Gut Microbiota’s Oxalate-Degrading Activity and Its Implications on Cardiovascular Health in Patients with Kidney Failure: A Pilot Prospective Study
Abstract
1. Introduction
2. Materials and Methods
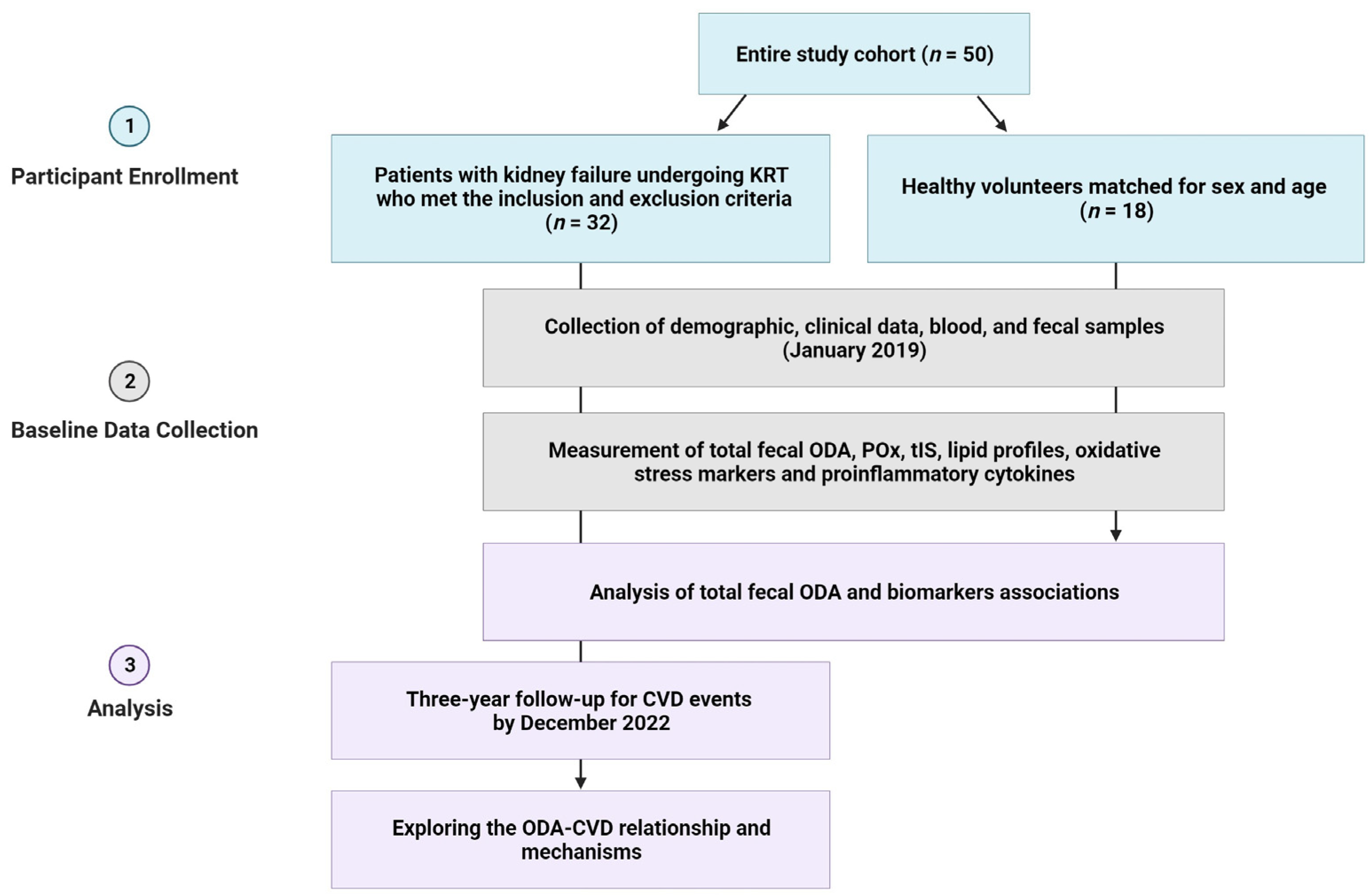
2.1. Study Design
2.2. Sample Size
2.3. Study Participants
2.4. Sample Collection
2.5. Clinical and Routine Laboratory Measurements
2.6. Determination of ODA in Feces
2.7. Measurement of POx Concentration
2.8. Determination of Plasma Malondialdehyde and Antioxidant Markers
2.9. Cytokine’s Measurements
2.10. Total Serum Indoxyl Sulfate Determination
2.11. Endpoint and Definition of CVD Events
2.12. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Participants
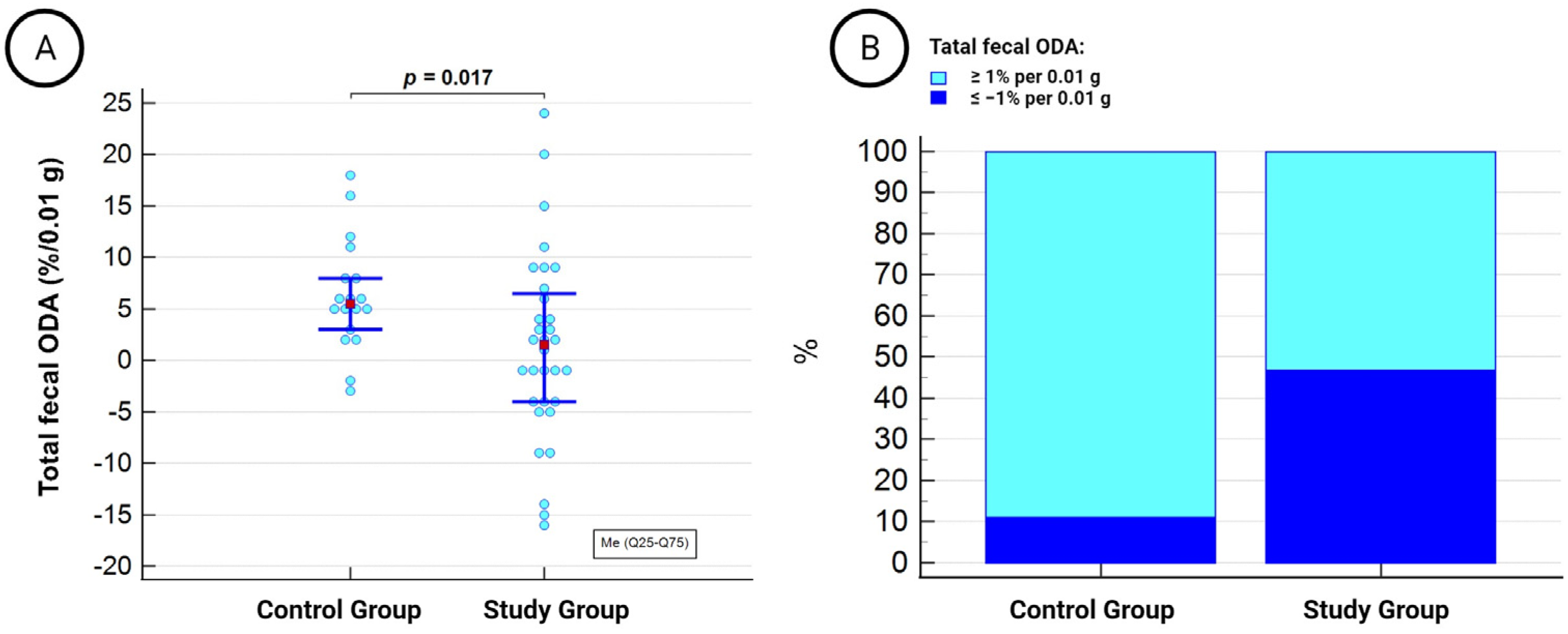
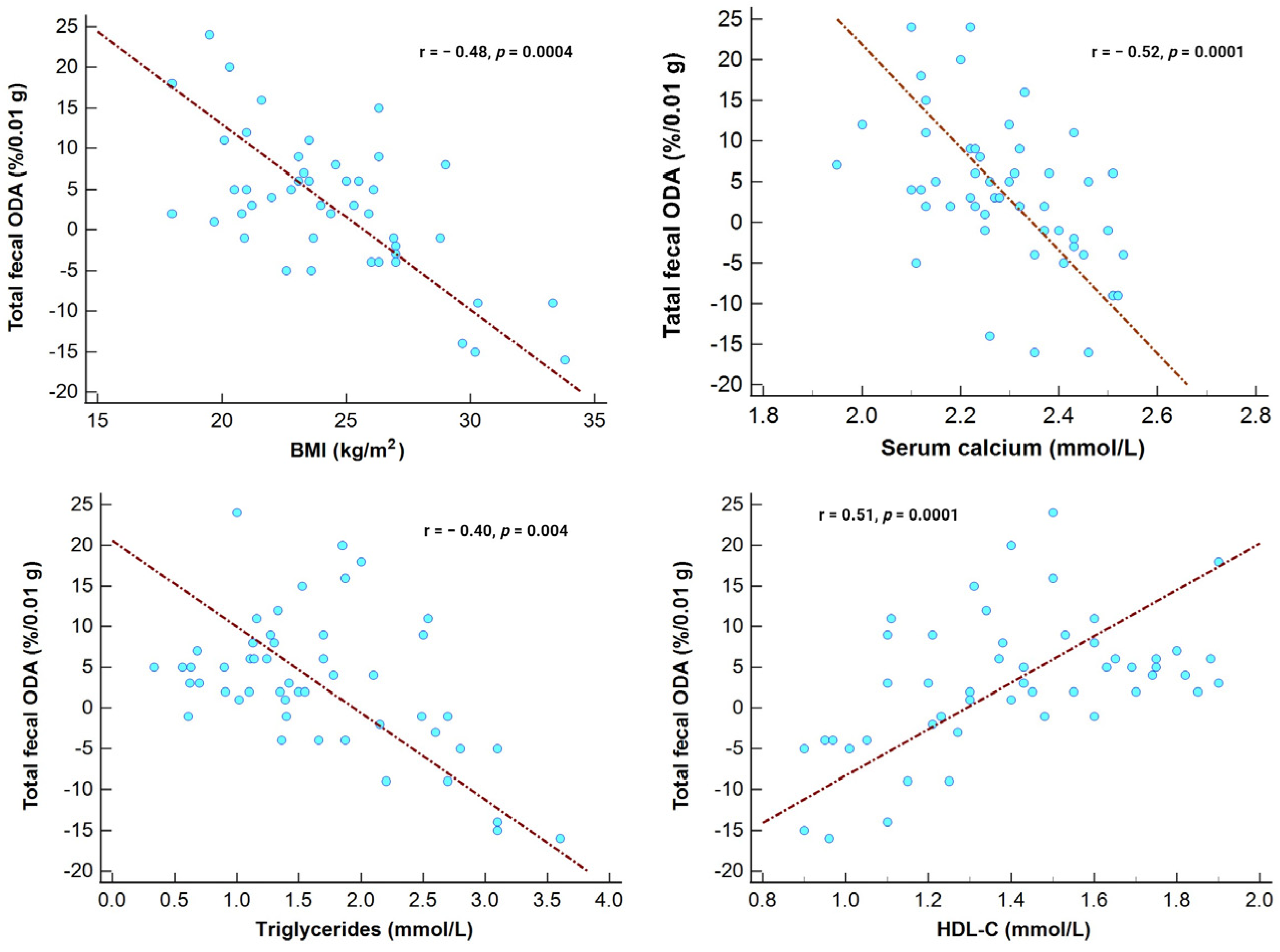
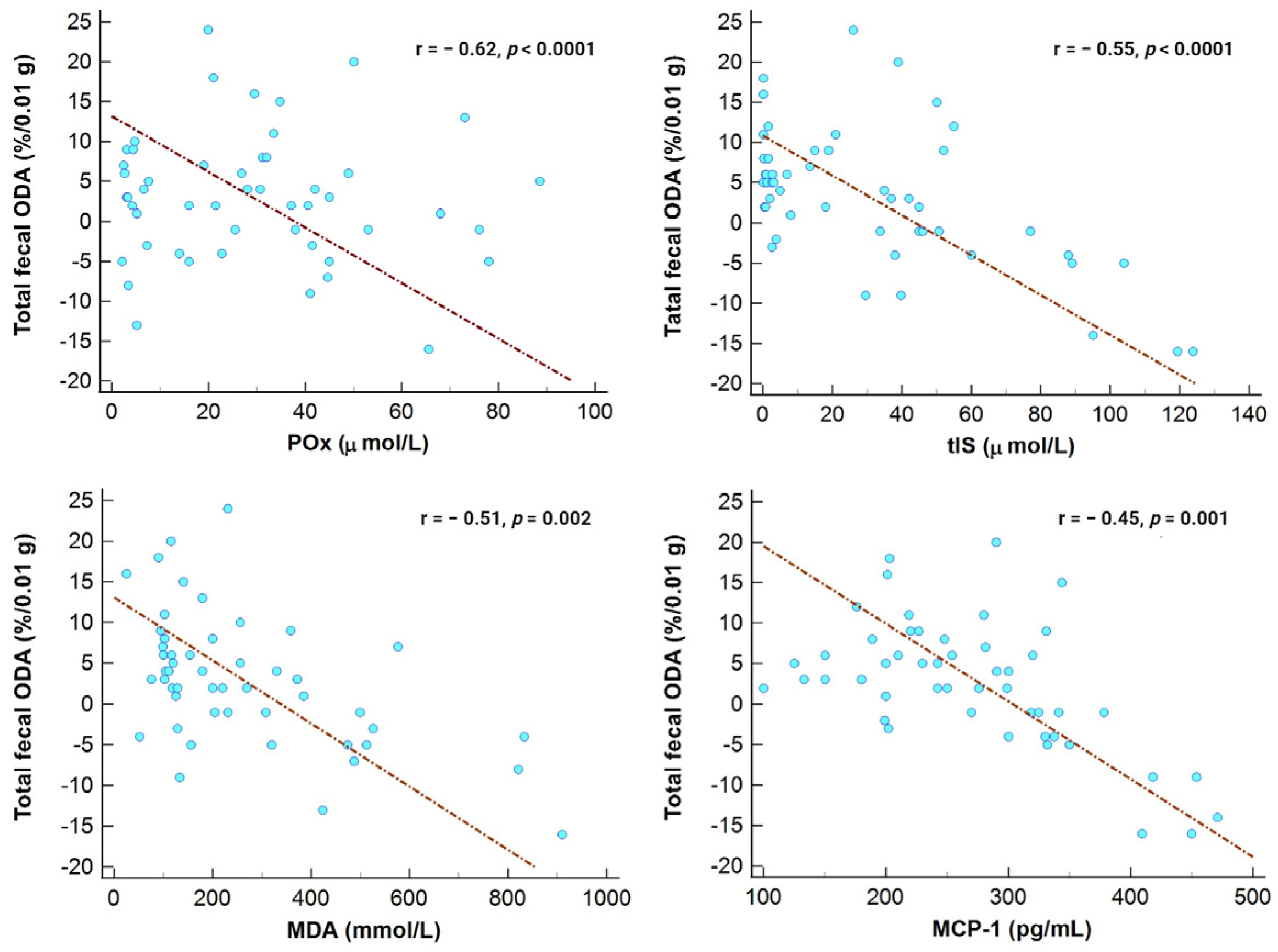
3.2. Microbiota’s ODA and Its Association with Examined Markers in the Entire Study Cohort
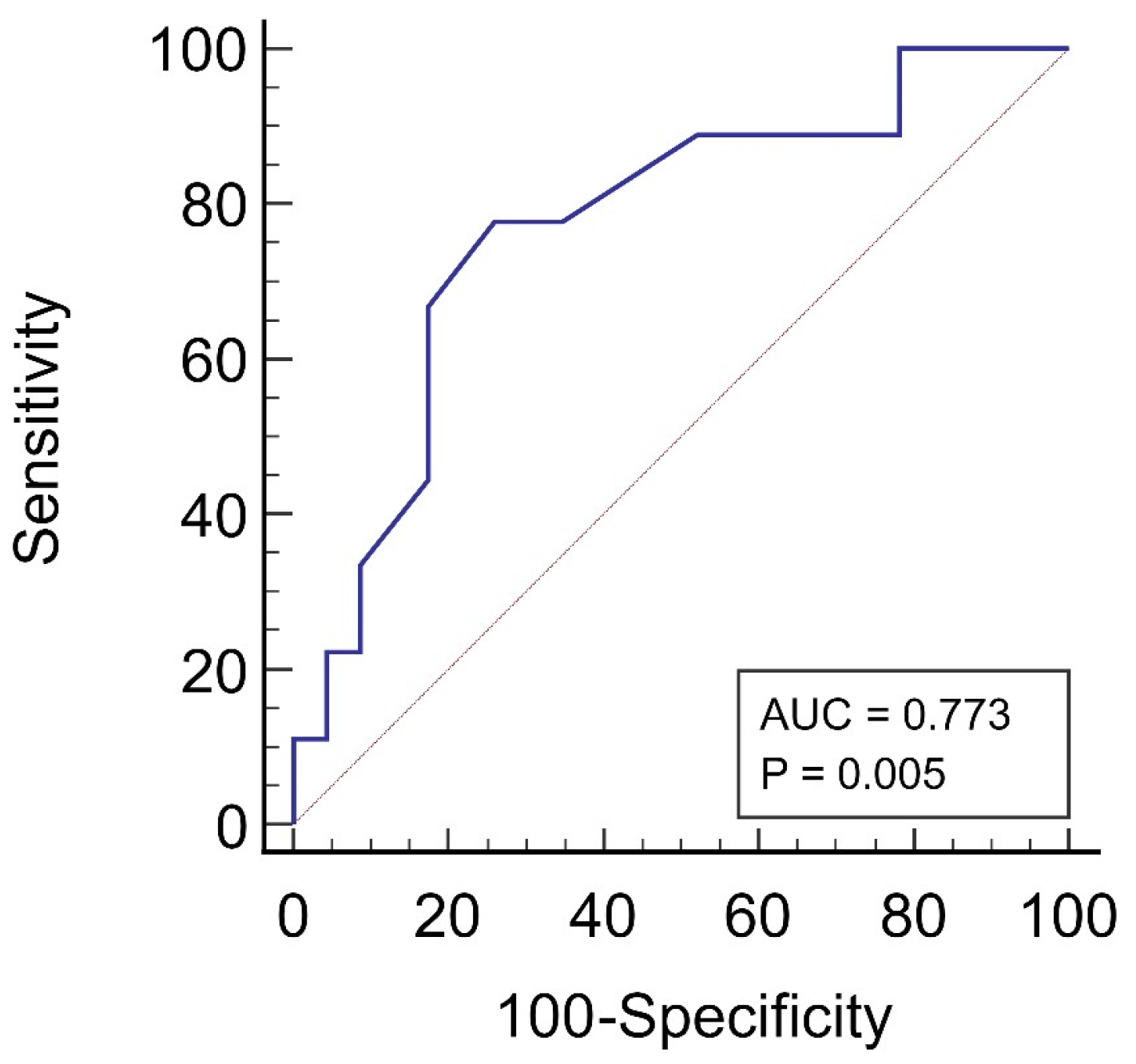
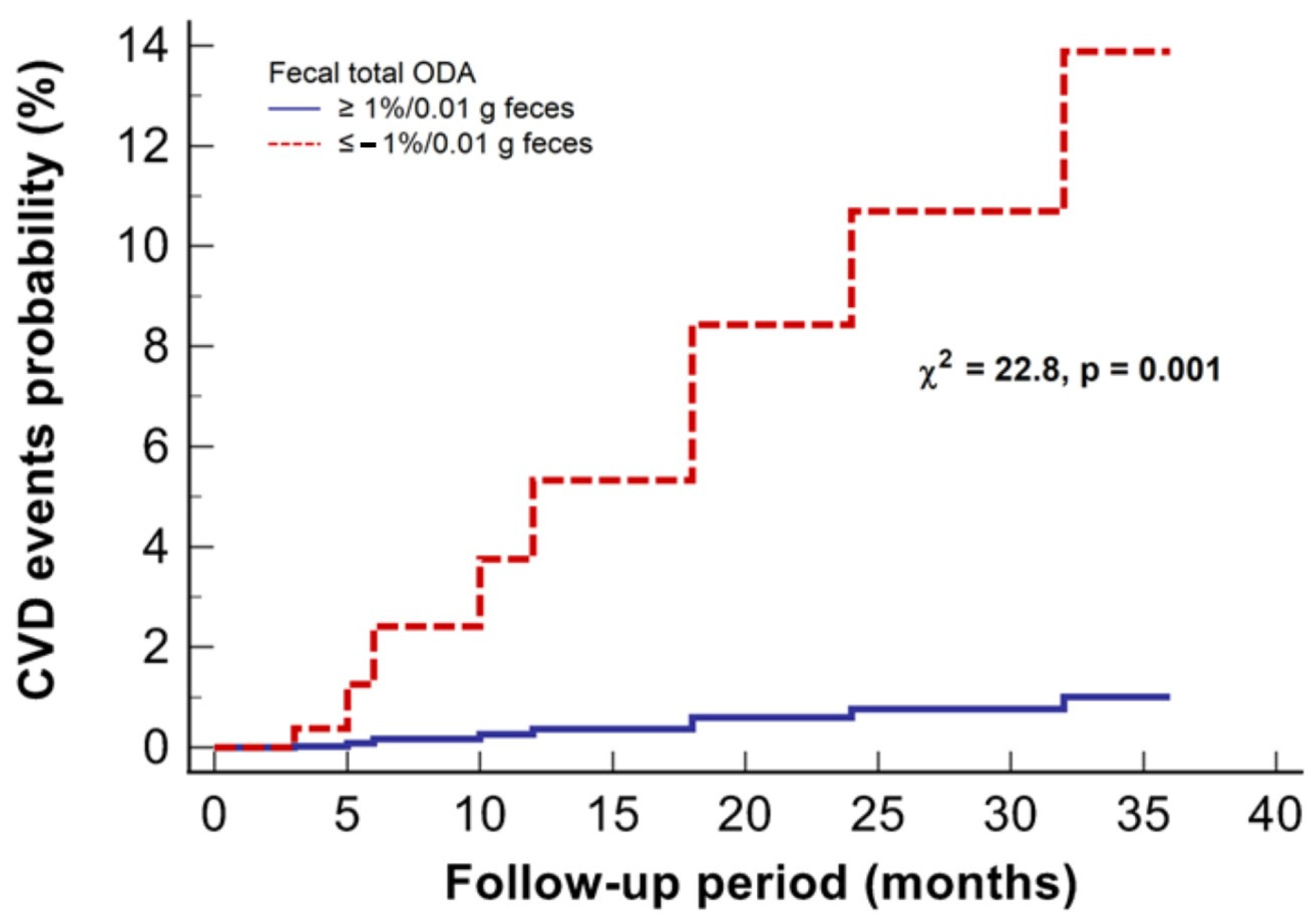
3.3. Microbiota’s ODA and CVD Events in Patients with Kidney Failure
3.4. Exploring the Link between Total Fecal ODA and CVD Events in Patients Undergoing KRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human Gut Microbiota in Health and Disease: Unveiling the Relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Rukavina Mikusic, N.L.; Kouyoumdzian, N.M.; Choi, M.R. Gut microbiota and chronic kidney disease: Evidences and mechanisms that mediate a new communication in the gastrointestinal-renal axis. Pflug. Arch. 2020, 472, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Wehedy, E.; Shatat, I.F.; Al Khodor, S. The Human Microbiome in Chronic Kidney Disease: A Double-Edged Sword. Front. Med. 2022, 8, 790783. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, M.; Weeks, T.L.; Hazen, S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020, 127, 553–570. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, N.; Mahmoudi, M.; Halperin, F.; Wu, J.C.; Pakpour, S. Gut Microbiota and Cardiovascular Disease: Opportunities and Challenges. Microbiome 2020, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M. Gut Microbiota and Oxalate Homeostasis. Ann. Transl. Med. 2017, 5, 36. [Google Scholar] [CrossRef]
- Ermer, T.; Nazzal, L.; Tio, M.C.; Waikar, S.; Aronson, P.S.; Knauf, F. Oxalate Homeostasis. Nat. Rev. Nephrol. 2023, 19, 123–138. [Google Scholar] [CrossRef]
- Matsushita, K.; Ballew, S.H.; Wang, A.Y.M.; Kalyesubula, R.; Schaeffner, E.; Agarwal, R. Epidemiology and Risk of Cardiovascular Disease in Populations with Chronic Kidney Disease. Nat. Rev. Nephrol. 2022, 18, 696–707. [Google Scholar] [CrossRef]
- Waikar, S.S.; Srivastava, A.; Palsson, R.; Shafi, T.; Hsu, C.Y.; Sharma, K.; Lash, J.P.; Chen, J.; He, J.; Lieske, J.; et al. Association of Urinary Oxalate Excretion with the Risk of Chronic Kidney Disease Progression. JAMA Intern. Med. 2019, 179, 542–551. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Shukha, Y.; Lu, H.; Wang, L.; Liu, Z.; Liu, C.; Zhao, Y.; Wang, H.; Zhao, G.; et al. Dysregulated Oxalate Metabolism Is a Driver and Therapeutic Target in Atherosclerosis. Cell Rep. 2021, 36, 109420. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary Oxalate to Calcium Ratio and Incident Cardiovascular Events: A 10-Year Follow-up among an Asian Population. Nutr. J. 2022, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Devlin, J.C.; Hu, J.; Volkova, A.; Battaglia, T.W.; Ho, M.; Asplin, J.R.; Byrd, A.; Loke, P.; Li, H.; et al. Title: Microbial Genetic and Transcriptional Contributions to Oxalate Degradation by the Gut Microbiota in Health and Disease. eLife 2021, 10, e63642. [Google Scholar] [CrossRef] [PubMed]
- Abratt, V.R.; Reid, S.J. Oxalate-Degrading Bacteria of the Human Gut as Probiotics in the Management of Kidney Stone Disease. Adv. Appl. Microbiol. 2010, 72, 63–87. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.W.; Dearing, D. The Metabolic and Ecological Interactions of Oxalate-Degrading Bacteria in the Mammalian Gut. Pathogens 2013, 2, 636–652. [Google Scholar] [CrossRef] [PubMed]
- Karamad, D.; Khosravi-Darani, K.; Khaneghah, A.M.; Miller, A.W. Probiotic Oxalate-Degrading Bacteria: New Insight of Environmental Variables and Expression of the Oxc and Frc Genes on Oxalate Degradation Activity. Foods 2022, 11, 2876. [Google Scholar] [CrossRef]
- Tolstanova, G.; Akulenko, I.; Serhiichuk, T.; Dovbynchuk, T.; Stepanova, N. Pro- Pre- and Synbiotic Supplementation and Oxalate Homeostasis in 3 PM Context: Focus on Microbiota Oxalate-Degrading Activity. In Microbiome in 3P Medicine Strategies; Advances in Predictive, Preventive and Personalised Medicine; Boyko, N., Golubnitschaja, O., Eds.; Springer: Cham, Switzerland, 2023; Volume 16, pp. 335–353. [Google Scholar] [CrossRef]
- Stepanova, N.; Akulenko, I.; Serhiichuk, T.; Dovbynchuk, T.; Savchenko, S.; Tolstanova, G. Synbiotic Supplementation and Oxalate Homeostasis in Rats: Focus on Microbiota Oxalate-Degrading Activity. Urolithiasis 2022, 50, 249–258. [Google Scholar] [CrossRef]
- Allison, M.; Cook, H.M.; Milne, D.B.; Gallagher, S.; Clayman, R.V. Oxalate Degradation by Gastrointestinal Bacteria from Humans. J. Nutr. 1986, 116, 455–460. [Google Scholar] [CrossRef]
- Ermer, T.; Kopp, C.; Asplin, J.R.; Granja, I.; Perazella, M.A.; Reichel, M.; Nolin, T.D.; Eckardt, K.U.; Aronson, P.S.; Finkelstein, F.O.; et al. Impact of Regular or Extended Hemodialysis and Hemodialfiltration on Plasma Oxalate Concentrations in Patients with End-Stage Renal Disease. Kidney Int. Rep. 2017, 2, 1050–1058. [Google Scholar] [CrossRef][Green Version]
- Metry, E.L.; Garrelfs, S.F.; Peters-Sengers, H.; Vaz, F.M.; Bijlsma, J.A.; Neradova, A.; Oosterveld, M.J.S.; Groothoff, J.W. Plasma Oxalate and Glycolate Concentrations in Dialysis Patients with and without Primary Hyperoxaluria Type 1. Nephrol. Dial. Transpl. 2023, 38, 1773–1775. [Google Scholar] [CrossRef]
- Pfau, A.; Wytopil, M.; Chauhan, K.; Reichel, M.; Coca, S.G.; Aronson, P.S.; Eckardt, K.U.; Knauf, F. Assessment of Plasma Oxalate Concentration in Patients With CKD. Kidney Int. Rep. 2020, 5, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, N. Oxalate Homeostasis in Non-Stone-Forming Chronic Kidney Disease: A Review of Key Findings and Perspectives. Biomedicines 2023, 11, 1654. [Google Scholar] [CrossRef] [PubMed]
- Amini Khiabani, S.; Asgharzadeh, M.; Samadi Kafil, H. Chronic Kidney Disease and Gut Microbiota. Heliyon 2023, 9, e18991. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, V.; Horvath, A.; Ribitsch, W.; Schmerböck, B.; Schilcher, G.; Lemesch, S.; Stiegler, P.; Rosenkranz, A.R.; Fickert, P.; Leber, B. Structural and Functional Differences in Gut Microbiome Composition in Patients Undergoing Haemodialysis or Peritoneal Dialysis. Sci. Rep. 2017, 7, 15601. [Google Scholar] [CrossRef] [PubMed]
- Bryniarski, M.A.; Hamarneh, F.; Yacoub, R. The Role of Chronic Kidney Disease-Associated Dysbiosis in Cardiovascular Disease. Exp. Biol. Med. 2019, 244, 514–525. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, P.H.; Lin, Y.T.; Hung, S.C. Gut Dysbiosis and Mortality in Hemodialysis Patients. NPJ Biofilms Microbiomes 2021, 7, 20. [Google Scholar] [CrossRef]
- Pfau, A.; Ermer, T.; Coca, S.G.; Tio, M.C.; Genser, B.; Reichel, M.; Finkelstein, F.O.; März, W.; Wanner, C.; Waikar, S.S.; et al. High Oxalate Concentrations Correlate with Increased Risk for Sudden Cardiac Death in Dialysis Patients. J. Am. Soc. Nephrol. 2021, 32, 2375–2385. [Google Scholar] [CrossRef]
- Guldris, S.C.; Parra, E.G.; Amenós, A.C. Gut Microbiota in Chronic Kidney Disease. Nefrología 2017, 37, 9–19. [Google Scholar] [CrossRef]
- Lau, W.L.; Kalantar-Zadeh, K.; Vaziri, N.D. The Gut as a Source of Inflammation in Chronic Kidney Disease. Nephron 2015, 130, 92–98. [Google Scholar] [CrossRef]
- Kumar, P.; Saini, K.; Saini, V.; Mitchell, T. Oxalate Alters Cellular Bioenergetics, Redox Homeostasis, Antibacterial Response, and Immune Response in Macrophages. Front. Immunol. 2021, 12, 694865. [Google Scholar] [CrossRef]
- Sun, K.; Tang, X.; Song, S.; Gao, Y.; Yu, H.; Sun, N.; Wen, B.; Mei, C. Hyperoxalemia Leads to Oxidative Stress in Endothelial Cells and Mice with Chronic Kidney Disease. Kidney Blood Press. Res. 2021, 46, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.; Lewis, M. The Size of a Pilot Study for a Clinical Trial Should Be Calculated in Relation to Considerations of Precision and Efficiency. J. Clin. Epidemiol. 2012, 65, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Korol, L.V.; Mygal, L.Y.; Stepanova, N.M. Intensity of Oxidative Stress and Activity of Angiotensin Converting Enzyme in Blood of Patients with Uncomplicated Pyelonephritis. Ukr. Biochem. J. 2017, 89, 99–105. [Google Scholar] [CrossRef][Green Version]
- Batislam, E.; Yilmaz, E.; Yuvanc, E.; Kisa, O.; Kisa, U. Quantitative Analysis of Colonization with Real-Time PCR to Identify the Role of Oxalobacter Formigenes in Calcium Oxalate Urolithiasis. Urol. Res. 2012, 40, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.W.; Dale, C.; Dearing, M.D. The Induction of Oxalate Metabolism In Vivo Is More Effective with Functional Microbial Communities than with Functional Microbial Species. mSystems 2017, 2, e00088-17. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.R. Reactive Oxygen Species, Inflammation and Calcium Oxalate Nephrolithiasis. Transl. Androl. Urol. 2014, 3, 256–276. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Ison, J.; Tyagi, S.C.; Tyagi, N. The Role of Gut Microbiota in Bone Homeostasis. Bone 2020, 135, 115317. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Zhang, Y.; Yang, J.; Hu, Z. Gut Microbiota and Calcium Balance. Front. Microbiol. 2022, 13, 1033933. [Google Scholar] [CrossRef]
- Liebman, M.; Al-Wahsh, I.A. Probiotics and Other Key Determinants of Dietary Oxalate Absorption. Adv. Nutr. 2011, 2, 254–260. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent Advances on the Mechanisms of Kidney Stone Formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Moe, S.M. Calcium as a Cardiovascular Toxin in CKD-MBD. Bone 2017, 100, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiang, F.; Ji, J.; Ding, X.; Shen, B.; Chen, J.; Chen, Y.; Xue, N.; Zhang, L.; Jiang, X.; et al. Indoxyl Sulfate and High-Density Lipoprotein Cholesterol in Early Stages of Chronic Kidney Disease. Ren. Fail. 2020, 42, 1157. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Tarng, D.C. Diet, Gut Microbiome and Indoxyl Sulphate in Chronic Kidney Disease Patients. Nephrology 2018, 23, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, S.; Wu, Q.J.; Xiao, J.; Wang, Z.H.; Mu, X.W.; Zhang, Y.; Wang, X.N.; You, L.L.; Wang, S.N.; et al. Serum Total Indoxyl Sulfate Levels and All-Cause and Cardiovascular Mortality in Maintenance Hemodialysis Patients: A Prospective Cohort Study. BMC Nephrol. 2022, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Fuller, D.S.; Komaba, H.; Nomura, T.; Massy, Z.A.; Bieber, B.; Robinson, B.; Pisoni, R.; Fukagawa, M. Serum Total Indoxyl Sulfate and Clinical Outcomes in Hemodialysis Patients: Results from the Japan Dialysis Outcomes and Practice Patterns Study. Clin. Kidney J. 2021, 14, 1236. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Chen, M.; Huang, Y.C.; Liou, H.H.; Fang, Y.W. The Protective Effects of Lipid-Lowering Agents on Cardiovascular Disease and Mortality in Maintenance Dialysis Patients: Propensity Score Analysis of a Population-Based Cohort Study. Front. Pharmacol. 2022, 12, 804000. [Google Scholar] [CrossRef] [PubMed]
- Ceja-Galicia, Z.A.; Aranda-Rivera, A.K.; Amador-Martínez, I.; Aparicio-Trejo, O.E.; Tapia, E.; Trujillo, J.; Ramírez, V.; Pedraza-Chaverri, J. The Development of Dyslipidemia in Chronic Kidney Disease and Associated Cardiovascular Damage, and the Protective Effects of Curcuminoids. Foods 2023, 12, 921. [Google Scholar] [CrossRef]
- Vourakis, M.; Mayer, G.; Rousseau, G. The Role of Gut Microbiota on Cholesterol Metabolism in Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef]
- Yu, Y.; Raka, F.; Adeli, K. The Role of the Gut Microbiota in Lipid and Lipoprotein Metabolism. J. Clin. Med. 2019, 8, 2227. [Google Scholar] [CrossRef]
- Andriamihaja, M.; Blachier, F.; De Simone, C.; Tennoune, N.; Andriamihaja, M.; Blachier, F. Production of Indole and Indole-Related Compounds by the Intestinal Microbiota and Consequences for the Host: The Good, the Bad, and the Ugly. Microorganisms 2022, 10, 930. [Google Scholar] [CrossRef]
- Foresto-Neto, O.; Ghirotto, B.; Câmara, N.O.S. Renal Sensing of Bacterial Metabolites in the Gut-Kidney Axis. Kidney360 2021, 2, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liang, W.; Li, L.; Xiong, Q.; He, S.; Zhao, J.; Guo, X.; Xiang, S.; Zhang, P.; Wang, H.; et al. The Accumulation of Gut Microbiome–Derived Indoxyl Sulfate and P-Cresyl Sulfate in Patients with End-Stage Renal Disease. J. Ren. Nutr. 2022, 32, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Bakhautdin, B.; Febbraio, M.; Goksoy, E.; De La Motte, C.A.; Gulen, M.F.; Childers, E.P.; Hazen, S.L.; Li, X.; Fox, P.L. Protective Role of Macrophage-Derived Ceruloplasmin in Inflammatory Bowel Disease. Gut 2013, 62, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xie, X.; Li, Y.; Liang, T.; Zhong, H.; Yang, L.; Xi, Y.; Zhang, J.; Ding, Y.; Wu, Q. Gut Microbiota as an Antioxidant System in Centenarians Associated with High Antioxidant Activities of Gut-Resident Lactobacillus. NPJ Biofilms Microbiomes 2022, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, J.; Akiyama, M.; Hase, K.; Kumagai, Y.; Kim, Y.G. Gut Microbiota Reinforce Host Antioxidant Capacity via the Generation of Reactive Sulfur Species. Cell Rep. 2022, 38, 110479. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal 2021, 35, 1426–1448. [Google Scholar] [CrossRef] [PubMed]
- Podkowińska, A.; Formanowicz, D. Chronic Kidney Disease as Oxidative Stress- and Inflammatory-Mediated Cardiovascular Disease. Antioxidants 2020, 9, 752. [Google Scholar] [CrossRef]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Kelly, J.T.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology Ii (Synergy Ii): A Feasibility Randomized Controlled Trial. Nutrients 2021, 13, 4481. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, J.; Qin, Y.; Wang, Y.; Zhang, Y.; Sun, S. Probiotics, Prebiotics, and Synbiotics Improve Uremic, Inflammatory, and Gastrointestinal Symptoms in End-Stage Renal Disease with Dialysis: A Network Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 850425. [Google Scholar] [CrossRef]
- Mitrović, M.; Stanković-Popović, V.; Tolinački, M.; Golić, N.; Soković Bajić, S.; Veljović, K.; Nastasijević, B.; Soldatović, I.; Svorcan, P.; Dimković, N. The Impact of Synbiotic Treatment on the Levels of Gut-Derived Uremic Toxins, Inflammation, and Gut Microbiome of Chronic Kidney Disease Patients—A Randomized Trial. J. Ren. Nutr. 2023, 33, 278–288. [Google Scholar] [CrossRef]
- Tian, N.; Li, L.; Ng, J.K.C.; Li, P.K.T. The Potential Benefits and Controversies of Probiotics Use in Patients at Different Stages of Chronic Kidney Disease. Nutrients 2022, 14, 4044. [Google Scholar] [CrossRef] [PubMed]
| Clinical and Laboratory Data | Control Group (n = 18) | Study Group (n = 32) | p-Value |
|---|---|---|---|
| Demographic and clinical parameters | |||
| Male sex, n (%) | 6 (33.4%) | 18 (56.2%) | 0.12 |
| Age, years | 46.4 ± 10.9 | 49.1 ± 13.4 | 0.54 |
| BMI, kg/m2 | 23.8 ± 4.2 | 24.4 ± 3.9 | 0.31 |
| Systolic blood pressure, mm Hg | 116.2 ± 10.9 | 128.9 ± 12.2 | 0.006 |
| Diastolic blood pressure, mm Hg | 73.2 ± 8.8 | 82.1 ± 10.3 | 0.003 |
| Time on dialysis, months | 38.5 (22–135) | ||
| Anuria, n (%) | 11 (34.4%) | ||
| Serum creatinine (µmol/L) | 87 (82.02–102.5) | 751 (575.7–968.4) | <0.0001 |
| Serum urea | 5.4 (4.3–6.1) | 22.5 (17.7–31.3) | <0.0001 |
| GFR (mL/min/1.73 m2) | 76.4 (66.1–79.8) | 4.5 (3.5–7.0) | <0.0001 |
| Serum albumin, g/L | 42.2 (41.05–46.1) | 40.6 (38.5–41.6) | 0.003 |
| Hb, g/L | 123 (119.5–133) | 105.5 (94–125) | 0.003 |
| Glucose, mmol/L | 5.4 (4.7–5.7) | 4.9 (4.02–5.9) | 0.47 |
| Calcium, mmol/L | 2.37 ± 0.1 | 2.29 ± 0.18 | 0.019 |
| Phosphorus, mmol/L | 1.05 (0.9–1.1) | 1.99 (1.5–2.6) | <0.0001 |
| Potassium, mmol/L | 4.1 (3.8–4.4) | 5.9 (5.2–6.9) | 0.0001 |
| Uric acid (mmol/L) | 286.7 (146.4–374) | 329.4 (294.5–389.3) | 0.007 |
| Lipid profile parameters | |||
| TC, mmol/L | 4.9 (4.6–5.3) | 5.3 (4.5–6.6) | 0.15 |
| Triglycerides, mmol/L | 1.12 (0.9–1.6) | 1.56 (1.1–2.5) | 0.05 |
| LDL-C, mmol/L | 2.3 (1.5–2.9) | 3.1 (2.5–3.5) | 0.05 |
| HDL-C, mmol/L | 1.5 (1.3–1.65) | 1.2 (1.07–1.56) | 0.04 |
| AIP | 2.7 (1.9–3.1) | 3.2 (2.5–4.9) | 0.01 |
| Oxidative stress markers | |||
| MDA, mmol/L | 119 (101.3–128.4) | 256.4 (134.6–480.7) | <0.0001 |
| Ceruloplasmin, g/L | 0.22 (0.16–0.31) | 0.13 (0.09–0.19) | 0.003 |
| Transferrin, g/L | 5.2 (4.5–5.8) | 2.2 (1.5–2.6) | <0.0001 |
| SH groups, mmol/L | 2.22 ± 1.1 | 1.65 ± 0.69 | 0.02 |
| OSI, CU | 1.04 ± 0.04 | 3.8 ± 2.1 | <0.0001 |
| Proinflammatory markers, tIS and POx concentrations | |||
| CRP, mg/L | 4.2 (2.9–10.9) | 11.6 (7.7–17.8) | 0.001 |
| IL-6, pg/mL | 0.8 (0.5–1.3) | 2.5 (1.9–6.1) | 0.0001 |
| MCP-1, pg/mL | 200.0 (159.7–239.1) | 327.5 (281.2–344) | <0.0001 |
| tIS, μmol/L | 0.85 (0.5–2.6) | 39.8 (20–51.3) | <0.0001 |
| POx, µmol/L | 4.5 (3.09–7.3) | 39.3 (26.2–49.5) | <0.0001 |
| Medications, n (%) | |||
| ACE inhibitors/RAAS blockers | 25 (78%) | ||
| Beta blockers | 18 (56.2%) | ||
| Calcium channel blockers | 7 (21.9%) | ||
| Iron supplementation | 19 (59.3%) | ||
| Erythropoietins | 23 (71.8%) | ||
| Calcium-based phosphate binders | 9 (28.1%) | ||
| Non-calcium phosphate binders | 3 (9.4%) | ||
| Statins | 11 (34.4%) | ||
| Univariate Cox Regression Analysis | Multivariate Cox Regression Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | Wald χ2 | p-Value | HR (95% CI) | Wald χ2 | p-Value | HR (95% CI) |
| Age, years | 2.15 | 0.041 | 1.05 (1.02; 1.09) | 1.11 | 0.290 | 1.01 (0.93; 1.26) |
| Systolic blood pressure, mm Hg | 0.09 | 0.765 | 0.99 (0.91–1.08) | |||
| Diastolic blood pressure, mm Hg | 0.90 | 0.343 | 0.95 (0.86; 1.05) | |||
| Male sex | 1.88 | 0.171 | 0.37 (0.09; 1.54) | |||
| Dialysis modality | 1.29 | 0.257 | 2.30 (0.55; 9.6) | |||
| Dialysis duration, months | 3.58 | 0.048 | 1.11 (1.00; 1.27) | 1.83 | 0.061 | 1.31 (0.95; 2.1) |
| Anuria | 2.03 | 0.064 | 0.86 (0.17; 4.3) | |||
| Obesity (BMI ≥ 30 kg/m2) | 6.28 | 0.012 | 6.22 (1.21; 10.1) | 5.21 | 0.018 | 1.63 (1.1; 2.4) |
| Glucose_blood | 0.05 | 0.825 | 0.93 (0.48; 1.79) | |||
| Ca, mmol/L | 2.81 | 0.082 | 0.93 (0.49; 1.77) | |||
| Triglycerides, mmol/L | 5.82 | 0.015 | 1.39 (1.09; 2.36) | 3.59 | 0.022 | 1.46 (1.1; 3.4) |
| HDL-C, mmol/L | 3.05 | 0.048 | 0.06 (0.008; 0.9) | 2.71 | 0.039 | 0.05 (0.01; 0.89) |
| MDA, mmol/L | 3.41 | 0.044 | 1.27 (1.0; 8.8) | 2.29 | 0.066 | 1.25 (0.97; 7.16) |
| Ceruloplasmin, g/L | 1.86 | 0.172 | 0.91 (0.54; 1.3) | |||
| tIS, μmol/L | 4.26 | 0.018 | 2.15 (1.18; 3.22) | 3.79 | 0.026 | 1.73 (1.06; 4.11) |
| POx (µmol/L) | 5.61 | 0.001 | 4.66 (1.2; 16.5) | 3.12 | 0.017 | 3.65 (1.3; 8.9) |
| IL-6, pg/mL | 1.83 | 0.045 | 1.13 (1.01; 1.28) | 1.56 | 0.068 | 2.43 (0.94; 6.2) |
| MCP-1, pg/mL | 1.61 | 0.038 | 1.11 (1.07; 1.19) | 1.37 | 0.172 | 1.05 (0.97; 1.13) |
| CRP, g/L | 0.17 | 0.680 | 1.02 (0.95; 1.08) | |||
| Total fecal ODA, %/0.01 g | 5.51 | 0.001 | 9.41 (2.3; 19.4) | 3.89 | 0.003 | 4.1 (1.4; 16.3) |
| Total Fecal ODA Status | p-Value | ||
|---|---|---|---|
| Positive (≥1%/0.01 g of Feces) (n = 17) | Negative (≤−1%/0.01 g of Feces) (n = 15) | ||
| Lipid profile parameters | |||
| TC, mmol/L | 5.3 (4.8–6.0) | 5.8 (4.1–6.6) | 0.89 |
| Triglycerides, mmol/L | 1.4 (102.0–1.7) | 2.6 (1.6–3.1) | 0.002 |
| LDL-C, mmol/L | 2.8 (2.4–3.5) | 2.9 (1.8–3.8) | 0.9 |
| HDL-C, mmol/L | 1.45 (1.3–1.8) | 1.03 (0.9–1.2) | 0.0005 |
| AIP | 2.8 (1.8–3.5) | 3.1 (2.3–4.0) | 0.007 |
| Oxidative stress markers | |||
| MDA, mmol/L | 179.5 (112.2–282.0) | 474.3 (250.1–522.4) | 0.008 |
| Ceruloplasmin, g/L | 0.18 (0.12–0.25) | 0.09 (0.07–0.14) | 0.003 |
| Transferrin, g/L | 2.1 (1.5–2.7) | 2.3 (1.8–2.6) | 0.74 |
| SH groups, mmol/L | 1.65 ± 0.4 | 1.66 ± 0.3 | 0.95 |
| OSI, CU | 2.6 ± 1.5 | 5.2 ± 2.6 | 0.001 |
| Proinflammatory markers, tIS and POx concentrations | |||
| CRP, mg/L | 4.9 (3.3–12.9) | 12.5 (10.3–15.1) | 0.03 |
| IL-6, pg/mL | 0.18 (0.1–1.4) | 2.1 (0.1–6.6) | 0.01 |
| MCP-1, pg/mL | 285.6 (234.4–315.6) | 342.0 (330.0–418.1) | 0.0003 |
| tIS, μmol/L | 23.5 (15.0–42.2) | 63.8 (39.8–95.0) | 0.0001 |
| POx, µmol/L | 19.4 (4.7–30.7) | 49.5 (30.3–70.5) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, N.; Tolstanova, G.; Aleksandrova, I.; Korol, L.; Dovbynchuk, T.; Driianska, V.; Savchenko, S. Gut Microbiota’s Oxalate-Degrading Activity and Its Implications on Cardiovascular Health in Patients with Kidney Failure: A Pilot Prospective Study. Medicina 2023, 59, 2189. https://doi.org/10.3390/medicina59122189
Stepanova N, Tolstanova G, Aleksandrova I, Korol L, Dovbynchuk T, Driianska V, Savchenko S. Gut Microbiota’s Oxalate-Degrading Activity and Its Implications on Cardiovascular Health in Patients with Kidney Failure: A Pilot Prospective Study. Medicina. 2023; 59(12):2189. https://doi.org/10.3390/medicina59122189
Chicago/Turabian StyleStepanova, Natalia, Ganna Tolstanova, Iryna Aleksandrova, Lesya Korol, Taisa Dovbynchuk, Victoria Driianska, and Svitlana Savchenko. 2023. "Gut Microbiota’s Oxalate-Degrading Activity and Its Implications on Cardiovascular Health in Patients with Kidney Failure: A Pilot Prospective Study" Medicina 59, no. 12: 2189. https://doi.org/10.3390/medicina59122189
APA StyleStepanova, N., Tolstanova, G., Aleksandrova, I., Korol, L., Dovbynchuk, T., Driianska, V., & Savchenko, S. (2023). Gut Microbiota’s Oxalate-Degrading Activity and Its Implications on Cardiovascular Health in Patients with Kidney Failure: A Pilot Prospective Study. Medicina, 59(12), 2189. https://doi.org/10.3390/medicina59122189








