Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in Dextran Sulfate Sodium-Induced Murine Model of Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
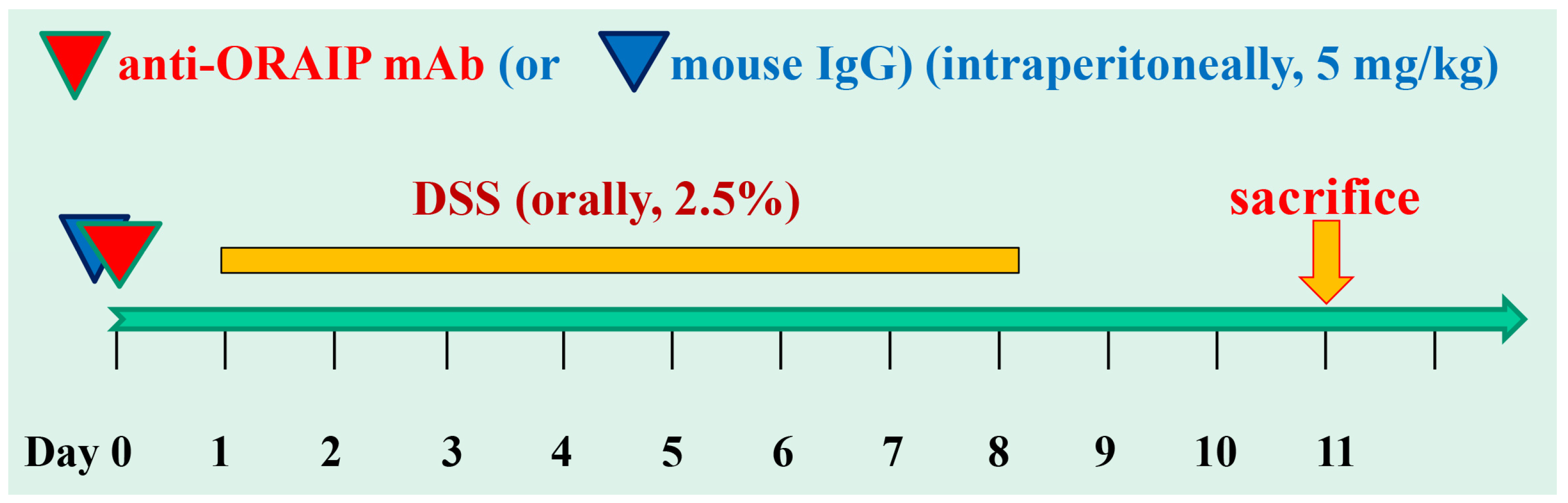
2.2. Dextran Sulfate Sodium (DSS)-Induced Colitis
2.3. Anti-ORAIP mAb
2.4. Immunofluorescence
2.5. TdT (Terminal Deoxynucleotidyl Transferase)-Mediated dUTP Nick End Labeling (TUNEL) Staining
2.6. Statistical Analysis
3. Results
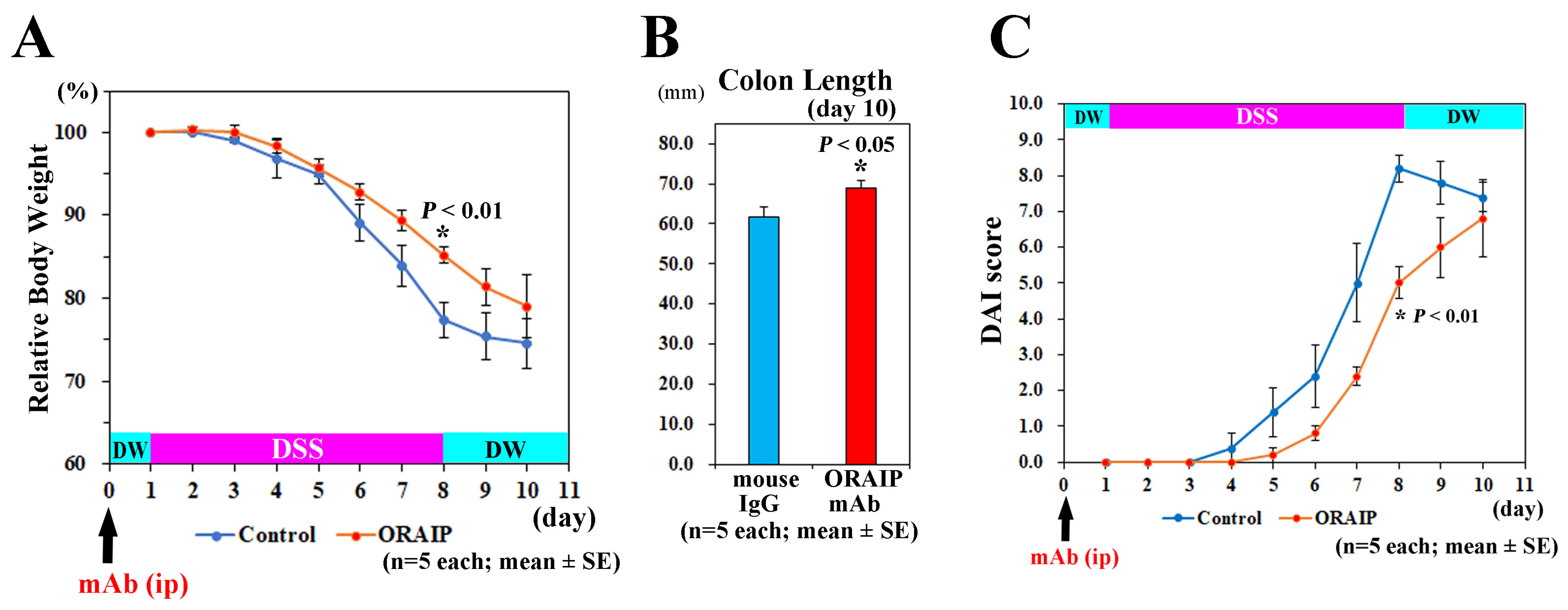
3.1. In Vivo Treatment with Anti-ORAIP mAb Suppressed Body Weight Loss, Colon Shortening, and Disease Activity
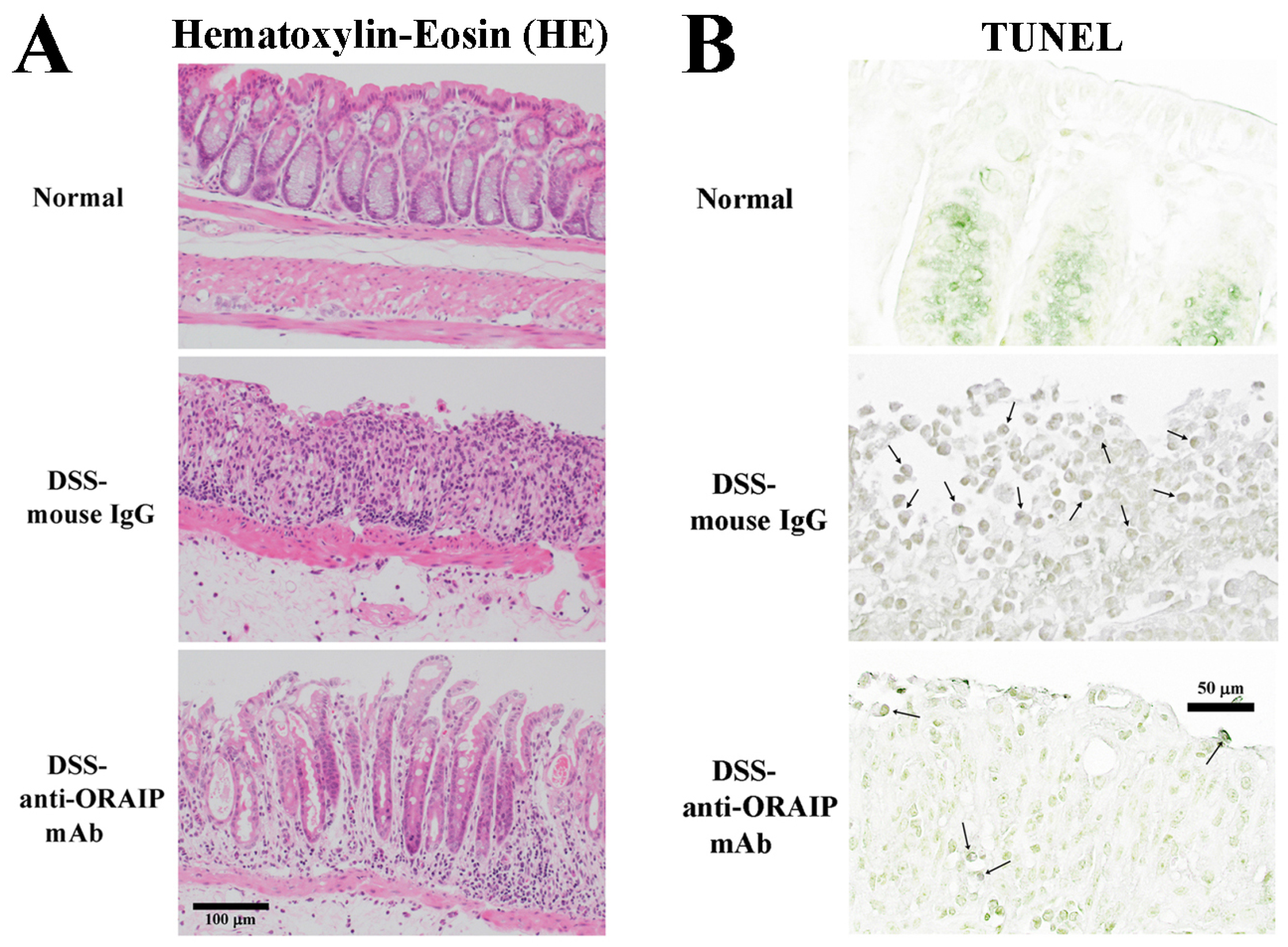
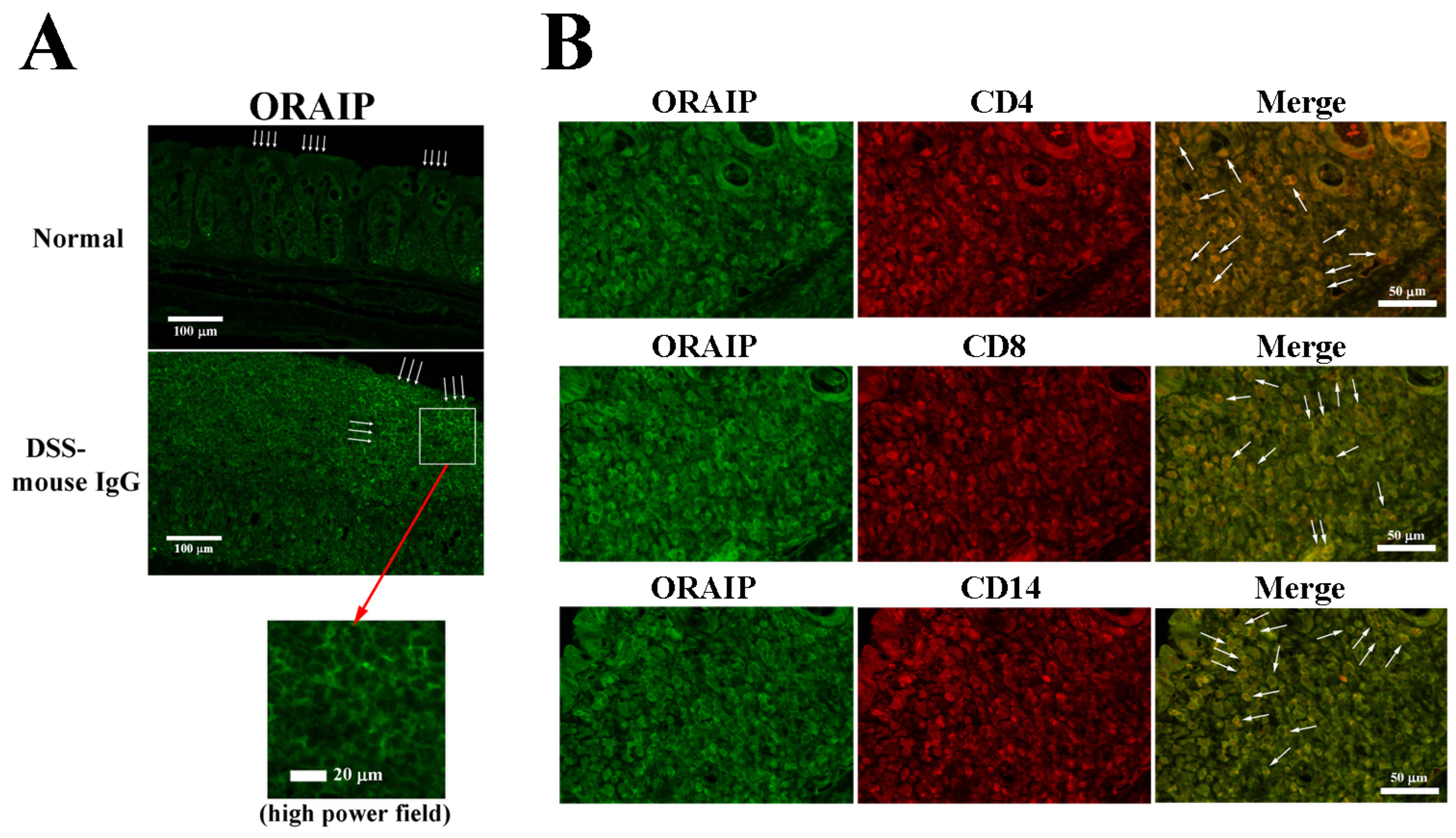
3.2. In Vivo Treatment with Anti-ORAIP mAb Suppressed Inflammation of Rectal Tissue and Apoptosis of IELs
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Shivashankar, R.; Tremaine, W.J.; Harmsen, W.S.; Loftus, E.V., Jr. Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota from 1970 through 2010. Clin. Gastroenterol. Hepatol. 2017, 15, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Nishiwaki, Y.; Oba, M.S.; Asakura, K.; Ohfuji, S.; Fukushima, W.; Suzuki, Y.; Nakamura, Y. Estimated prevalence of ulcerative colitis and Crohn’s disease in Japan in 2014: An analysis of a nationwide survey. J. Gastroenterol. 2019, 54, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Su, C.; Sands, B.E.; D’Haens, G.R.; Vermeire, S.; Schreiber, S.; Danese, S.; Feagan, B.G.; Reinisch, W.; Niezychowski, W.; et al. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Danese, S.; Loftus, E.V., Jr.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): A phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: Results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V., Jr.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef] [PubMed]
- D’haens, G.; Dubinsky, M.; Kobayashi, T.; Irving, P.M.; Howaldt, S.; Pokrotnieks, J.; Krueger, K.; Laskowski, J.; Li, X.; Lissoos, T.; et al. Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2023, 388, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.-F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: Results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet 2022, 399, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Kruidenier, L.; Kuiper, I.; Lamers, C.B.; Verspaget, H.W. Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification localization, and association with mucosal antioxidants. J. Pathol. 2003, 201, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Sadar, S.S.; Vyawahare, N.S.; Bodhankar, S.L. Ferulic acid ameliorates TNBS-induced ulcerative colitis through modulation of cytokines, oxidative stress, inos, cox-2, and apoptosis in laboratory rats. EXCLI J. 2016, 15, 482–499. [Google Scholar] [PubMed]
- Shafik, N.M.; Gaber, R.A.; Mohamed, D.A.; Ebeid, A.M. Hesperidin modulates dextran sulfate sodium-induced ulcerative colitis in rats: Targeting sphingosine kinase-1- sphingosine 1 phosphate signaling pathway, mitochondrial biogenesis, inflammation, and apoptosis. J. Biochem. Mol. Toxicol. 2019, 33, e22312. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, D.; Yang, C.; Chen, Z.; Liu, M. Research on extraction technology, antibacterial and antioxidant activity of ethanol extract from leaves of abutilon theophrasti medic. Acta Pol. Pharm. 2017, 74, 881–890. [Google Scholar] [PubMed]
- Arab, H.H.; Al-Shorbagy, M.Y.; Abdallah, D.M.; Nassar, N.N. Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS ONE 2014, 9, e97193. [Google Scholar] [CrossRef] [PubMed]
- Amrouche-Mekkioui, I.; Djerdjouri, B. N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur. J. Pharmacol. 2012, 691, 209–217. [Google Scholar] [CrossRef]
- Haddad, J.J. Antioxidant and prooxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal 2002, 14, 879–897. [Google Scholar] [CrossRef]
- Gonzalez-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutvin, S.A.; Troost, F.J.; Hamer, H.M.; Lindsey, P.J.; Koek, G.H.; Jonkers, D.M.; Kodde, A.; Venema, K.; Brummer, R.J. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS ONE 2009, 4, e6759. [Google Scholar] [CrossRef] [PubMed]
- Chien, C.T.; Lee, P.H.; Chen, C.F.; Ma, M.C.; Lai, M.K.; Hsu, S.M. De Novo demonstration and co-localization of free-radical production and apoptosis formation in rat kidney subjected to ischemia/reperfusion. J. Am. Soc. Nephrol. 2001, 12, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Hao, Y.; Guan, Y.; Bu, H.; Wang, H. Effects of vitamin D supplementation on blood markers in ulcerative colitis patients: A systematic review and meta-analysis. Eur. J. Nutr. 2020, 61, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Fujimura, T.; Yao, T.; Taka, H.; Mineki, R.; Okumura, K.; Murayama, K. Secreted tyrosine sulfated-eIF5A mediates oxidative stress-induced apoptosis. Sci. Rep. 2015, 5, 13737. [Google Scholar] [CrossRef]
- Kishimoto, M.; Suenaga, J.; Takase, H.; Araki, K.; Yao, T.; Fujimura, T.; Murayama, K.; Okumura, K.; Ueno, R.; Shimizu, N.; et al. Oxidative stress-responsive apoptosis inducing protein (ORAIP) plays a critical role in cerebral ischemia/reperfusion injury. Sci. Rep. 2019, 9, 13512. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Yao, T.; Fujimura, T.; Murayama, K.; Okumura, K.; Hagiwara, N.; Seko, Y. Oxidative stress-responsive apoptosis-inducing protein in patients with heterozygous familial hypercholesterolemia. Heart Vessel. 2021, 36, 1923–1932. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yao, T.; Okumura, K.; Seko, Y.; Kitano, S. Elevation of vitreous body concentrations of oxidative stress-responsive apoptosis inducing protein (ORAIP) in diabetic retinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Shibuya, T.; Sasaki, T.; Lu, Y.J.; Ishikawa, D.; Haga, K.; Takahashi, M.; Kaga, N.; Osada, T.; Sato, N.; et al. Nicotine oral administration attenuates DSS-induced colitis through upregulation of indole in the distal colon and rectum in mice. Front. Med. 2021, 8, 789037. [Google Scholar] [CrossRef]
- Horiguchi, T.; Shimizu, K.; Ogino, M.; Suga, S.; Inamasu, J.; Kawase, T. Postischemic hypothermia inhibits the generation of hydroxyl radical following ransient forebrain ischemia in rats. J. Neurotrauma 2003, 20, 511–520. [Google Scholar] [CrossRef]
- Piechota-Polanczyk, A.; Fichna, J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 605–620. [Google Scholar] [CrossRef]
- Yao, T.; Fujimura, T.; Murayama, K.; Okumura, K.; Seko, Y. Oxidative stress-responsive apoptosis inducing protein (ORAIP) plays a critical role in doxorubicin-induced apoptosis in rat cardiac myocytes. Int. J. Cardiol. 2022, 348, 119–124. [Google Scholar] [CrossRef]
- Jena, G.; Trivedi, P.P.; Sandala, B. Oxidative stress in ulcerative colitis: An old concept but a new concern. Free Radic. Res. 2012, 46, 1339–1345. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease: Mechanisms and management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef]
- Ohnishi, S.; Ma, N.; Thanan, R.; Pinlaor, S.; Hammam, O.; Murata, M.; Kawanishi, S. DNA damage in inflammation-related carcinogenesis and cancer stem cells. Oxid. Med. Cell. Longev. 2013, 2013, 87014. [Google Scholar] [CrossRef]
- Srinivasa, U.S.; Tan, B.W.Q.; Vellayappanb, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Nakamura, N.; Takada, A. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Wen, S.-Y.; Ali, A.; Huang, I.-C.; Liu, J.-S.; Chen, P.-Y.; Viswanadha, V.P.; Huang, C.-Y.; Kuo, W.-W. Doxorubicin induced ROS-dependent HIF1α activation mediates blockage of IGF1R survival signaling by IGFBP3 promotes cardiac apoptosis. Aging 2023, 15, 164–178. [Google Scholar] [CrossRef]
- Gros, B.; Kaplan, G.G. Ulcerative colitis in adults: A review. JAMA 2023, 330, 951–965. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakajima, A.; Shibuya, T.; Yao, T.; Fujimura, T.; Murayama, K.; Okumura, K.; Nagahara, A.; Seko, Y. Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in Dextran Sulfate Sodium-Induced Murine Model of Ulcerative Colitis. Medicina 2024, 60, 539. https://doi.org/10.3390/medicina60040539
Nakajima A, Shibuya T, Yao T, Fujimura T, Murayama K, Okumura K, Nagahara A, Seko Y. Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in Dextran Sulfate Sodium-Induced Murine Model of Ulcerative Colitis. Medicina. 2024; 60(4):539. https://doi.org/10.3390/medicina60040539
Chicago/Turabian StyleNakajima, Akihito, Tomoyoshi Shibuya, Takako Yao, Tsutomu Fujimura, Kimie Murayama, Ko Okumura, Akihito Nagahara, and Yoshinori Seko. 2024. "Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in Dextran Sulfate Sodium-Induced Murine Model of Ulcerative Colitis" Medicina 60, no. 4: 539. https://doi.org/10.3390/medicina60040539
APA StyleNakajima, A., Shibuya, T., Yao, T., Fujimura, T., Murayama, K., Okumura, K., Nagahara, A., & Seko, Y. (2024). Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in Dextran Sulfate Sodium-Induced Murine Model of Ulcerative Colitis. Medicina, 60(4), 539. https://doi.org/10.3390/medicina60040539





