am. J. Obstet. Gynecol. 2012, 206, 351.e1–351.e8. [Google Scholar] [CrossRef] [PubMed]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.J.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2016, CD012179. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Suisse, S.; Puchar, A.; Delbos, L.; Poilblanc, M.; Descamps, P.; Golfier, F.; Jornea, L.; Bouteiller, D.; Touboul, C.; et al. Salivary MicroRNA Signature for Diagnosis of Endometriosis. J. Clin. Med. 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Bendifallah, S.; Dabi, Y.; Suisse, S.; Delbos, L.; Spiers, A.; Poilblanc, M.; Golfier, F.; Jornea, L.; Bouteiller, D.; Fernandez, H.; et al. Validation of a Salivary miRNA Signature of Endometriosis—Interim Data. NEJM Evid. 2023, 2, EVIDoa2200282. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Carbonnel, M.; Ceccaldi, P.-F.; Feki, A.; Ayoubi, J.-M. Postmenopausal endometriosis: A challenging condition beyond menopause. Menopause 2024, 31, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, C.V.; Shapiro, E.Y.; Ahn, J.J.; Van Batavia, J.P.; Silva, M.V.; Tan, Y.; Gupta, M. Endoscopic Management of Intraluminal Ureteral Endometriosis. Urology 2013, 82, 307–312. [Google Scholar] [CrossRef]
- Neto, J.N.; Melo, V.G.; Lima, L.C.S.; Costa, M.V.L.R.; Silva, L.C.; Gomes, L.M.R.d.S.; Freire, G.I.d.M.; Leal, P.d.C.; de Oliveira, C.M.B.; Moura, E.C.R. Improved quality of life (EHP-30) in patients with endometriosis after surgical treatment. Rev. Assoc. Médica Bras. 2023, 69, e20230316. [Google Scholar] [CrossRef] [PubMed]
- Deecher, D.C.; Dorries, K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch. Women’s Ment. Health 2007, 10, 247–257. [Google Scholar] [CrossRef]
- Khan, S.J.; Kapoor, E.; Faubion, S.S.; Kling, J.M. Vasomotor Symptoms During Menopause: A Practical Guide on Current Treatments and Future Perspectives. Int. J. Women’s Health 2023, 15, 273–287. [Google Scholar] [CrossRef]
- Eisenberger, A.; Westhoff, C. Hormone replacement therapy and venous thromboembolism. J. Steroid Biochem. Mol. Biol. 2014, 142, 76–82. [Google Scholar] [CrossRef]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [PubMed]
- Mikkola, T.S.; Savolainen-Peltonen, H.; Tuomikoski, P.; Hoti, F.; Vattulainen, P.; Gissler, M.; Ylikorkala, O. Reduced risk of breast cancer mortality in women using postmenopausal hormone therapy: A Finnish nationwide comparative study. Menopause 2016, 23, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Anderson, G.L.; Aragaki, A.K.; Manson, J.E.; Stefanick, M.L.; Pan, K.; Barrington, W.; Kuller, L.H.; Simon, M.S.; Lane, D.; et al. Association of Menopausal Hormone Therapy with Breast Cancer Incidence and Mortality During Long-term Follow-up of the Women’s Health Initiative Randomized Clinical Trials. JAMA 2020, 324, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Trespidi, L.; Colombo, A.; Vendola, N.; Marchini, M.; Crosignani, P.G. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil. Steril. 1993, 60, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Moen, M.H.; Rees, M.; Brincat, M.; Erel, T.; Gambacciani, M.; Lambrinoudaki, I.; Schenck-Gustafsson, K.; Tremollieres, F.; Vujovic, S.; Rozenberg, S. EMAS position statement: Managing the menopause in women with a past history of endometriosis. Maturitas 2010, 67, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Al Kadri, H.; Hassan, S.; Al-Fozan, H.M.; Hajeer, A. Hormone therapy for endometriosis and surgical menopause. Cochrane Database Syst. Rev. 2009, 1, CD005997. [Google Scholar] [CrossRef] [PubMed]
- Zanello, M.; Borghese, G.; Manzara, F.; Degli Esposti, E.; Moro, E.; Raimondo, D.; Abdullahi, L.O.; Arena, A.; Terzano, P.; Meriggiola, M.C.; et al. Hormonal Replacement Therapy in Menopausal Women with History of Endometriosis: A Review of Literature. Medicina 2019, 55, 477. [Google Scholar] [CrossRef] [PubMed]
- Gemmell, L.C.; Webster, K.E.; Kirtley, S.; Vincent, K.; Zondervan, K.T.; Becker, C.M. The management of menopause in women with a history of endometriosis: A systematic review. Hum. Reprod. Update 2017, 23, 481–500. [Google Scholar] [CrossRef] [PubMed]
- Guy, J.M. Edgar Haydon (1859–1942): General practitioner and radium pioneer. J. Med. Biogr. 2009, 17, 127–134. [Google Scholar] [CrossRef]
- Streuli, I.; Gaitzsch, H.; Wenger, J.-M.; Petignat, P. Endometriosis after menopause: Physiopathology and management of an uncommon condition. Climacteric 2017, 20, 138–143. [Google Scholar] [CrossRef]
- Vorstman, B.; Lynne, C.; Politano, V.A. Postmenopausal vesical endometriosis. Urology 1983, 22, 540–542. [Google Scholar] [CrossRef]
- Ismail, S.M.; Maulik, T.G. Tamoxifen-associated post-menopausal endometriosis. Histopathology 1997, 30, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Kurioka, H.; Takahashi, K.; Okada, M.; Ozaki, T.; Miyazaki, K.; Maruyama, R.; Yoshida, M. A case of postmenopausal endometriosis unrelated to neoplasm. Int. J. Fertil. Women’s Med. 1999, 44, 160–162. [Google Scholar]
- Deval, B.; Rafii, A.; Dachez, M.F.; Kermanash, R.; Levardon, M. Sigmoid endometriosis in a postmenopausal woman. Am. J. Obstet. Gynecol. 2002, 187, 1723–1725. [Google Scholar] [CrossRef]
- Goumenou, A.G.; Chow, C.; Taylor, A.; Magos, A. Endometriosis arising during estrogen and testosterone treatment 17 years after abdominal hysterectomy: A case report. Maturitas 2003, 46, 239–241. [Google Scholar] [CrossRef]
- Popoutchi, P.; Lemos, C.R.d.R.; e Silva, J.C.R.; Nogueira, A.A.; Feres, O.; da Rocha, J.J.R. Postmenopausal intestinal obstructive endometriosis: Case report and review of the literature. Sao Paulo Med. J. 2008, 126, 190–193. [Google Scholar] [CrossRef]
- Manero, M.G.; Royo, P.; Olartecoechea, B.; Alcázar, J.L. Endometriosis in a postmenopausal woman without previous hormonal therapy: A case report. J. Med. Case Rep. 2009, 3, 135. [Google Scholar] [CrossRef]
- Maeda, T.; Uchida, Y.; Nakajima, F. Vesical endometriosis following the menopause. Int. Urogynecol J. Pelvic Floor. Dysfunct. 2009, 20, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Lee, E.Y.P.; Vardhanabhuti, V.; Khong, P.-L.; Ngu, S.-F. Unusual Case of Postmenopausal Diffuse Endometriosis Mimicking Metastastic Ovarian Malignancy. Clin. Nucl. Med. 2016, 41, e120–e122. [Google Scholar] [CrossRef]
- Ianieri, M.; Buca, D.; Panaccio, P.; Cieri, M.; Francomano, F.; Liberati, M. Retroperitoneal endometriosis in postmenopausal woman causing deep vein thrombosis: Case report and review of the literature. Clin. Exp. Obstet. Gynecol. 2017, 44, 148–150. [Google Scholar] [CrossRef]
- Solima, E.; Pino, I.; Scagnelli, G.; Biasoni, D.; Vignali, M. “When You Hear Hoofbeats, Think of Horses, Not Zebras:” A Case of Bladder Endometriosis in Menopause. J. Minim. Invasive Gynecol. 2019, 26, 796–797. [Google Scholar] [CrossRef] [PubMed]
- Naem, A.; Shamandi, A.; Al-Shiekh, A.; Alsaid, B. Free large sized intra-abdominal endometrioma in a postmenopausal woman: A case report. BMC Women’s Health 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Raju, P.D.D.; Lamture, Y.; Deshpande, S.G.; Gattani, R.G. Endometrial Cyst Presenting as a Vague Abdominal Lump in a Postmenopausal Woman. Cureus 2022, 14, e29807. [Google Scholar] [CrossRef]
- Zografou, M.T.; Naem, A.; Laganà, A.S.; Krentel, H. A Large Ovarian Endometrioma Occupying the Abdominal Cavity in a Postmenopausal Patient: A Case Report. Medicina 2023, 59, 1398. [Google Scholar] [CrossRef] [PubMed]
- Ishizaka, A.; Taguchi, A.; Tsuruga, T.; Maruyama, M.; Kawata, A.; Miyamoto, Y.; Tanikawa, M.; Ikemura, M.; Sone, K.; Mori, M.; et al. Endometrial cancer with concomitant endometriosis is highly associated with ovarian endometrioid carcinoma: A retrospective cohort study. BMC Women’s Health 2022, 22, 332. [Google Scholar] [CrossRef] [PubMed]
- Garavaglia, E.; Sigismondi, C.; Ferrari, S.; Candiani, M. The origin of endometriosis-associated ovarian cancer from uterine neoplastic lesions. Med. Hypotheses 2018, 110, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, N.; Banys-Paluchowski, M.; Schmidt, D.; Ulrich, U.; Fehm, T. Endometriosis-associated Malignancy. Geburtshilfe Und Frauenheilkd. 2016, 76, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Magoga, G.; Pasciuto, T.; Mascilini, F.; Moruzzi, M.C.; Fischerova, D.; Savelli, L.; Giunchi, S.; Mancari, R.; Franchi, D.; et al. Imaging in gynecological disease (13): Clinical and ultrasound characteristics of endometrioid ovarian cancer. Ultrasound Obstet. Gynecol. 2018, 52, 535–543. [Google Scholar] [CrossRef]
- Minamikawa, T.; Yachida, N.; Takahashi, K.; Saito, K.; Sekizuka, T.; Akashi, H.; Suzuki, M.; Mori, Y.; Yamawaki, K.; Suda, K.; et al. Endometrial Cancer with and without Endometriosis: Clinicopathological Differences. Cancers 2023, 15, 5635. [Google Scholar] [CrossRef]
- Chene, G.; Ouellet, V.; Rahimi, K.; Barres, V.; Provencher, D.; Mes-Masson, A.M. The ARID1A pathway in ovarian clear cell and endometrioid carcinoma, contiguous endometriosis, and benign endometriosis. Int. J. Gynecol. Obstet. 2015, 130, 27–30. [Google Scholar] [CrossRef]
- Hollis, R.L.; Thomson, J.P.; Stanley, B.; Churchman, M.; Meynert, A.M.; Rye, T.; Bartos, C.; Iida, Y.; Croy, I.; Mackean, M.; et al. Molecular stratification of endometrioid ovarian carcinoma predicts clinical outcome. Nat. Commun. 2020, 11, 4995. [Google Scholar] [CrossRef] [PubMed]
- Giannella, L.; Marconi, C.; Di Giuseppe, J.; Carpini, G.D.; Fichera, M.; Grelloni, C.; Giuliani, L.; Montanari, M.; Insinga, S.; Ciavattini, A. Malignant Transformation of Postmenopausal Endometriosis: A Systematic Review of the Literature. Cancers 2021, 13, 4026. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, T.H.; Chung, H.H.; Song, Y.S. Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br. J. Cancer 2014, 110, 1878–1890. [Google Scholar] [CrossRef]
- Centini, G.; Schettini, G.; Pieri, E.; Giorgi, M.; Lazzeri, L.; Martire, F.G.; Mancini, V.; Raimondo, D.; Seracchioli, R.; Habib, N.; et al. Endometriosis-Related Ovarian Cancer: Where Are We Now? A Narrative Review towards a Pragmatic Approach. J. Clin. Med. 2024, 13, 1933. [Google Scholar] [CrossRef] [PubMed]
- Mihailovici, A.; Rottenstreich, M.; Kovel, S.; Wassermann, I.; Smorgick, N.; Vaknin, Z. Endometriosis-associated malignant transformation in abdominal surgical scar: A PRISMA-compliant systematic review. Medicine 2017, 96, e9136. [Google Scholar] [CrossRef]
- Ye, J.; Peng, H.; Huang, X.; Qi, X. The association between endometriosis and risk of endometrial cancer and breast cancer: A meta-analysis. BMC Women’s Health 2022, 22, 455. [Google Scholar] [CrossRef]
- Ladanyi, C.; Boyd, S.; Sticco, P.; Mohling, S. Postmenopausal endometriosis, where are we now? Curr. Opin. Obstet. Gynecol. 2019, 31, 267–278. [Google Scholar] [CrossRef]
| Condition | Definition | Management |
|---|---|---|
| Genitourinary syndrome of menopause | An array of symptoms caused by hypoestrogenic changes to the vulva, vagina, urethra, or bladder that are typically seen in postmenopausal women. Also referred to as vulvovaginal atrophy. | Estrogen vaginal cream, vaginal lubricants, and moisturizers |
| Pelvic floor tension myalgia | A common type of pelvic pain specific to the pelvic floor. A type of myofascial pain that typically arises from the spasticity, hypersensitivity, and dysfunction of the pelvic floor muscles; fascia/abdominal wall; trigger points; and back pain. | Pelvic floor physical therapy, vaginal suppositories, pelvic floor trigger point injections, and pelvic floor Botox injections |
| Pudendal neuralgia | Pelvic pain associated with hypersensitivity to the pudendal nerves which can cause nerve entrapment and inflammation. Pain is typically increased with sitting and can be caused by previous pelvic trauma, childbirth, or changes related to age. | Pelvic floor physical therapy, vaginal suppositories, pudendal nerve block, and pudendal neurolysis surgery |
| Pelvic adhesions | Scar tissue and adhesions in the pelvis from prior surgery or inflammatory conditions such as endometriosis. | Consideration of surgery for the lysis of adhesions (controversial) |
| Vulvodynia | Vulvar pain, likely idiopathic in nature, that occurs for three months or more. Hypersensitivity to the vulva with sitting, palpation, and with activity. | Estrogen vaginal cream, topical lidocaine, topical neuromodulating medications, and vestibulectomy |
| Pelvic congestion syndrome | Pelvic pain associated with dilated or engorged pelvic vascular structures. This can also be associated with Nutcracker syndrome and vulvovaginal varicostities. | Progestins, embolization or coiling of abnormal vasculature, and consideration of surgery |
| Painful bladder syndrome | Chronic bladder pain, also known as interstitial cystitis, which significantly impacts the quality of life in men and women. Etiology is unknown and likely multi-factorial. | Lifestyle modifications and avoidance of bladder irritants, anti-histamine medications, bladder instillations, bladder Botox injections, and bladder hydrodistention |
| Coccydynia | Chronic coccyx and/or tailbone pain causing tenderness and localized pain to the coccyx primarily with sitting and the supine position. Symptoms may also lead to pelvic floor dysfunction, dyspareunia, pelvic floor tension myalgia, and defecation disorders. | Pelvic floor physical therapy, coccyx trigger point injections, and ganglion impar block |
| Endometriosis | Endometrial glands and stomas found outside of the endometrial cavity that can cause pelvic pain, dyspareunia, menorrhagia, dysmenorrhea, potential infertility, and chronic pain. | Hormone suppression (OCPs, progestins, annd GnRH analogs), and surgical excision |
| Adnexal masses | Masses typically seen in the adnexal space or on the ovary; may be cystic lesions or tumors that range from simple to complex, benign to malignant. | Surgical excision |
| Vulvar dermatoses | Dermatitis to the vulva that is typically inflammatory in nature. Symptoms can include erythema, prurutitis, chronic irritation, and/or vulvar lesions or rash. | Estrogen vaginal cream, topical steroids, vaginal laser therapy, and vaginal platelet-rich plasma (experimental) |
| Vaginal cuff pain | Persistent tenderness, burning, or discomfort at the center or apexes of the vaginal cuff. This may be related to vaginal adhesions, previous pelvic radiation, or atrophy. | Pelvic floor physical therapy, vaginal suppositories, vaginal cuff trigger point injections, consideration of surgical revision of cuff (controversial) |
| Ovarian remnant syndrome | Ovarian tissue seen in a patient who previously underwent oophorectomy. This can lead to pelvic pain and adnexal masses. | Surgical excision of the remnant ovarian tissue |
| O-RADS Score | Risk Category | U/s Features | MRI Features |
|---|---|---|---|
| O-RADS 0 | Incomplete evaluation | N/a | N/a |
| O-RADS 1 | Normal ovary | Risk of malignancy: N/a
| Risk of malignancy: N/a
|
| O-RADS 2 | Almost certainly benign | Risk of malignancy: <1%
| Risk of malignancy: <0.5%
|
| O-RADS 3 | Low risk | Risk of malignancy: 1–10%
| Risk of malignancy: <5%
|
| O-RADS 4 | Intermediate risk | Risk of malignancy: 10–50%
| Risk of malignancy: 50%
|
| O-RADS 5 | High risk | Risk of malignancy: ≥50%
| Risk of malignancy: 90%
|
| Author | Year | Pathology | Case Description |
|---|---|---|---|
| Vorstman [63] | 1983 | Benign bladder endometrioma | A 64-year-old female with prior hysterectomy and no current HRT use presented with hematuria and stress urinary incontinence. A cystic mass was noted at the bladder dome and confirmed to be endometriosis with a bladder biopsy. She underwent partial cystectomy, left oophorectomy, and Marshall-Marchetti procedure. |
| Ismail [64] | 1997 | Benign ovarian endometrioma | A 52-year-old female with breast cancer (using tamoxifen)and no prior history of endometriosis or HRT use underwent hysterectomy with BSO due to vaginal bleeding. She had a cystic 10 cm ovarian mass. |
| Kurioka [65] | 1999 | Benign ovarian endometrioma | A 55-year-old postmenopausal woman without known endometriosis or HRT use presented with a partially solid ovarian mass. She underwent an abdominal hysterectomy with BSO. |
| Deval [66] | 2002 | Benign endometriosis | A 69-year-old woman with pelvic pain, constipation, and progressive weight loss with a 15 cm lesion causing compression of the sigmoid colon. There was no prior history of endometriosis or HRT use, and tumor markers were normal. She underwent laparotomy with radical hysterectomy, BSO, colon resection with sigmoid end-colostomy and Harmann’s pouch creation. |
| Goumenou [67] | 2003 | Benign endometriosis | A 67-year-old woman with pelvic pain and deep dyspareunia, prior hysterectomy, and she had been using estrogen HRT along with testosterone implants. Imaging showed a 2.9 cm cystic left ovarian mass, with normal Ca-125. Laparoscopy showed left ovarian endometriosis, extensive peritoneal implants, a large retroperitoneal mass, and obliteration of the posterior cul-de-sac. |
| Popoutchi [68] | 2008 | Benign rectal endometriosis | A 74-year-old female presented hematochezia and tenesmus; with prior hysterectomy and BSO and no prior HRT use. Imaging revealed a large bowel obstruction, and colonoscopy showed a friable rectal tumor, with pathology consistent with endometriosis. The patient underwent rectosigmoidectomy with protective transversotomy, and ultimately returned for end colostomy due to the stenosing recurrence of the rectum. |
| Manero [69] | 2009 | Benign ovarian endometrioma | A 62-year-old-female with no prior history of endometriosis or HRT use presented with pelvic pain. Imaging revealed a 4.4 cm left ovarian mass, with normal tumor markers. Laparoscopic BSO was performed. |
| Maeda [70] | 2009 | Benign bladder endometriosis | A 65-year-old woman with painless hematuria and abdominal pain, with prior hysterectomy and no prior HRT use. Imaging showed a vesical polypoid mass, and cystoscopic biopsy and transurethral resection of the mass revealed endometriosis. |
| Agarwal Sharma [71] | 2016 | Benign ovarian endometrioma | A 69-year-old woman with abdominal distention and short-term leg swelling. Ca-125 was elevated to 120 u/mL, and imaging showed a 25 cm cystic pelvic mass which was suggestive of malignancy with peritoneal carcinomatosis. She underwent exploratory laparotomy with cytoreduction, and findings showed diffuse endometriosis along the peritoneal surfaces in addition to the large ovarian mass. |
| Ianieri [72] | 2017 | Benign endometriosis | A 63-year-old female with abdominal pain and limb swelling, who was noted to have a retroperitoneal mass causing deep vein thrombosis because of the extrinsic compression of the left iliac vein. Laparotomy with endometriosis excision was performed. |
| Solima [73] | 2019 | Benign endometriosis | A 60-year-old postmenopausal woman with no prior HRT use, and no known endometriosis or chronic pelvic pain presented with a rectovaginal mass. Imaging revealed a mass involving the uterus, posterior bladder wall, and rectum, and a cystic lesion was seen in the bladder trigone during cystoscopy. She underwent laparoscopic BSO and bladder biopsy. |
| Naem [74] | 2020 | Benign abdominal endometrioma | A 67-year-old woman presented with bowel obstruction and right-sided hydronephrosis in the setting of a 17 × 26 cm abdominal mass. The patient underwent laparotomy with the en-bloc resection of the mass. |
| Devasilpa [75] | 2022 | Benign ovarian endometrioma | A 52-year-old postmenopausal patient presented with abdominal distention, and imaging showed a 30 × 13 × 20 cm right ovarian cystic mass. Ca-125 was normal and she was not previously using HRT. Laparotomy with right salpingo-oophorectomy was performed, with extrusion of 5 L of chocolate cyst fluid. |
| Zografou [76] | 2023 | Benign ovarian endometrioma | A 60-year-old previously healthy female presented with a 26 cm ovarian mass. Pre-operative Ca-125 was 512.9 U/mL, and all other labs were normal. The patient underwent laparotomy with BSO. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dave, D.; Page, H.E.; Carrubba, A.R. Clinical Management of Endometriosis in Menopause: A Narrative Review. Medicina 2024, 60, 1341. https://doi.org/10.3390/medicina60081341
Dave D, Page HE, Carrubba AR. Clinical Management of Endometriosis in Menopause: A Narrative Review. Medicina. 2024; 60(8):1341. https://doi.org/10.3390/medicina60081341
Chicago/Turabian StyleDave, Dhruva, Heidi E. Page, and Aakriti R. Carrubba. 2024. "Clinical Management of Endometriosis in Menopause: A Narrative Review" Medicina 60, no. 8: 1341. https://doi.org/10.3390/medicina60081341
APA StyleDave, D., Page, H. E., & Carrubba, A. R. (2024). Clinical Management of Endometriosis in Menopause: A Narrative Review. Medicina, 60(8), 1341. https://doi.org/10.3390/medicina60081341






