Abstract
Background and Objectives: Peripheral arterial stiffness (PAS), assessed by brachial-ankle pulse wave velocity (baPWV), is an independent biomarker of cardiovascular diseases (CVD) in patients on maintenance hemodialysis (HD). Malondialdehyde-modified low-density lipoprotein (MDA-LDL), an oxidative stress marker, has been linked to atherosclerosis and CVD. However, the association between serum MDA-LDL and PAS among HD patients has not been fully elucidated. This study aimed to examine the association of serum MDA-LDL with PAS in HD patients and to identify the optimal cutoff value of serum MDA-LDL for predicting PAS. Materials and Methods: A cross-sectional study was conducted in 100 HD patients. Serum MDA-LDL was quantified using an enzyme-linked immunosorbent assay (ELISA), and baPWV was measured using a volume plethysmographic device. Patients were divided into the PAS group (baPWV > 18.0 m/s) and the non-PAS group (baPWV ≤ 18.0 m/s). The associations of baPWV and other clinical and biochemical parameters with serum MDA-LDL were assessed by multivariable logistic regression analyses. A receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutoff value of serum MDA-LDL for predicting PAS. Results: In multivariable logistic regression analysis, higher serum MDA-LDL, older age, and higher serum C-reactive protein [odds ratios (ORs) and 95% confidence intervals: 1.014 (1.004–1.025), 1.044 (1.004–1.085) and 3.697 (1.149–11.893)] were significantly associated with PAS. In the ROC curve analysis, the optimal cutoff value of MDA-LDL for predicting PAS was 80.91 mg/dL, with a sensitivity of 79.25% and a specificity of 59.57%. Conclusions: Greater serum MDA-LDL levels, particularly ≥80.91 mg/dL, were independently associated with PAS in HD patients. The findings suggest that oxidative stress plays a crucial role in the pathogenesis of PAS, and targeting MDA-LDL may be a potential therapeutic strategy for reducing cardiovascular risk in HD patients.
1. Introduction
Cardiovascular disease (CVD) is the primary cause of morbidity and mortality among patients with end-stage renal disease (ESRD) receiving maintenance hemodialysis (HD) [1,2,3]. The increased cardiovascular risk in this population is attributed to a complex interplay of traditional risk factors, e.g., hypertension, diabetes mellitus, and dyslipidemia, and non-traditional risk factors, including oxidative stress, inflammation, and mineral bone disorders [4,5,6]. Identifying novel biomarkers and therapeutic targets for CVD in HD patients is crucial for improving their clinical outcomes and quality of life.
Peripheral arterial stiffness (PAS), characterized by reduced arterial elasticity with increased pulse wave velocity (PWV), has emerged as an independent predictor of cardiovascular events and all-cause mortality in HD patients [7,8]. Brachial-ankle PWV (baPWV), a non-invasive measure of arterial stiffness, has been widely used to assess PAS in HD patients [9]. Several factors such as old age, hypertension, diabetes mellitus, and chronic inflammation, have been associated with higher baPWV among HD patients [10,11,12]. However, the role of oxidative stress in the pathogenesis of PAS in this population remains poorly understood.
In the general population, triglycerides (TG), among the blood lipid parameters, have been revealed to have the strongest associations with PAS, while low-density lipoprotein cholesterol (LDL-C) levels have been more strongly associated with atherosclerosis than with PAS [13]. Notably, a few modified LDLs, such as oxidized LDL (oxLDL), were found to be associated with both atherosclerosis and PAS [14]. Malondialdehyde (MDA)-modified LDL, a biomarker of oxidative stress, is another kind of modified LDL that is formed by the reaction of MDA with the amino groups of apolipoprotein B in LDL particles [15,16]. In prior population-based studies, MDA-LDL has been implicated in the development of atherosclerosis and clinical CVD in the general population [16,17,18]. In experimental studies, MDA-LDL was observed to promote endothelial dysfunction, vascular inflammation, and foam cell formation, subsequently leading to the progression of atherosclerotic lesions [16,19,20]. Additionally, elevated serum MDA-LDL has been associated with a higher risk of PAS in patients with hypertension or diabetes mellitus [13,21,22]. However, in HD patients, there were rare studies to examine the effects of various lipids on aortic stiffness and PAS. To the best of our knowledge, MDA-LDL was the most commonly studied lipid target in HD patients, which has been found to be associated with aortic wall stiffness [21], whereas its relationship with PAS among HD patients has not been fully elucidated. Since both aortic wall stiffness and PAS have been recognized as crucial risk factors of clinical CVD in HD patients, the present study aimed to examine the association between serum MDA-LDL levels and PAS, as assessed by baPWV, in a sample of HD patients. We hypothesized that elevated serum MDA-LDL levels would be independently associated with increased baPWV, and that serum MDA-LDL levels could serve as a useful biomarker for predicting PAS in this high-risk population.
To investigate our hypotheses, we performed a cross-sectional study involving 100 HD patients. We assessed the associations between serum MDA-LDL levels, baPWV, and other pertinent clinical and biochemical parameters. Additionally, we aimed to determine the optimal cutoff value of serum MDA-LDL levels for predicting PAS in this population. Our results may offer novel insights into the role of oxidative stress in PAS pathogenesis and emphasize the potential utility of serum MDA-LDL as an innovative marker and therapeutic target for CVD in HD patients.
2. Materials and Methods
2.1. Participants and Study Protocol
This observational study was conducted at a single dialysis center in eastern Taiwan from June to August 2022. According to the patient enrollment flowchart (Figure 1), there were 232 adult hemodialysis patients who had received maintenance hemodialysis for more than 3 months at the time of screening. Patients who had a limb amputated (n = 12), acute infection (n = 6), malignancy (n = 16), liver cirrhosis (n = 4), chronic obstructive lung disease (COPD) (n = 8), stroke (n = 10), acute heart failure (n = 1) or were bedridden (n = 12) were excluded. Patients who used other than a high-flux dialyzer (n = 33) or refused to provide informed consent (n = 30) were also excluded.
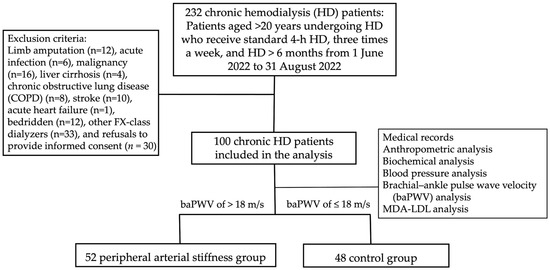
Figure 1.
Flowchart of the selection of HD patients receiving measurements of both brachial-ankle pulse wave velocity and Malondialdehyde (MDA)-modified LDL.
The study enrolled 100 patients who met the following criteria: age ≥ 20 years, receiving regular HD sessions (4 h per session, 3 times per week) for more than 3 months, and using high-flux dialyzers (FX-class, Fresenius Medical Care, Germany). Patients’ demographic and clinical information, including the presence of comorbidities, e.g., diabetes mellitus and hypertension, was collected from their medical records. The study protocol was reviewed and approved by the Tzu Chi Hospital Institutional Review Board (IRB108–219-A) and adhered to the principles of the Declaration of Helsinki. Informed written consent was obtained from all study participants.
2.2. Assessment of Anthropometric Parameters
Anthropometric measurements were carried out by a trained staff member, with patients dressed in lightweight clothing and barefoot. Body weight and height were measured and rounded up to the nearest 0.5 kg and 0.5 cm, respectively. Body mass index (BMI) was calculated by dividing the participant’s weight in kilograms by the square of the participant’s height in meters.
2.3. Laboratory Investigations
Following an overnight fast of 8–12 h, blood samples (approximately 5 mL) were drawn from each participant and centrifuged at 3000× g for 10 min. An automated analyzer (Siemens Advia 1800; Siemens Healthcare GmbH, Henkestr, Germany) was employed to measure serum concentrations of blood urea nitrogen (BUN), creatinine, total cholesterol, triglycerides (TG), glucose, total calcium, phosphorus, and C-reactive protein (CRP). Enzyme-linked immunosorbent assay kits were used to determine serum levels of intact parathyroid hormone (iPTH) (Abcam, Cambridge, MA, USA) and MDA-LDL (Sekisui Diagnostics GmbH, Kaplaneigasse, Pfungstadt, Germany) [21].
2.4. Evaluation of Blood Pressure and Brachial-Ankle Pulse Wave Velocity
Following blood sample collection, patients rested in a supine position for 10 min. The trained staff member took the participants’ morning blood pressure (BP) of the upper arm using an automatic oscillometric device. Three measurements of systolic BP (SBP) and diastolic BP (DBP) were taken at 5-min intervals, and the mean values were used for analysis. Hypertension was defined as SBP ≥ 140 mmHg, DBP ≥ 90 mmHg, or the use of antihypertensive medications for at least two weeks, based on the Eighth Joint National Committee (JNC 8) guidelines. Assessment of baPWV was performed by a volume plethysmographic device (VP-2000, Omron, Japan) with four pneumatic cuffs coupled with oscillometric and plethysmographic sensors wrapped around the upper arms and ankles [23]. Patients with left or right baPWV values > 18.0 m/s were classified as the PAS group, according to the cutoff value established by the Physiological Diagnosis Criteria for Vascular Failure Committee of Japan [24,25].
2.5. Data Analysis
The normality of data distribution was assessed using the Kolmogorov-Smirnov test. Normally distributed continuous variables were expressed as mean ± standard deviation and compared using the two-tailed Student’s independent t-test. Non-normally distributed variables, including TG, glucose, iPTH, and CRP levels, were presented as median and interquartile range and compared using the Mann-Whitney U test. These non-normally distributed variables were log-transformed to achieve normality for further analysis. The chi-square test was used to analyze categorical variables. The associations between serum MDA-LDL and other variables were assessed using Spearman’s rank-order correlation coefficient. Variables significantly correlated with PAS were then included in a multivariable logistic regression analysis. A receiver operating characteristic (ROC) curve was constructed to identify the optimal cutoff value of serum MDA-LDL for predicting PAS, and the area under the curve (AUC) was calculated to assess its predictive ability. Statistical analyses were performed using IBM SPSS Version 19.0 (SPSS, Inc., Chicago, IL, USA), with statistical significance set at p < 0.05.
3. Results
Demographic, clinical, and biochemical characteristics of the study population are shown in Table 1. The study comprised 100 HD patients with a mean age of 63.84 ± 13.51 years. The median HD duration was 55.92 months (interquartile range [IQR]: 21.96–123.60 months). Of the patients, 47 (47.0%) were women, 46 (46.0%) had diabetes mellitus, and 46 (46.0%) had hypertension. The mean left and right baPWV were 18.04 ± 3.25 m/s and 18.14 ± 3.29 m/s, respectively. The median serum MDA-LDL level was 88.67 mg/dL (IQR: 69.96–148.52 mg/dL).

Table 1.
Clinical variables of chronic hemodialysis patients with baPWV ≤ 18.0 m/s or baPWV > 18.0 m/s.
Compared to the non-PAS group (n = 48), the PAS group (n = 52) were older (67.37 ± 12.11 vs. 57.94 ± 13.35 years, p < 0.001), had lower serum albumin levels (4.06 ± 0.43 vs. 4.25 ± 0.41 g/dL, p = 0.022), lower serum creatinine levels (8.99 ± 1.67 vs. 9.85 ± 1.98 mg/dL, p = 0.021), higher serum MDA-LDL levels (119.67 [81.65–176.54] vs. 78.38 [59.41–98.35] mg/dL, p < 0.001), and higher serum CRP levels (0.35 [0.10–1.01] vs. 0.16 [0.05–0.40] mg/dL, p = 0.007).
Table 2 demonstrated the multivariable logistic regression analysis results that higher serum MDA-LDL levels (odds ratio [OR] =1.014, 95% confidence interval [CI]: 1.004–1.025, p = 0.009), older age (OR = 1.044, 95% CI: 1.004–1.085, p = 0.031), and higher serum CRP levels (OR = 3.697, 95% CI: 1.149–11.893, p = 0.028) were independently associated with PAS in HD patients.

Table 2.
Multivariable logistic regression analysis of the factors correlated to peripheral arterial stiffness among chronic hemodialysis patients.
Table 3 shows the correlation between baPWV, serum MDA-LDL, and clinical variables. Spearman’s rank-order correlation analysis revealed that left baPWV values were positively correlated with age (r = 0.284, p = 0.004), serum MDA-LDL levels (r = 0.385, p < 0.001), log-transformed glucose levels (r = 0.227, p = 0.023), and log-transformed CRP levels (r = 0.307, p = 0.002) and negatively correlated with serum creatinine levels (r = −0.221, p = 0.027). Right baPWV values were positively correlated with age (r = 0.308, p = 0.002), serum MDA-LDL levels (r = 0.390, p < 0.001), and log-transformed CRP levels (r = 0.249, p = 0.013) and negatively correlated with serum creatinine levels (r = −0.216, p = 0.031). Log-transformed serum MDA-LDL levels were positively correlated with waist circumference (r = 0.225, p = 0.025), total cholesterol (r = 0.218, p = 0.029), and log-transformed CRP levels (r = 0.197, p = 0.049).

Table 3.
Spearman correlation coefficients between left baPWV, right baPWV, log-transformed malondialdehyde-modified low-density lipoprotein, and clinical variables in chronic hemodialysis patients.
The predictive value of serum MDA-LDL levels for PAS. The ROC curve analysis reveals that the optimal cut-off value of serum MDA-LDL for predicting PAS among HD patients was 80.91 mg/dL, with a sensitivity of 79.25%, specificity of 59.57%, positive predictive value of 68.85%, and negative predictive value of 71.80% (Table 4). The area under the ROC curve was 0.717 (95% CI: 0.618–0.803, p < 0.001) (Figure 2).

Table 4.
The optimal cut-off value of serum MDA-LDL and related diagnostic performances for predicting peripheral arterial stiffness among chronic hemodialysis patients.
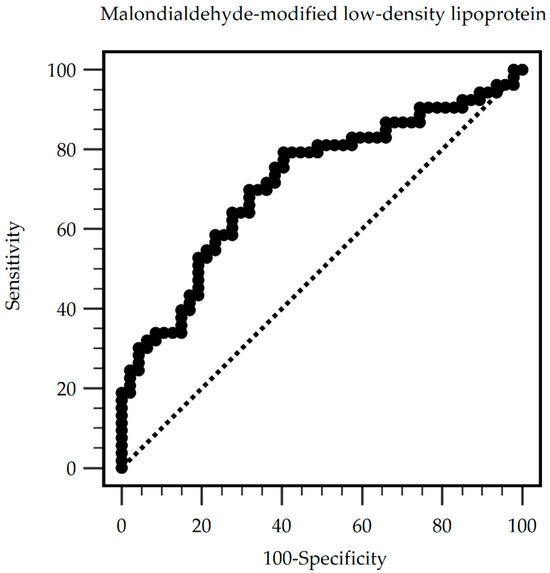
Figure 2.
Peripheral arterial stiffness can be predicted by serum malondialdehyde-modified low-density lipoprotein levels among chronic hemodialysis patients, as indicated by the area under the receiver operating characteristic curve. Area under the ROC curve (AUC): 0.717. 95% Confidence interval: 0.618–0.803. p < 0.001. Cut-off level 80.91 mg/dL. (Sensitivity: 79.25%; specificity: 59.57%; positive predictive values: 68.85%; and negative predictive values: 71.80%).
4. Discussion
In this cross-sectional study, we investigated the association between serum MDA-LDL levels and PAS among HD patients. Using multivariable logistic regression analysis, we found that higher serum MDA-LDL levels, older age, and elevated serum CRP levels were independently associated with PAS in this population. Furthermore, ROC curve analysis revealed that serum MDA-LDL levels greater than 80.91 mg/dL could potentially predict PAS in HD patients.
Our findings highlight the association between serum MDA-LDL levels and PAS in HD patients, suggesting that MDA-LDL potentially plays a role in the development of PAS in this population. Oxidative stress as reflected by serum MDA-LDL levels plays an important role in the pathogenesis of arterial stiffness and atherosclerosis [26,27]. MDA-LDL, a surrogate of oxidative stress, has been associated with endothelial dysfunction and atherosclerosis in various populations [28,29]. In the present study, we observed that higher serum MDA-LDL levels were independently associated with PAS in HD patients, even with adjusting for potential confounders such as age, CRP, albumin, and pre-dialysis creatinine levels. This finding is consistent with previous studies reporting an association between MDA-LDL and arterial stiffness in other populations [13,21].
The mechanisms underlying the association between MDA-LDL and PAS in HD patients were likely multifactorial. MDA-LDL has been shown to promote endothelial dysfunction by increasing the expression of adhesion molecules, facilitating leukocyte recruitment, and impairing nitric oxide bioavailability [20,30,31]. Additionally, MDA-LDL may contribute to arterial stiffening by stimulating the proliferation and migration of vascular smooth muscle cells and promoting the synthesis of extracellular matrix components, such as collagen [13,32]. Moreover, the uremic milieu in ESRD patients may exacerbate the adverse effects of MDA-LDL on the arterial wall leading to accelerated arterial stiffening [33]. The ROC curve analysis identified a serum MDA-LDL level of 80.91 mg/dL as an optimal cut-off value for predicting the presence of PAS in our cohort of HD patients. Further prospective studies are needed to validate the predictive value of this cut-off level and to investigate the potential utility of serum MDA-LDL as a biomarker for risk stratification and management of PAS in HD patients.
As stated above, most of the prior studies on the association between various kinds of lipids such as modified LDLs and PAS and aortic wall stiffness were carried out in the general population [34,35,36,37,38,39], while there were rare studies conducted for HD patients [21]. Among HD patients, serum LDL levels are commonly normal or lower, whereas TG-rich lipoproteins levels are higher as compared to non-HD individuals [40]. Elevated TG levels, frequently found in CKD patients, could contribute to oxidative stress, oxLDL, vascular inflammation, and endothelial dysfunction which could be regarded as a critical precursor of PAS [41,42]. In a study conducted by An et al. [43], oxLDL to LDL ratio was identified as an independent predictor of vascular calcification in the feet, a representative of PAS, of HD patients [44]. Based on currently available evidence including our prior study for MDA-LDL and its association with increased aortic wall stiffness in HD patients, modified LDLs might be potential contributors to reducing both the large and small arterial elasticity in HD patients. While studies in non-HD populations have revealed that pharmacological interventions, such as statins, can reduce oxLDL, oxidative stress, and arterial stiffness [40], their effects on MDA-LDL levels and PAS in HD patients remain to be established.
Aging is a potent risk factor for PAS in the general population and in patients with chronic kidney disease (CKD) [45], and this association is also observed in HD patients. This association can be attributed to age-related changes in the arterial wall, including increased collagen content, decreased elastin content, and accumulation of glycation end-product [46]. The cumulative exposure to both traditional and non-traditional CVD risk factors in older HD patients may further contribute to the development of PAS [23]. Inflammation has been involved in the development of arterial stiffness and CVD events in CKD patients [42,47]. It has been well known that serum CRP may be a mediator of the development of CVD events and post-statin therapeutic CRP levels rather than LDL were associated with subsequent CVD events [48]. In our study, elevated serum CRP was independently associated with PAS in HD patients. This finding is consistent with many prior reports that chronic inflammation contributes to arterial stiffening in HD patients [46,49]. Modified LDLs have been found to cause vascular inflammation and thus increase serum CRP levels [50,51]. In a systemic review, a mild to moderate correlation between CRP levels and PWV (Pearson r = 0.33 to r = 0.624) was observed in individuals with dyslipidemia in most of the relevant studies [52]. As the levels of various modified LDLs may be increased in advanced CKD or HD patients, the association of CRP levels with PAS independent of MDA-LDL was possibly related to increases in other modified LDLs which were not assessed in this study. The mechanisms linking inflammation to PAS in HD patients are complex and involve the direct effects of pro-inflammatory cytokines on the arterial wall, as well as the indirect effects of inflammation on oxidative stress, endothelial dysfunction, and vascular calcification [53]. A combination of serum levels of MDA-LDL and CRP may be a good target to assess the status of PAS in HD patients in future studies.
The potential therapeutic implication of our findings merits more consideration. Strategies aimed at reducing oxidative stress and inflammation levels may be effective in preventing or slowing the progression of PAS among HD patients. Antioxidant therapies, such as vitamin E supplementation, have demonstrated promise in reducing CVD risk in some populations [54], although their efficacy in HD patients remains to be established. Similarly, anti-inflammatory agents, e.g., statins and angiotensin-converting enzyme inhibitors, have been shown to improve PAS in CKD patients [55], while their beneficial effects in HD patients require further investigation. Lifestyle modifications, e.g., regular exercise and dietary interventions, may reduce oxidative stress and chronic inflammation in HD patients. Exercise training has been shown to improve PAS and endothelial function in CKD patients [56], and dietary approaches, e.g., the Mediterranean diet, have been associated with reduced chronic inflammation and improved CVD outcomes in the general population [57]. However, the optimal exercise regimen and dietary strategy for preventing PAS in HD patients remain to be determined.
Several limitations of this study should be acknowledged. First, the cross-sectional design precludes the establishment of a causal relationship between serum MDA-LDL levels and PAS. Prospective studies are needed to confirm our findings and to evaluate the prognostic value of serum MDA-LDL levels in predicting CVD events in HD patients. Second, the relatively small sample size and single-center setting may limit the generalizability of our results. Larger, multicenter studies are warranted to validate our findings. Finally, we did not assess other biomarkers of oxidative stress or inflammation, which may have provided additional insights into the pathogenesis of PAS in this population.
5. Conclusions
Our study demonstrated that higher serum MDA-LDL, older age, and elevated serum CRP were independently associated with PAS in HD patients. Moreover, serum MDA-LDL levels ≥80.91 mg/dL may serve as a useful biomarker for predicting PAS in this population. The mechanisms linking inflammation to PAS in HD patients are complex and involve the direct effects of pro-inflammatory cytokines on the arterial wall, as well as the indirect effects of inflammation on oxidative stress, endothelial dysfunction, and vascular calcification. While our study demonstrates the independent associations of MDA-LDL and CRP with PAS in HD patients, further cohort studies with larger sample sizes and clinical trials are needed to evaluate the potential therapeutic implications of the findings to develop effective strategies for reducing CVD in this vulnerable population.
Author Contributions
Writing—original draft preparation, W.-N.L. and Y.-C.H.; data curation, C.-W.L. and S.-C.L.; methodology, T.-J.W.; software, C.-W.L.; formal analysis, investigation, C.-W.L., S.-C.L., Y.-C.H., T.-J.W. and G.-M.L.; resources, T.-J.W.; writing—review and editing, G.-M.L.; visualization, G.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by a grant from Hualien Armed Forces General Hospital, Hualien, Taiwan, grant number HAFGH-D-112013.
Institutional Review Board Statement
The research conducted herein has been reviewed and approved by the Research Ethics Committee, Hualien Tzu Chi Hospital, adhering to the ethical standards for research involving human subjects. (IRB108–219-A and approval on 1 January 2020).
Informed Consent Statement
Informed consent was procured from all study participants.
Data Availability Statement
Upon request, the corresponding author can provide the data utilized in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Golembiewska, E.; Lindholm, B.; Stenvinkel, P. End-stage renal disease, inflammation and cardiovascular outcomes. Expand. Hemodial. 2017, 191, 32–43. [Google Scholar]
- Wang, Y.; Gao, L. Inflammation and cardiovascular disease associated with hemodialysis for end-stage renal disease. Front. Pharmacol. 2022, 13, 800950. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress-and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Gluba-Brzózka, A. Oxidative stress in ESRD patients on dialysis and the risk of cardiovascular diseases. Antioxidants 2020, 9, 1079. [Google Scholar] [CrossRef]
- Sarafidis, P.A.; Loutradis, C.; Karpetas, A.; Tzanis, G.; Piperidou, A.; Koutroumpas, G.; Raptis, V.; Syrgkanis, C.; Liakopoulos, V.; Efstratiadis, G. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension 2017, 70, 148–157. [Google Scholar] [CrossRef]
- Matschkal, J.; Mayer, C.C.; Sarafidis, P.A.; Lorenz, G.; Braunisch, M.C.; Guenthner, R.; Angermann, S.; Steubl, D.; Kemmner, S.; Bachmann, Q. Comparison of 24-hour and office pulse wave velocity for prediction of mortality in hemodialysis patients. Am. J. Nephrol. 2019, 49, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Munakata, M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: Recent evidence and clinical applications. Curr. Hypertens. Rev. 2014, 10, 49–57. [Google Scholar] [CrossRef]
- Chen, S.-C.; Huang, J.-C.; Su, H.-M.; Chiu, Y.-W.; Chang, J.-M.; Hwang, S.-J.; Chen, H.-C. Prognostic cardiovascular markers in chronic kidney disease. Kidney Blood Press. Res. 2018, 43, 1388–1407. [Google Scholar] [CrossRef]
- Tsai, J.-P.; Hsu, B.-G. Arterial stiffness: A brief review. Tzu Chi Med. J. 2021, 33, 115–121. [Google Scholar]
- Wung, C.-H.; Wang, Y.-H.; Lee, Y.-C.; Chang, C.-W.; Wu, P.-Y.; Huang, J.-C.; Tsai, Y.-C.; Chen, S.-C.; Chang, J.-M.; Hwang, S.-J. Association between flow-mediated dilation and skin perfusion pressure with peripheral artery disease in hemodialysis patients. J. Pers. Med. 2021, 11, 1251. [Google Scholar] [CrossRef]
- Baba, M.; Maris, M.; Jianu, D.; Luca, C.T.; Stoian, D.; Mozos, I. The impact of the blood lipids levels on arterial stiffness. J. Cardiovasc. Dev. Dis. 2023, 10, 127. [Google Scholar] [CrossRef]
- Wu, C.-F.; Liu, P.-Y.; Wu, T.-J.; Hung, Y.; Yang, S.-P.; Lin, G.-M. Therapeutic modification of arterial stiffness: An update and comprehensive review. World J. Cardiol. 2015, 7, 742. [Google Scholar] [CrossRef]
- Busch, C.J.; Binder, C.J. Malondialdehyde epitopes as mediators of sterile inflammation. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 398–406. [Google Scholar] [CrossRef]
- Gianazza, E.; Brioschi, M.; Martinez Fernandez, A.; Casalnuovo, F.; Altomare, A.; Aldini, G.; Banfi, C. Lipid peroxidation in atherosclerotic cardiovascular diseases. Antioxid. Redox Signal. 2021, 34, 49–98. [Google Scholar] [CrossRef]
- Ichikawa, K.; Miyoshi, T.; Osawa, K.; Miki, T.; Ito, H. Increased circulating malondialdehyde-modified low-density lipoprotein level is associated with high-risk plaque in coronary computed tomography angiography in patients receiving statin therapy. J. Clin. Med. 2021, 10, 1480. [Google Scholar] [CrossRef]
- Prasad, A.; Clopton, P.; Ayers, C.; Khera, A.; De Lemos, J.A.; Witztum, J.L.; Tsimikas, S. Relationship of autoantibodies to MDA-LDL and ApoB-immune complexes to sex, ethnicity, subclinical atherosclerosis, and cardiovascular events. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1213–1221. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis; Endotext: Dartmouth, MA, USA, 2019. [Google Scholar]
- Hou, J.-S.; Wang, C.-H.; Lai, Y.-H.; Kuo, C.-H.; Lin, Y.-L.; Hsu, B.-G.; Tsai, J.-P. Serum malondialdehyde-modified low-density lipoprotein is a risk factor for central arterial stiffness in maintenance hemodialysis patients. Nutrients 2020, 12, 2160. [Google Scholar] [CrossRef]
- Mozos, I.; Luca, C.T. Crosstalk between oxidative and nitrosative stress and arterial stiffness. Curr. Vasc. Pharmacol. 2017, 15, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Hsu, B.-G.; Wang, C.-H.; Lin, Y.-L.; Lai, Y.-H.; Tsai, J.-P. Serum trimethylamine N-oxide level is associated with peripheral arterial stiffness in advanced non-dialysis chronic kidney disease patients. Toxins 2022, 14, 526. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H. Physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, H.; Shiina, K. State of the art review: Brachial-ankle PWV. J. Atheroscler. Thromb. 2020, 27, 621–636. [Google Scholar] [CrossRef] [PubMed]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Martin-Ventura, J.L.; Rodrigues-Diez, R.; Martinez-Lopez, D.; Salaices, M.; Blanco-Colio, L.M.; Briones, A.M. Oxidative stress in human atherothrombosis: Sources, markers and therapeutic targets. Int. J. Mol. Sci. 2017, 18, 2315. [Google Scholar] [CrossRef]
- Takamura, T.-A.; Tsuchiya, T.; Oda, M.; Watanabe, M.; Saito, R.; Sato-Ishida, R.; Akao, H.; Kawai, Y.; Kitayama, M.; Kajinami, K. Circulating malondialdehyde-modified low-density lipoprotein (MDA-LDL) as a novel predictor of clinical outcome after endovascular therapy in patients with peripheral artery disease (PAD). Atherosclerosis 2017, 263, 192–197. [Google Scholar] [CrossRef]
- Piqueras, L.; Sanz, M.-J. Angiotensin II and leukocyte trafficking: New insights for an old vascular mediator. Role of redox-signaling pathways. Free. Radic. Biol. Med. 2020, 157, 38–54. [Google Scholar] [CrossRef]
- Suciu, C.F.; Prete, M.; Ruscitti, P.; Favoino, E.; Giacomelli, R.; Perosa, F. Oxidized low density lipoproteins: The bridge between atherosclerosis and autoimmunity. Possible implications in accelerated atherosclerosis and for immune intervention in autoimmune rheumatic disorders. Autoimmun. Rev. 2018, 17, 366–375. [Google Scholar] [CrossRef]
- Amirfakhryan, H. Vaccination against atherosclerosis: An overview. Hell. J. Cardiol. 2020, 61, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, J.; Alique, M.; Vida, C.; Bodega, G.; Ceprián, N.; Morales, E.; Praga, M.; de Sequera, P.; Ramírez, R. Mechanisms of cardiovascular disorders in patients with chronic kidney disease: A process related to accelerated senescence. Front. Cell Dev. Biol. 2020, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Hsu, S.H.-J.; Wu, Y.-J.; Su, T.-C. Additive effects of postchallenge hyperglycemia and low-density lipoprotein particles on the risk of arterial stiffness in healthy adults. Lipids Health Dis. 2014, 13, 179. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orekhov, A.N.; Bobryshev, Y.V.; Sobenin, I.A.; Melnichenko, A.A.; Chistiakov, D.A. Modified low density lipoprotein and lipoprotein-containing circulating immune complexes as diagnostic and prognostic biomarkers of atherosclerosis and type 1 diabetes macrovascular disease. Int. J. Mol. Sci. 2014, 15, 12807–12841. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Maeda, S.; Mukai, J.; Ohki, M.; Nakanishi, M.; Yoshikawa, T. Association between plasma sLOX-1 concentration and arterial stiffness in middle-aged and older individuals. J. Clin. Biochem. Nutr. 2015, 57, 151–155. [Google Scholar] [CrossRef]
- Matsui, S.; Kajikawa, M.; Hida, E.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Oda, N.; Kishimoto, S.; Hidaka, T.; Kihara, Y. Optimal target level of low-density lipoprotein cholesterol for vascular function in statin naïve individuals. Sci. Rep. 2017, 7, 8422. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, H.-K.; Wu, X.-W.; Cao, Z.; Tu, Y.-C.; Ma, Y.; Wang, W.-Q.; Cheng, J.; Zhou, Z.-H. Small dense low density lipoprotein-cholesterol and cholesterol ratios to predict arterial stiffness progression in normotensive subjects over a 5-year period. Lipids Health Dis. 2018, 17, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takaeko, Y.; Kajikawa, M.; Kishimoto, S.; Yamaji, T.; Harada, T.; Han, Y.; Kihara, Y.; Hida, E.; Chayama, K.; Goto, C. Low levels of low-density lipoprotein cholesterol and endothelial function in subjects without lipid-lowering therapy. J. Clin. Med. 2020, 9, 3796. [Google Scholar] [CrossRef]
- Scarpioni, R.; Ricardi, M.; Melfa, L.; Cristinelli, L. Dyslipidemia in chronic kidney disease: Are statins still indicated in reduction cardiovascular risk in patients on dialysis treatment? Cardiovasc. Ther. 2010, 28, 361–368. [Google Scholar] [CrossRef]
- Moradi, H.; Streja, E.; Vaziri, N.D. ESRD-induced dyslipidemia—Should management of lipid disorders differ in dialysis patients? In Seminars in Dialysis; Whiley: Hoboken, NJ, USA, 2018; pp. 398–405. [Google Scholar]
- Zanoli, L.; Lentini, P.; Briet, M.; Castellino, P.; House, A.A.; London, G.M.; Malatino, L.; McCullough, P.A.; Mikhailidis, D.P.; Boutouyrie, P. Arterial stiffness in the heart disease of CKD. J. Am. Soc. Nephrol. 2019, 30, 918–928. [Google Scholar] [CrossRef]
- An, W.S.; Kim, S.-E.; Kim, K.-H.; Bae, H.-R.; Rha, S.-H. Associations between oxidized LDL to LDL ratio, HDL and vascular calcification in the feet of hemodialysis patients. J. Korean Med. Sci. 2009, 24, S115. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Wu, H. Arterial stiffness: A focus on vascular calcification and its link to bone mineralization. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E. Arterial stiffness as a risk factor for clinical hypertension. Nat. Rev. Cardiol. 2018, 15, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mishra, M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int. J. Angiol. 2017, 26, 001–011. [Google Scholar]
- Lin, G.-M.; Wu, C.-F.; Liu, P.-Y.; Han, C.-L. Modified low-density lipoprotein may moderate the association of baseline hs-CRP with incident cardiac events in the Asian populations. J. Cardiol. 2016, 68, 178–179. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.-M.; Liu, K.; Colangelo, L.A.; Lakoski, S.G.; Tracy, R.P.; Greenland, P. Low-density lipoprotein cholesterol concentrations and association of high-sensitivity C-reactive protein concentrations with incident coronary heart disease in the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2016, 183, 46–52. [Google Scholar] [CrossRef] [PubMed]
- London, G.M. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018, 45, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. Vascular inflammation and low-density lipoproteins: Is cholesterol the link? A lesson from the clinical trials. Br. J. Pharmacol. 2017, 174, 3973–3985. [Google Scholar] [CrossRef] [PubMed]
- Hoogeveen, R.C.; Ballantyne, C.M. Residual cardiovascular risk at low LDL: Remnants, lipoprotein (a), and inflammation. Clin. Chem. 2021, 67, 143–153. [Google Scholar] [CrossRef]
- Aminuddin, A.; Lazim, M.R.M.; Hamid, A.A.; Hui, C.K.; Mohd Yunus, M.H.; Kumar, J.; Ugusman, A. The association between inflammation and pulse wave velocity in dyslipidemia: An evidence-based review. Mediat. Inflamm. 2020, 2020, 4732987. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial dysfunction in chronic kidney disease, from biology to clinical outcomes: A 2020 update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and metabolic protection by vitamin E: A matter of treatment strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Breyer, M.D.; Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 2016, 15, 568–588. [Google Scholar] [CrossRef] [PubMed]
- Sprick, J.D.; Mammino, K.; Jeong, J.; DaCosta, D.R.; Hu, Y.; Morison, D.G.; Nocera, J.R.; Park, J. Aerobic exercise training improves endothelial function and attenuates blood pressure reactivity during maximal exercise in chronic kidney disease. J. Appl. Physiol. 2022, 132, 758–793. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean diet and cardiovascular health: A critical review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).