Abstract
Background and objectives: Age-related macular degeneration (AMD) is a complex and multifactorial condition that can lead to permanent vision loss once it progresses to the neovascular exudative stage. This review aims to summarize the use of deep learning in neovascular AMD. Materials and Methods: Pubmed search. Results: Deep learning has demonstrated effectiveness in analyzing structural OCT images in patients with neovascular AMD. This review outlines the role of deep learning in identifying and measuring biomarkers linked to an elevated risk of transitioning to the neovascular form of AMD. Additionally, deep learning techniques can quantify critical OCT features associated with neovascular AMD, which have prognostic implications for these patients. Incorporating deep learning into the assessment of neovascular AMD eyes holds promise for enhancing clinical management strategies for affected individuals. Conclusion: Several studies have demonstrated effectiveness of deep learning in assessing neovascular AMD patients and this has a promising role in the assessment of these patients.
1. Introduction
Age-related macular degeneration (AMD) represents a major cause of visual impairment worldwide, with its prevalence steadily rising owing to demographic shifts and the continuous growth of the elderly population. Projections indicate that by 2040, approximately 288 million individuals will be affected [1]. Numerous factors are involved in the pathogenesis of this disease, including aging, genetic predisposition, and environmental factors such as smoking, diet, higher body mass index (BMI), and cardiovascular disease. Consequently, AMD presents itself as a complex array of disorders with a multifactorial etiology [2]. Regardless of the etiology, AMD is ultimately considered as a disease characterized by the dysfunction of the unit comprised of photoreceptors, retinal pigment epithelium (RPE), Bruch’s membrane and choriocapillaris [3,4,5,6,7,8,9,10,11,12,13].
A multimodal imaging approach is essential in diagnosing and treating AMD, utilizing several modalities including fluorescein angiography (FA), indocyanine green angiography (ICGA), structural optical coherence tomography (OCT), and OCT angiography (OCTA). With approximately 30 million scans per year, structural OCT provides high-resolution images of the retinal choroidal structures and anatomy, making it the most important imaging employed in ophthalmology for follow-up and predicting treatment response [14].
The advanced form of AMD is characterized by the development of either macular neovascularization (MNV) or geographic atrophy (GA), which are both responsible for the majority of cases of visual impairment in eyes with AMD [15,16,17,18,19,20,21,22]. MNV is characterized by the proliferation of abnormal blood vessels and subsequent leakage due to their immature vascular network. The neovascularization can manifest in various retinal layers: between the RPE and Bruch’s membrane (i.e., Type 1 MNV, the predominant subtype), within the sub-retinal space (i.e., Type 2 MNV), and within the intra-retinal space (i.e., Type 3 MNV or retinal angiomatous proliferation—RAP, the second most common subtype) [23,24]. Type 1 and 2 MNV originate from the choroid, whereas Type 3 MNV arises from the deep retinal capillary plexus or the deep vascular complex, extending downward toward the RPE [21,25,26,27,28,29,30].
In this review, we will delve into the primary biomarkers linked to neovascular AMD. The discovery of these biomarkers has been facilitated by advancements in imaging technologies, notably the advent of structural OCT and OCTA. Furthermore, we will explore the utilization of deep learning algorithms to detect and measure these biomarkers. This aspect holds significant clinical implications, as deep learning algorithms streamline the process of identifying and quantifying these biomarkers, addressing the time constraints associated with manual assessment and reducing reliance on the expertise of individual readers.
A PubMed engine search was conducted utilizing the keyword “neovascular AMD” in conjunction with “artificial intelligence” and “deep learning”. All studies published in between January 2016 and February 2024 were reviewed. The relevant publications identified through this process and selected by the authors have been incorporated into this review (Figure 1).
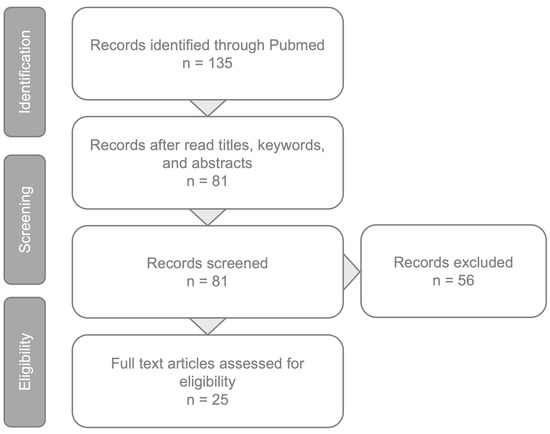
Figure 1.
Article review and selection process.
2. OCT Biomarkers in Neovascular AMD
Biomarkers are considered as morphological signs which are useful in clinical practice in the diagnostic process, assessing the disease progression and predicting or monitoring the effect of therapeutic decisions. In this scenario, structural OCT is greatly useful as it can identify retinal biomarkers that are fundamental in the management of neovascular AMD [14,31].
In neovascular AMD, there are several biomarkers that are considered extremely important in the diagnosis and management of these patients:
- Intraretinal fluid (IRF) is characterized by the presence of round- or oval-shaped cysts within the inner retinal layers, appearing typically hyporeflective on OCT. IRF is more frequently associated with Type 2 and Type 3 MNV. Numerous studies suggest that IRF serves as a crucial negative prognostic biomarker, correlating not only with reduced visual acuity at baseline and less improvement after treatment, but also with a higher risk of fibrosis and atrophy development [32,33,34].
- Subretinal fluid (SRF) occurs when exudative fluid accumulates between the neuroretina and RPE. SRF is more frequently associated with Type 1 MNV. In contrast to IRF, SRF tends to indicate a more favorable prognosis. It is often associated with better visual acuity at baseline and after intravitreal therapy, as well as a reduced risk of atrophy [34,35]. However, it is important to note that the presence of SRF is considered as a negative biomarker in eyes with Type 3 MNV.
- Pigmented epithelium detachment (PED) occurs when there is a splitting between the RPE and Bruch’s membrane. The literature lacks consensus on the prognostic significance of PED. The latter aspect could be attributed to the existence of different types of PEDs, including fibrovascular, serous, drusenoid, and hemorrhagic, each potentially exerting distinct effects on visual acuity [34,35,36,37].
- Subretinal hyperreflective material (SHRM) refers to a hyperreflective material observed on structural OCT, situated beneath the neurosensory retina and above the RPE. SHRM could indicate various substances including fluid, blood, scar tissue, fibrin, vitelliform material, or neovascularization [38,39]. Previous studies have indicated that SHRM is a negative prognostic biomarker, correlating with a reduced response to anti-VEGF treatment and poorer visual outcomes [40,41,42].
- The disruption of the outer retinal layers refers to a notable OCT sign occurring when damage to the outer hyperreflective retinal layers is evident, including the ellipsoid zone (EZ) and external limiting membrane (ELM). Such disruptions in these hyperreflective bands have been associated with compromised visual acuity both at baseline and following anti-VEGF therapy [43,44,45].
- Retinal hyperreflective foci (HRF) are defined as hyperreflective spots on structural OCT, displaying a reflectivity akin to or higher than the retinal pigment epithelium (RPE), typically measuring between 20 to 40 microns and often exhibiting clear boundaries [46]. While intraretinal HRF may be the imaging surrogate of different cells/lesions, in AMD they are mostly associated with migrating RPE cells [47,48]. In a previous study, it was demonstrated that the number of HRF decreased after anti-VEGF therapy in responders, while they persisted in non-responders, and as a result their persistence despite treatment is considered a negative prognostic factor associated with poor VA [49]. Neuroretinal HRF were also suggested as an imaging indicator of inflammation in neovascular AMD, showing a decrease in number following effective anti-VEGF treatment [50]. Of note, HRF detection in neovascular AMD was considered a reliable predictor of poor visual prognosis after anti-VEGF treatment [51].
3. Artificial Intelligence in AMD
Artificial intelligence (AI) represents a new emerging tool in the medical field, offering expedited assistance in diagnosing, categorizing, and managing various prevalent diseases.
Image-based deep learning holds promise in swiftly analyzing vast image datasets, and this may be particularly beneficial in those medical fields characterized by a continual rise in patient numbers. Deep learning may aid in biomedical image interpretation and facilitate therapeutic decision-making across a number of medical specialties, including radiology, pathology, dermatology, and ophthalmology. Ophthalmology, especially the retinal field, heavily relies on image-based diagnostics such as OCT and color fundus photography. This reliance renders it highly suitable for the incorporation of computer-assisted diagnostic algorithms. Early applications of deep learning in retinal disorders utilized color fundus photography to classify conditions like diabetic retinopathy, AMD, and retinopathy of prematurity [52,53,54,55]. Subsequently, significant efforts have been dedicated to applying deep learning to OCT images. This focus stems from the recognition that the identification and quantification of the biomarkers of progression could greatly aid in monitoring patients undergoing treatment.
4. Deep Learning to Predict Progression from Intermediate to Neovascular AMD
In a previous important study, researchers developed a deep learning algorithm aimed at assessing the risk of disease progression from intermediate AMD to late AMD, including both geographic atrophy and neovascular AMD, in individuals with unilateral neovascular AMD. For the conversion to neovascular AMD, the authors evaluated different pathognomonic biomarkers and ranked them according to their prognostic significance. Among these biomarkers, the most relevant included drusen volume, area and average thickness, as well as the vertical extension of HRF in the outer nuclear layer (ONL). The study reported a 2-year area under the curve (AUC) for predicting neovascular AMD conversion of 0.68, with a specificity of 0.46 achieved at a sensitivity level of 0.80 [56].
Russakoff et al. [57], in a recent work, aimed to use deep learning algorithms to predict the conversion from early/intermediate AMD to neovascular AMD, based on OCT biomarkers. In particular, they analyzed OCT images of 71 eyes of patients with intermediate AMD and wAMD in the fellow eye, at baseline, in year 1 and year 2, using two different deep learning networks: for AMDnet the AUC was 0.89 on OCT b-scans and 0.91 on OCT volumes, while for VGG16 the AUC was 0.82 on b-scans and 0.87 at the volume level. Moreover, for non-progressors, areas around RPE seemed to have the larger impact on the final score, while for progressors choroid and under-RPE appeared to be more relevant [57].
Banerjee et al. [58] proposed a hybrid modeling approach, which incorporates the same platform radiomics, visual acuity and demographic data with deep learning: they first presented a sequential model for temporal prediction of exudation, from 3 to 21 months, in eyes with early or intermediate AMD, using the previous visits data. They obtained the best model performance at 3 months with an AUC of 0.96 ± 0.02, an AUC of 0.83 ± 0.04 at 6 months, and a decrease in the performance at 12 months (0.77 ± 0.06), followed by an improvement at 18 and 21 months (respectively AUC of 0.9 ± 0.06 and 0.97 ± 0.02) [58].
Similarly, Yim et al. [59]. proposed an AI system to predict conversion to exudative AMD in patients already treated in one eye for wAMD and with early–intermediate AMD in the fellow eye, during a 6 month follow up. This group showed that when using a single OCT scan for foreseeing conversion, the AI system outperformed five experts and matched one optometrist, plus, when the specialist had additional data, (OCT historic, fundus images, demographic and BCVA data) the model still showed a better performance than five experts and equal to one retinal specialist [59].
5. Deep Learning to Segment OCT Features in Patients with Neovascular AMD
Identifying and quantifying the OCT biomarkers linked to neovascular AMD may play a crucial role in determining patient outcomes in cases involving this condition [60,61].
In 2017, Lee and colleagues [62] were the first to utilize deep learning in OCT imaging, showing the capability of a deep learning model to differentiate between AMD and normal OCT images, yielding promising outcomes. Successively, Kermany and colleagues [63] utilized a deep learning algorithm to classify OCT images as normal or, alternatively, as affected by drusen, neovascular AMD, or diabetic macular edema. The latter may be considered as one of the first attempts to identify distinct OCT features in diseased eyes.
One of the first studies evaluating the different OCT biomarkers in neovascular AMD eyes was conducted by Schlegl and colleagues [64]. In the latter study, the authors introduced a fully automated deep learning approach not only for the detection but also for the quantification of fluid (i.e., IRF, SRF). Their model achieved an AUC of 0.93 for detecting IRF and an AUC of 0.98 for detecting SRF. The volumes of fluid assessed manually exhibited a strong correlation with the volumes segmented automatically, indicating excellent agreement between expert assessments and the AI model [64].
The number of OCT features detected and quantified in neovascular AMD eyes was successively increased in following studies. Lee et al. [65]. constructed a dataset including 930 B-scans from 93 eyes of 93 patients with neovascular AMD and validated a 2D trained network, showing a high agreement with manual labeling in the detection of IRF, SRF, SHRM and DEP in 930 OCT B-scans.
Subsequently, a three-dimensional CNN network algorithm known as 3D U-NET was introduced for the segmentation of retinal OCT images. This model took into account spatial–temporal correlations between adjacent scans. The 3D U-NET demonstrated superior segmentation accuracy, achieving a remarkable overall accuracy of 99.56%. Particularly noteworthy were its results in retinal fluid segmentation, with a Kappa coefficient of 98.47% and an F1 score of 95.50%. These outcomes closely resembled the manually labeled annotations provided by ophthalmology specialists [66].
The amount of OCT biomarkers detected and quantified using a deep learning 3D segmentation network was expanded in a study by Moraes et al. [67]. They assessed 2966 OCT scans from the Moorfield AMD database, comprising eyes with treatment-naive neovascular AMD yet to undergo anti-VEGF therapy or previously treated eyes. The latter study analyzed various segmented features, including central foveal thickness, RPE atrophy, IRF, SRF, SHRM, HRF, drusenoid, fibrovascular and serous PED. Except for drusen, the eyes first treated generally exhibited greater volumes across all analyzed features. Fibrovascular PED and SHRM volumes showed a positive correlation with each other and with SRF volumes, but a weaker correlation with IRF volume. HRF demonstrated a strong volumetric correlation with IRF. Regarding VA, all biomarkers exhibited a negative volumetric correlation with VA in treatment-naive eyes, while only SHRM, RPE atrophy, central foveal thickness, and IRF showed a statistically significant negative correlation in second treated eyes. Notably, SHRM volume displayed the most substantial negative correlation with VA for both first and second treated eyes, and IRF showed a stronger association with VA compared to SRF, highlighting the importance of distinguishing between different fluid types [67].
Liefers et al. [68], in a recent study, proposed a deep learning segmentation model for 13 biomarkers commonly found in neovascular AMD. They compared the performance of the model with the performance obtained from four experienced graders. Overall, the model performed better than the manual grading in the quantification of IRF, SHRM, RPE loss and ellipsoid zone loss, while the manual labeling was slightly better in the quantification of drusen and drusenoid PED. For the other features, the manual and deep learning-based assessments performed similarly. This study represents a pioneering effort in the comprehensive analysis of multiple OCT features, extending beyond fluid-based biomarkers to include features of atrophy such as RPE loss and EZ loss. Remarkably, this study illustrates that the model’s performance is comparable to, and occasionally surpasses, that of experienced human graders.
Our group has extensively worked on the application of deep learning to quantify critical OCT biomarkers associated with neovascular AMD. First, we performed a pilot study on 50 eyes (50 patients) to develop and validate a deep learning algorithm for automated IRF, SRF and neovascular PED segmentation in neovascular AMD [69]. In the latter study, we validated a deep learning algorithm for automated IRF and SRF segmentation in neovascular AMD. Successively, we adopted a methodology similar to that used in the aforementioned paper, although the current investigation was conducted on a large real-world dataset and an extended feature set [70]. Three-hundred OCT volumes from subject eyes with neovascular AMD were collected. The images were manually segmented for the presence of five crucial OCT features: IRF, SRF, SHRM, drusen/drusenoid PED, and neovascular PED. A deep learning architecture based on a U-Net was trained to perform automatic segmentation of these retinal biomarkers and evaluated on the sequestered data. The model obtained a mean (±SD) AUC of 0.93 (±0.04) per slice and 0.88 (±0.07) per volume for fluid detection. The correlation score (R2) between automatic and manual segmentation obtained by the model resulted in a mean (±SD) of 0.89 (±0.05). The mean (±SD) 2-D correlation score was 0.69 (±0.04). The mean (±SD) Dice score resulted in 0.61 (±0.10). Overall, this model, for five features related to neovascular AMD, performs at the level of experienced graders.
6. Deep Learning to Predict Anti-VEGF Treatment in Patients with Neovascular AMD
Bogunovic et al. [71] introduced a machine learning approach aimed at predicting low and high injection requirements in patients with neovascular AMD undergoing a 2-year pro re nata (PRN) treatment regimen. The objective was to evaluate biomarker presence during the initial visits following the initiation of anti-VEGF therapy, including baseline, 1-month follow-up, and 2-month follow-up visits. They employed a convolutional neural network (CNN) trained on 20,000 OCT B-scans. Their findings revealed that the AUCs for predicting the categories steadily increased over time, starting at 0.60 at baseline, reaching 0.68 at month 1, 0.70 at month 2 for low treatment requirements, and 0.61 at baseline, escalating to 0.74 at month 1, and 0.77 at month 2 for high treatment requirements. Thus, the retinal morphology at baseline before intravitreal injection appears to be less predictive of future treatment needs compared to the retinal morphology after the initial anti-VEGF treatment. According to this study, the three most crucial features in predicting the number of injections required are: SRF in the central 3 mm at month 2, inner retinal thickness in the fovea at month 1, and inner retinal thickness in the central 3 mm at month 2. The model’s predictive performance was comparable to that of human graders for low requirements, and 50% better than expert grading for the high requirements category.
Similarly, Pfau et al. [72] utilized three distinct machine learning algorithms to anticipate the frequency of intravitreal treatments over the subsequent 12 months for neovascular AMD patients undergoing PRN or treat-and-extend treatment regimens. The mean absolute error (MAE) predictions were 2.76 injections per year (range: 2.39–3.14) using lasso regression, 2.74 injections per year (range: 2.38–3.11) using principal component regression, and 2.60 injections per year (range: 2.25–2.96) for random forest regression. All models predicted a higher number of injections than necessary for the lower treatment group and underestimated the required injection frequency for the higher treatment group.
Feng et al. [73] introduced a deep learning algorithm designed to assess the effectiveness of anti-VEGF treatment in patients with neovascular AMD, solely relying on OCT images before treatment initiation. They employed the ResNet-50 architecture, pretrained on ImageNet, and trained it using four distinct datasets. A comparative analysis against other established models revealed that their model achieved the highest AUC (0.81), indicating its superior capability in predicting the effectiveness of anti-VEGF treatment. Additionally, they observed improved outcomes when utilizing OCT full images compared to solely focusing on pathological regions [73].
Two recent studies [74,75] have introduced a model for predicting OCT post-therapeutic images by employing generative adversarial network (GAN) technology. Initially, the model was trained using both pre- and post-therapeutic images, after which it generated synthetic OCT scans for comparison with actual scans. Both of these studies demonstrate the significant potential of GAN in generating post-anti-VEGF OCT scans with high accuracy and quality. Similarly, Moon et al. [76] developed an AI model aimed at predicting anatomical treatment outcomes specific to anti-VEGF agents in neovascular AMD, assisting clinicians in selecting the most appropriate agent for individual patients. This retrospective study involved patients diagnosed with neovascular AMD who underwent three loading injections of either ranibizumab or aflibercept, training the utilized OCT images with an attention GAN model. To assess the AI model’s performance, the sensitivity and specificity in predicting the presence of retinal fluid post-treatment were calculated for the AI model, an experienced examiner (Examiner 1), and a less experienced examiner (Examiner 2). The training set comprised 1684 OCT images from 842 patients (419 ranibizumab-treated and 423 aflibercept-treated), while testing employed images from 98 patients. For patients treated with ranibizumab, the AI model demonstrated a sensitivity and specificity of 0.615 and 0.667, respectively, while Examiner 1 showed 0.385 sensitivity and 0.861 specificity, and Examiner 2 exhibited 0.231 sensitivity and 0.806 specificity. In aflibercept-treated patients, the AI model achieved a sensitivity and specificity of 0.857 and 0.881, respectively, compared to 0.429 sensitivity and 0.976 specificity for Examiner 1, and 0.429 sensitivity and 0.857 specificity for Examiner 2. Furthermore, in 18.5% of cases, the fluid status differed between synthetic post-treatment images of ranibizumab and aflibercept. The AI model utilizing GAN showed potential in predicting agent-specific short-term treatment outcomes, with higher sensitivity than human examiners. Additionally, variations in efficacy were observed in fluid resolution between the anti-VEGF agents, underscoring the potential of AI in personalized medicine for neovascular AMD patients [76].
Finally, Ursula Schmidt-Erfurth et al. [77] used machine learning regression using random forests to predict final BCVA at 12 months in patients with active neovascular AMD undergoing anti VEGF treatment with ranibizumab. In the latter study, the authors revealed that BCVA at 3 months and, among imaging biomarkers, the horizontal extension of IRF both centrally within 3 mm and in the parafoveal area were the most significant predictors for the final BCVA outcome.
7. Conclusions
This review highlights the emerging applications of deep learning in neovascular AMD.
Considering the continual aging of the population, the prevalence of neovascular AMD patients is steadily increasing, with the latter necessitating a corresponding rise in ophthalmology examinations. As multimodal imaging techniques evolve, deep learning may play a crucial role in extracting relevant data, processing information, and alleviating the workload of physicians, thereby enhancing patient management and therapeutic decision-making.
The integration of deep learning and OCT image analysis holds potential for predicting the conversion to neovascular AMD and promptly referring patients to specialists for the expedited initiation of anti-VEGF therapy, thus mitigating permanent damage and optimizing visual outcomes and quality of life. Additionally, the analysis of imaging biomarkers using deep learning models promises to furnish more precise functional prognoses, enabling clinicians to tailor patient visit schedules optimally, thereby maximizing resource utilization and improving treatment outcomes.
Author Contributions
Conceptualization: E.B., S.S., F.R. and M.R.; Writing—original draft preparation: E.B. and S.S.; Writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Daien, V.; Finger, R.P.; Talks, J.S.; Mitchell, P.; Wong, T.Y.; Sakamoto, T.; Eldem, B.M.; Korobelnik, J.F. Evolution of treatment paradigms in neovascular age-related macular degeneration: A review of real-world evidence. Br. J. Ophthalmol. 2020, 105, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Souied, E.H.; Benlian, P.; Amouyel, P.; Feingold, J.; Lagarde, J.P.; Munnich, A.; Kaplan, J.; Coscas, G.; Soubrane, G. The ε4 allele of the Apolipoprotein E gene as a potential protective factor for exudative age-related macular degeneration. Am. J. Ophthalmol. 1998, 125, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Reibaldi, M.; Barresi, C.; Berni, A.; Introini, U.; Bandello, F. Choroidal Hyper-Reflective Foci in Geographic Atrophy. Investig. Ophthalmol. Vis. Sci. 2023, 64, 5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borrelli, E.; Berni, A.; Mastropasqua, L.; Querques, G.; Sadda, S.R.; Sarraf, D.; Bandello, F. Pushing Retinal Imaging Forward: Innovations and Their Clinical Meaning—The 2022 Ophthalmologica Lecture. Ophthalmologica 2023, 246, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Barresi, C.; Chhablani, J.; Dolz-Marco, R.; Gallego-Pinazo, R.; Berni, A.; Bandello, F.; Borrelli, E. Retinal neurodegeneration in age-related macular degeneration. Eur. J. Ophthalmol. 2023, 34, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, P.; Grassi, M.O.; Pignataro, M.; Boscia, G.; Borrelli, E.; Molfetta, T.; Evangelista, F.; Alessio, G.; Boscia, F. Topographical Analysis of the Choriocapillaris Reperfusion after Loading Anti-VEGF Therapy in Neovascular AMD. Transl. Vis. Sci. Technol. 2022, 11, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borrelli, E.; Bandello, F.; Souied, E.H.; Barresi, C.; Miere, A.; Querques, L.; Sacconi, R.; Querques, G. Neovascular age-related macular degeneration: Advancement in retinal imaging builds a bridge between histopathology and clinical findings. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Sacconi, R.; Zuccaro, B.; Cavalleri, M.; Bordato, A.; Zucchiatti, I.; Querques, L.; Bandello, F.; Querques, G. Photoreceptor alteration in intermediate age-related macular degeneration. Sci. Rep. 2020, 10, 21036. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Borrelli, E.; Shi, Y.; Uji, A.; Balasubramanian, S.; Nassisi, M.; Sarraf, D.; Sadda, S.R. Topographic Analysis of the Choriocapillaris in Intermediate Age-related Macular Degeneration. Am. J. Ophthalmol. 2018, 196, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Sarraf, D.; Freund, K.B.; Sadda, S.R. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog. Retin. Eye Res. 2018, 67, 30–55. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Mastropasqua, R.; Senatore, A.; Palmieri, M.; Toto, L.; Sadda, S.R.; Mastropasqua, L. Impact of Choriocapillaris Flow on Multifocal Electroretinography in Intermediate Age-Related Macular Degeneration Eyes. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD25–AMD30. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Souied, E.H.; Freund, K.B.; Querques, G.; Miere, A.; Gal-Or, O.; Sacconi, R.; Sadda, S.R.; Sarraf, D. Reduced choriocapillaris flow in eyes with type 3 neovascularization and age-related macular degeneration. Retina 2018, 38, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Uji, A.; Sarraf, D.; Sadda, S.R. Alterations in the Choriocapillaris in Intermediate Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4792–4798. [Google Scholar] [CrossRef] [PubMed]
- Phadikar, P.; Saxena, S.; Ruia, S.; Lai, T.Y.; Meyer, C.H.; Eliott, D. The potential of spectral domain optical coherence tomography imaging based retinal biomarkers. Int. J. Retin. Vitr. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Jaffe, G.J.; Sarraf, D.; Freund, K.B.; Sadda, S.R.; Staurenghi, G.; Waheed, N.K.; Chakravarthy, U.; Rosenfeld, P.J.; Holz, F.G.; et al. Consensus Nomenclature for Reporting Neovascular Age-Related Macular Degeneration Data: Consensus on Neovascular Age-Related Macular Degeneration Nomenclature Study Group. Ophthalmology 2020, 127, 616–636. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.L., III; Wilkinson, C.P.; Bird, A.; Chakravarthy, U.; Chew, E.; Csaky, K.; Sadda, S.R.; Beckman Initiative for Macular Research Classification Committee. Clinical classification of age-related macular degeneration. Ophthalmology 2013, 120, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Ricardi, F.; Borrelli, E.; Boscia, G.; Gelormini, F.; Marica, V.; Conte, F.; Viggiano, P.; Marolo, P.; Bandello, F.; Reibaldi, M. Relationship of Topographic Distribution of Macular Atrophy Secondary to Neovascular AMD and Reading Performance. Investig. Ophthalmol. Vis. Sci. 2024, 65, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boscia, G.; Ricardi, F.; Gelormini, F.; Marica, V.; Conte, F.; Ghilardi, A.; Viggiano, P.; Marolo, P.; Bandello, F.; Borrelli, E.; et al. Inter-session repeatability of reading performance measures in patients with neovascular AMD. Retina 2023, 44, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Barresi, C.; Lari, G.; Berni, A.; Battista, M.; Reibaldi, M.; Cascavilla, M.L.; Bandello, F. Capturing the Transition From Intermediate to Neovascular AMD: Longitudinal Inner Retinal Thinning and Factors Associated With Neuronal Loss. Investig. Ophthalmol. Vis. Sci. 2023, 6, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barresi, C.; Borrelli, E.; Fantaguzzi, F.; Grosso, D.; Sacconi, R.; Bandello, F.; Querques, G. Complications Associated with Worse Visual Outcomes in Patients with Exudative Neovascular Age-Related Macular Degeneration. Ophthalmologica 2021, 244, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Mastropasqua, L.; Souied, E.; Sadda, S.; Vella, G.; Toto, L.; Miere, A.; Corradetti, G.; Sacconi, R.; Ferro, G.; et al. Longitudinal assessment of type 3 macular neovascularization using 3D volume-rendering OCTA. Can. J. Ophthalmol. 2022, 57, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Grosso, D.; Vella, G.; Sacconi, R.; Battista, M.; Querques, L.; Zucchiatti, I.; Prascina, F.; Bandello, F.; Querques, G. Short-term outcomes of patients with neovascular exudative AMD: The effect of COVID-19 pandemic. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2621–2628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grossniklaus, H.E.; Green, W.R. Choroidal neovascularization. Am. J. Ophthalmol. 2004, 137, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Freund, K.B.; Yannuzzi, L.A.; Sorenson, J.A. Age-related macular degeneration and choroidal neovascularization. Am. J. Ophthalmol. 1993, 115, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Nagiel, A.; Sarraf, D.; Sadda, S.R.; Spaide, R.F.; Jung, J.J.; Bhavsar, K.V.; Ameri, H.; Querques, G.; Freund, K.B. Type 3 neovascularization: Evolution, association with pigment epithelial detachment, and treatment response as revealed by spectral domain optical coherence tomography. Retina 2015, 35, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Kuehlewein, L.; Dansingani, K.K.; De Carlo, T.E.; Bonini Filho, M.A.; Iafe, N.A.; Lenis, T.L.; Freund, K.B.; Waheed, N.K.; Duker, J.S.; Sadda, S.R.; et al. Optical Coherence Tomography Angiography of Type 3 Neovascularization Secondary To Age-Related Macular Degeneration. Retina 2015, 35, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Barresi, C.; Ricardi, F.; Berni, A.; Grosso, D.; Viggiano, P.; Marolo, P.; Introini, U.; Reibaldi, M.; Bandello, F. Distinct Pathways of Macular Atrophy in Type 3 Macular Neovascularization Associated With AMD. Investig. Ophthalmol. Vis. Sci. 2024, 65, 18. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Zerbini, G.; Maestroni, S.; Sacconi, R.; Querques, L.; Zucchiatti, I.; Bandello, F.; Querques, G. Multimodal Imaging to Detect in vivo Responses to Aflibercept Therapy in a Mouse Model of Type 3 Neovascularization. Ophthalmologica 2021, 244, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Sacconi, R.; Battista, M.; Borrelli, E.; Miere, A.; Corbelli, E.; Capuano, V.; Querques, L.; Souied, E.H.; Bandello, F.; Querques, G. OCT-A characterisation of recurrent type 3 macular neovascularisation. Br. J. Ophthalmol. 2021, 105, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Sacconi, R.; Klose, G.; de Sisternes, L.; Bandello, F.; Querques, G. Rotational Three-dimensional OCTA: A Notable New Imaging Tool to Characterize Type 3 Macular Neovascularization. Sci. Rep. 2019, 9, 17053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Metrangolo, C.; Donati, S.; Mazzola, M.; Fontanel, L.; Messina, W.; D’alterio, G.; Rubino, M.; Radice, P.; Premi, E.; Azzolini, C. OCT Biomarkers in Neovascular Age-Related Macular Degeneration: A Narrative Review. J. Ophthalmol. 2021, 2021, 9994098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, T.T.; Hsieh, Y.T.; Yang, C.M.; Ho, T.C.; Yang, C.H. Biomarkers of optical coherence tomography in evaluating the treatment outcomes of neovascular agerelated macular degeneration: A real-world study. Sci. Rep. 2019, 9, 529. [Google Scholar] [CrossRef]
- Waldstein, S.M.; Simader, C.; Staurenghi, G.; Chong, N.V.; Mitchell, P.; Jaffe, G.J.; Lu, C.; Katz, T.A.; Schmidt-Erfurth, U. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology 2016, 123, 1521–1529. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Deak, G.G.; Kundi, M.; Simader, C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology 2015, 122, 822–832. [Google Scholar] [CrossRef]
- Sadda, S.R.; Tuomi, L.L.; Ding, B.; Fung, A.E.; Hopkins, J.J. Macular atrophy in the HARBOR study for 12 Journal of Ophthalmology neovascular age-related macular degeneration. Ophthalmology 2018, 125, 878–886. [Google Scholar] [CrossRef]
- Ach, T.; Hoeh, A.E.; Ruppenstein, M.; Kretz, F.T.; Dithmar, S. Intravitreal bevacizumab in vascular pigment epithelium detachment as a result of subfoveal occult choroidal neovascularization in age-related macular degeneration. Retina 2010, 30, 1420–1425. [Google Scholar] [CrossRef]
- Borrelli, E.; Barresi, C.; Berni, A.; Viggiano, P.; Reibaldi, M.; Introini, U.; Bandello, F. OCT risk factors for 2-year foveal involvement in non-treated eyes with extrafoveal geographic atrophy and AMD. Graefe’s Arch. Clin. Exp. Ophthalmol. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, A.S.; Ying, G.S.; Toth, C.A.; Maguire, M.G.; Burns, R.E.; Grunwald, J.E.; Ebenezer, D.; Jaffe, G.J. Subretinal hyperreflective material in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2015, 122, 1846.e5–1853.e5. [Google Scholar] [CrossRef]
- Charafeddin, W.; Nittala, M.G.; Oregon, A.; Sadda, S.R. Relationship between subretinal hyperreflective material reflectivity and volume in patients with neovascular agerelated macular degeneration following anti-vascular endothelial growth factor treatment. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 523–530. [Google Scholar] [CrossRef]
- Kawashima, Y.; Hata, M.; Oishi, A.; Ooto, S.; Yamashiro, K.; Tamura, H.; Miyata, M.; Uji, A.; Ueda-Arakawa, N. Association of vascular versus avascular subretinal hyperreflective material with aflibercept response in age-related macular degeneration. Am. J. Ophthalmol. 2017, 181, 61–70. [Google Scholar] [CrossRef]
- Kumar, J.B.; Stinnett, S.; Han, J.I.; Jaffe, G.J. Correlation of subretinal hyperreflective material morphology and visual acuity in neovascular age-related macular degeneration. Retina 2020, 40, 845–856. [Google Scholar] [CrossRef]
- Pokroy, R.; Mimouni, M.; Barayev, E.; Segev, F.; Geffen, N.; Nemet, A.Y.; Segal, O. Prognostic value of subretinal hyperreflective material in neovascular age-related macular degeneration treated with bevacizumab. Retina 2018, 38, 1485–1491. [Google Scholar] [CrossRef]
- Woronkowicz, M.; Lightman, S.; Tomkins-Netzer, O. The prognostic value of total macular external limiting membrane and ellipsoid zone damage for clinical outcome in treatment-resistant neovascular age-related macular degeneration. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2373–2378. [Google Scholar] [CrossRef]
- FCoscas, F.; Coscas, G.; Lupidi, M.; Dirani, A.; Srour, M.; Semoun, O.; Français, C.; Souied, E.H. Restoration of outer retinal layers after aflibercept therapy in exudative AMD: Prognostic value. Investig. Opthalmology Vis. Sci. 2015, 56, 4129–4134. [Google Scholar] [CrossRef]
- Shin, H.J.; Chung, H.; Kim, H.C. Association between foveal microstructure and visual outcome in age-related macular degeneration. Retina 2011, 31, 1627–1636. [Google Scholar] [CrossRef]
- Kang, J.-W.; Lee, H.; Chung, H.; Kim, H.C. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after intravitreal bevacizumab for macular edema in branch retinal vein occlusion. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 252, 1413–1421. [Google Scholar] [CrossRef]
- Omri, S.; Behar-Cohen, F.; De Kozak, Y.; Sennlaub, F.; Verissimo, L.M.; Jonet, L.; Savoldelli, M.; Omri, B.; Crisanti, P. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: Role of PKCζ in the Goto Kakizaki rat model. Am. J. Pathol. 2011, 179, 942–953. [Google Scholar] [CrossRef]
- Bolz, M.; Schmidt-Erfurth, U.; Deak, G.; Mylonas, G.; Kriechbaum, K.; Scholda, C.; Diabetic Retinopathy Research Group Vienna. Optical coherence tomographic hyperreflective foci: A morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology 2009, 116, 914–920. [Google Scholar] [CrossRef]
- Coscas, G.; De Benedetto, U.; Coscas, F.; Li Calzi, C.I.; Vismara, S.; Roudot-Thoraval, F.; Bandello, F.; Souied, E. Hyperreflective dots: A new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica 2013, 229, 32–37. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Yang, Q.; Xie, H.; Zhang, J.; Qiu, Q.; Liu, K.; Luo, D.; Liu, F.; Zhang, J. Imaging Hyperreflective Foci as an Inflammatory Biomarker after Anti-VEGF Treatment in Neovascular Age-Related Macular Degeneration Patients with Optical Coherence Tomography Angiography. BioMed Res. Int. 2021, 2021, 6648191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Ji, B.; Chung, H.; Kim, H.C. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after anti-vegf treatment in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Retina 2016, 36, 465–475. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Lou, Y.; Erginay, A.; Clarida, W.; Amelon, R.; Folk, J.C.; Niemeijer, M. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5200–5206. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Fondon, I.; Sarmiento, A.; Jiménez, S.; Alemany, P. Automatic recognition of severity level for diagnosis of diabetic retinopathy using deep visual features. Med. Biol. Eng. Comput. 2017, 55, 1959–1974. [Google Scholar] [CrossRef]
- Takahashi, H.; Tampo, H.; Arai, Y.; Inoue, Y.; Kawashima, H. Applying artificial intelligence to disease staging: Deep learning for improved staging of diabetic retinopathy. PLoS ONE 2017, 12, e0179790. [Google Scholar] [CrossRef]
- Burlina, P.M.; Joshi, N.; Pekala, M.; Pacheco, K.D.; Freund, D.E.; Bressler, N.M. Automated grading of age- related macular degeneration from color fundus images using deep convolutional neural networks. JAMA Ophthalmol. 2017, 135, 1170–1176. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Hu, X.; Gerendas, B.S.; Osborne, A.; Bogunovic, H. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3199–3208. [Google Scholar] [CrossRef] [PubMed]
- Russakoff, D.B.; Lamin, A.; Oakley, J.D.; Dubis, A.M.; Sivaprasad, S. Deep Learning for Prediction of AMD Progression: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Osborne, A.; Rosenfeld, P.J.; Gregori, G.; Durbin, M.; Rubin, D. Prediction of age-related macular degeneration disease using a sequential deep learning approach on longitudinal SD-OCT imaging biomarkers. Sci. Rep. 2020, 10, 15434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yim, J.; Chopra, R.; Spitz, T.; Winkens, J.; Obika, A.; Kelly, C.; Askham, H.; Lukic, M.; Huemer, J.; Fasler, K.; et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med. 2020, 2, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Sahni, J.; Campa, C.; Stangos, A.N.; Raj, A.; Harding, S.P. Computerized assessment of intraretinal and subretinal fluid regions in spectral-domain optical coherence tomography images of the retina. Am. J. Ophthalmol. 2013, 155, 277–286.e1. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Vogl, W.D.; Jampol, L.M.; Bogunović, H. Application of Automated Quantification of Fluid Volumes to Anti-VEGF Therapy of Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Baughman, D.M.; Lee, A.Y. Deep learning is effective for the classification of OCT images of normal versus Age-related Macular Degeneration. Ophthalmol. Retin. 2017, 1, 322–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kermany, D.S.; Goldbaum, M.; Cai, W.; Valentim, C.C.; Liang, H.; Baxter, S.L.; McKeown, A.; Yang, G.; Wu, X.; Yan, F.; et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell 2018, 172, 1122–1131.e9. [Google Scholar] [CrossRef] [PubMed]
- Schlegl, T.; Waldstein, S.M.; Bogunovic, H.; Endstraßer, F.; Sadeghipour, A.; Philip, A.M.; Podkowinski, D.; Gerendas, B.S.; Langs, G.; Schmidt-Erfurth, U. Fully Automated Detection and Quantification of Macular Fluid in OCT Using Deep Learning. Ophthalmology 2018, 125, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, K.E.; Chung, H.; Kim, H.C. Automated Segmentation of Lesions Including Subretinal Hyperreflective Material in Neovascular Age-related Macular Degeneration. Am. J. Ophthalmol. 2018, 191, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Yu, S.Q.; Zhang, W.; Zhou, H.; Xu, X.; Qian, T.W.; Wan, Y.J. Segmentation of retinal fluid based on deep learning: Application of three-dimensional fully convolutional neural networks in optical coherence tomography images. Int. J. Ophthalmol. 2019, 12, 1012–1020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moraes, G.; Fu, D.J.; Wilson, M.; Khalid, H.; Wagner, S.K.; Korot, E.; Ferraz, D.; Faes, L.; Kelly, C.J.; Spitz, T.; et al. Quantitative Analysis of OCT for Neovascular Age-Related Macular Degeneration Using Deep Learning. Ophthalmology 2021, 128, 693–705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liefers, B.; Taylor, P.; Alsaedi, A.; Bailey, C.; Balaskas, K.; Dhingra, N.; Egan, C.A.; Rodrigues, F.G.; Gonzalo, C.G.; Heeren, T.F.C.; et al. Quantification of Key Retinal Features in Early and Late Age-Related Macular Degeneration Using Deep Learning. Am. J. Ophthalmol. 2021, 226, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Oakley, J.D.; Iaccarino, G.; Russakoff, D.B.; Battista, M.; Grosso, D.; Borghesan, F.; Barresi, C.; Sacconi, R.; Bandello, F.; et al. Deep-learning based automated quantification of critical optical coherence tomography features in neovascular age-related macular degeneration. Eye 2024, 38, 537–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ricardi, F.; Oakley, J.; Russakoff, D.; Boscia, G.; Caselgrandi, P.; Gelormini, F.; Ghilardi, A.; Pintore, G.; Tibaldi, T.; Marolo, P.; et al. Validation of a deep learning model for automatic detection and quantification of five OCT critical retinal features associated with neovascular age-related macular degeneration. Br. J. Ophthalmol. 2024, bjo-2023-324647. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, H.; Waldstein, S.M.; Schlegl, T.; Langs, G.; Sadeghipour, A.; Liu, X.; Gerendas, B.S.; Osborne, A.; Schmidt-Erfurth, U. Prediction of Anti-VEGF Treatment Requirements in Neovascular AMD Using a Machine Learning Approach. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3240–3248. [Google Scholar] [CrossRef] [PubMed]
- Pfau, M.; Sahu, S.; Rupnow, R.A.; Romond, K.; Millet, D.; Holz, F.G.; Schmitz-Valckenberg, S.; Fleckenstein, M.; Lim, J.I.; de Sisternes, L.; et al. Probabilistic Forecasting of Anti-VEGF Treatment Frequency in Neovascular Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, D.; Chen, X.; Zhou, Z.; Liu, H.; Wang, Y.; Bai, L.; Zhang, S.; Mou, X. A Preliminary Study of Predicting Effectiveness of Anti-VEGF Injection Using OCT Images Based on Deep Learning. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2020, 2020, 5428–5431. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, S.; Kim, M.A.; Chung, H.; Kim, H.C. Post-treatment prediction of optical coherence tomography using a conditional generative adversarial network in age-related macular degeneration. Retina 2021, 41, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, J.; Zhou, Y.; Wang, W.; Zhao, J.; Yu, W.; Zhang, D.; Ding, D.; Li, X.; Chen, Y. Prediction of OCT images of short-term response to anti-VEGF treatment for neovascular age-related macular degeneration using generative adversarial network. Br. J. Ophthalmol. 2020, 104, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Lee, Y.; Hwang, J.; Kim, C.G.; Kim, J.W.; Yoon, W.T.; Kim, J.H. Prediction of anti-vascular endothelial growth factor agent-specific treatment outcomes in neovascular age-related macular degeneration using a generative adversarial network. Sci. Rep. 2023, 13, 5639. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Bogunovic, H.; Sadeghipour, A.; Schlegl, T.; Langs, G.; Gerendas, B.S.; Osborne, A.; Waldstein, S.M. Machine Learning to Analyze the Prognostic Value of Current Imaging Biomarkers in Neovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2018, 2, 24–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).