Regeneration of Rabbit Calvarial Defects with Combination of Stem Cells and Enamel Matrix Derivative: A Microcomputed Tomography and Histological Evaluation Comparing Two- and Three-Dimensional Cell Constructs
Abstract
1. Introduction
2. Materials and Methods
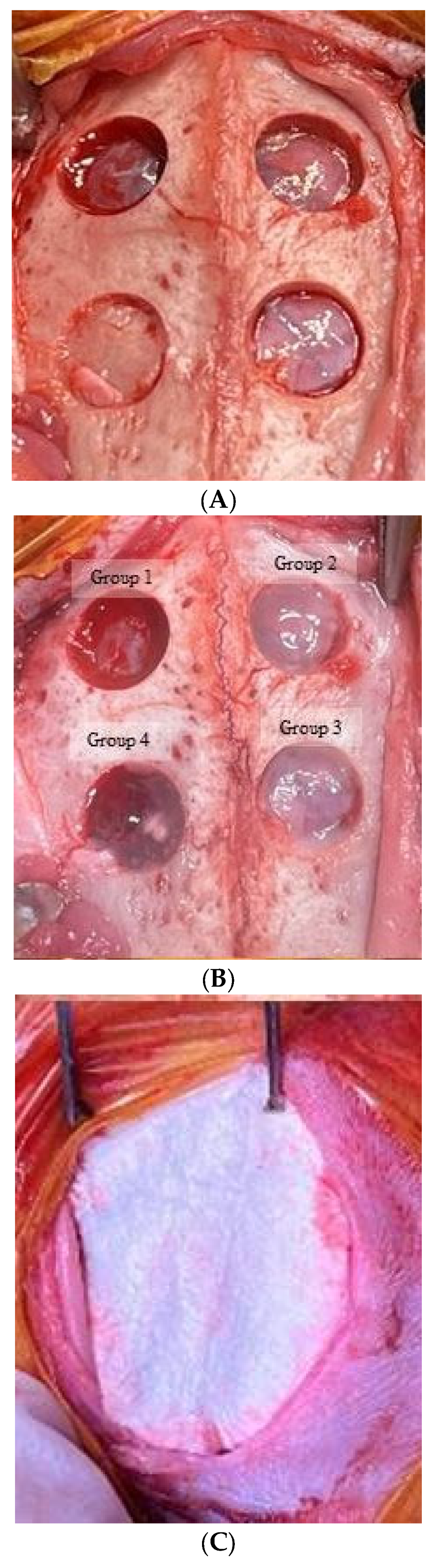
2.1. Manufacturing Stem cell Spheroids and Designing Animal Model and Groups
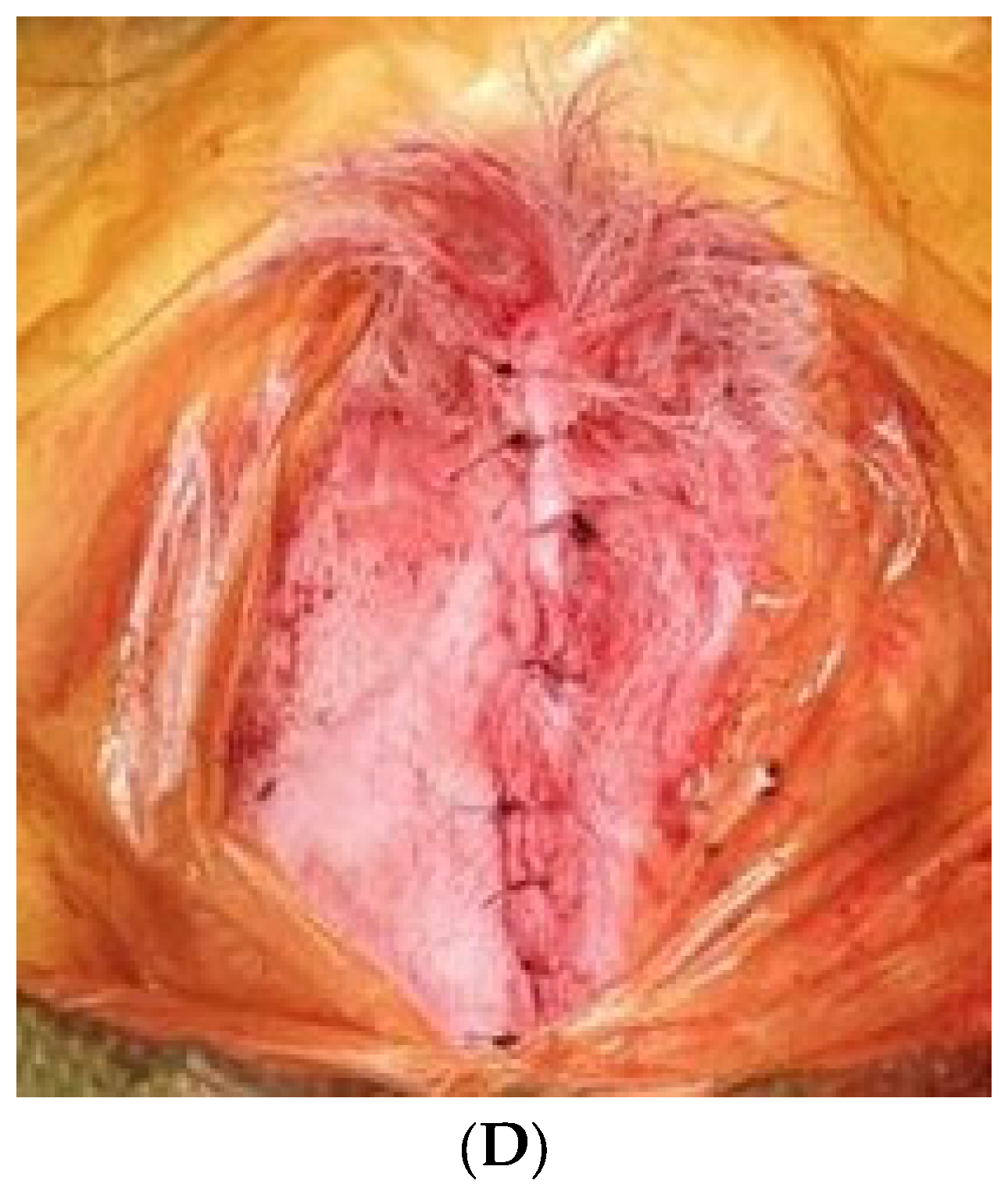
2.2. Surgical Protocol
2.3. Reconstruction of Computed Tomography Image and Measurements
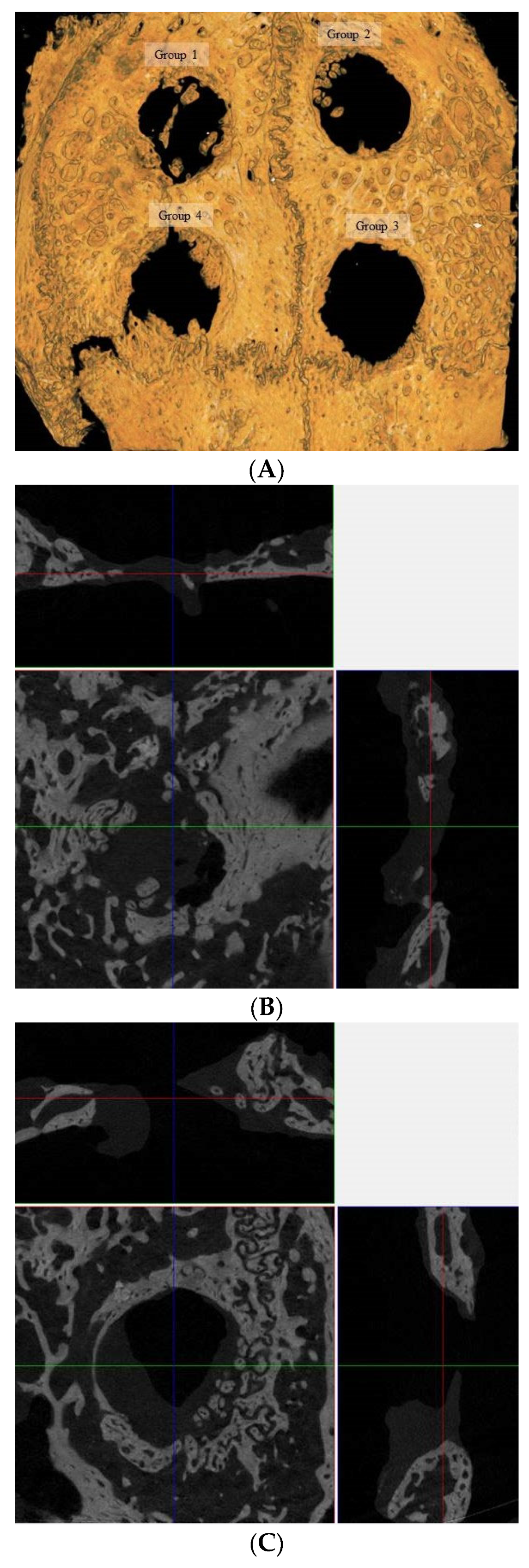
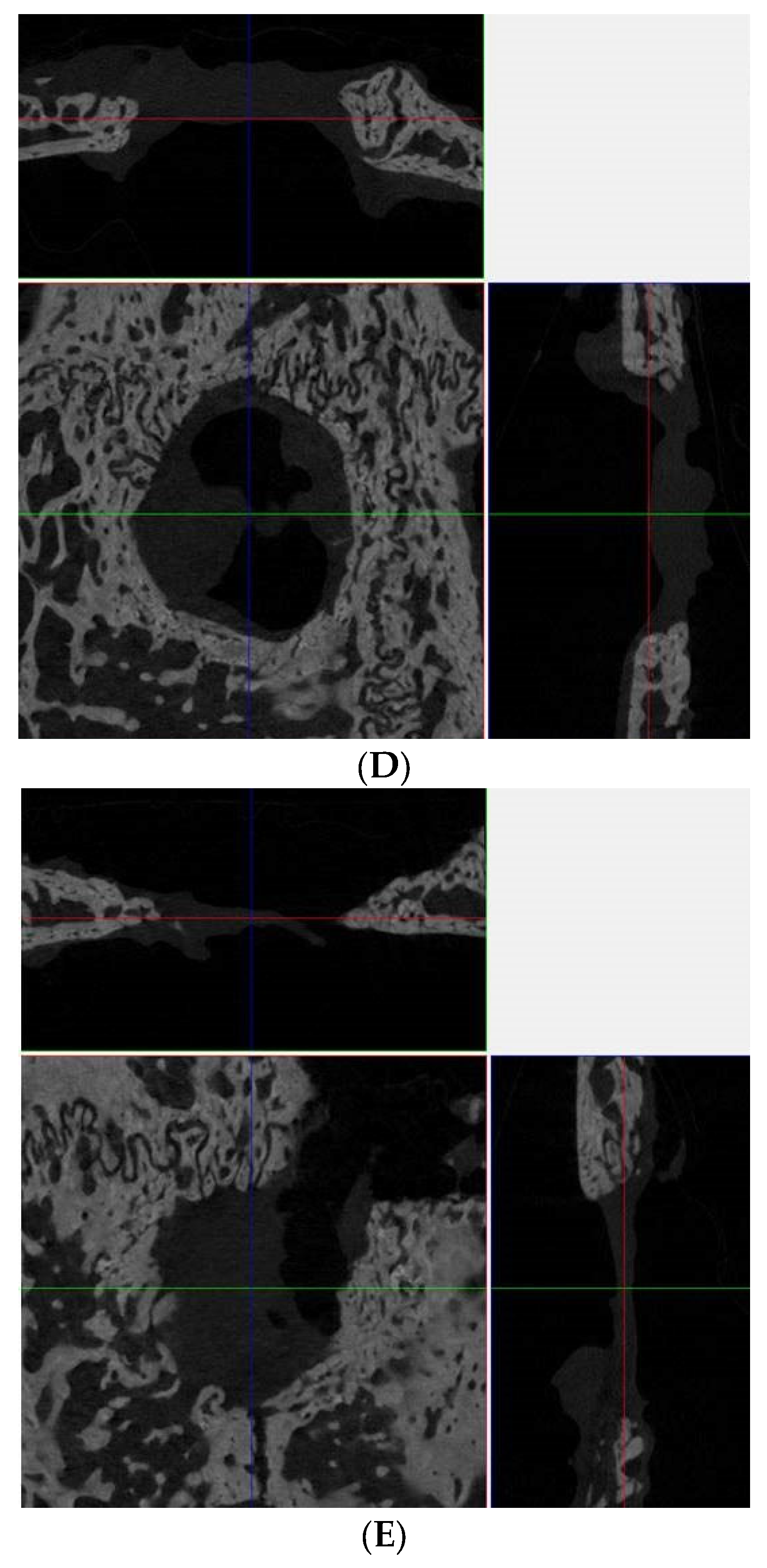
2.4. Microcomputed Tomography Analysis
2.5. Immunohistochemical Staining Process and Histological Assessment
2.6. Statistical Analysis
3. Results
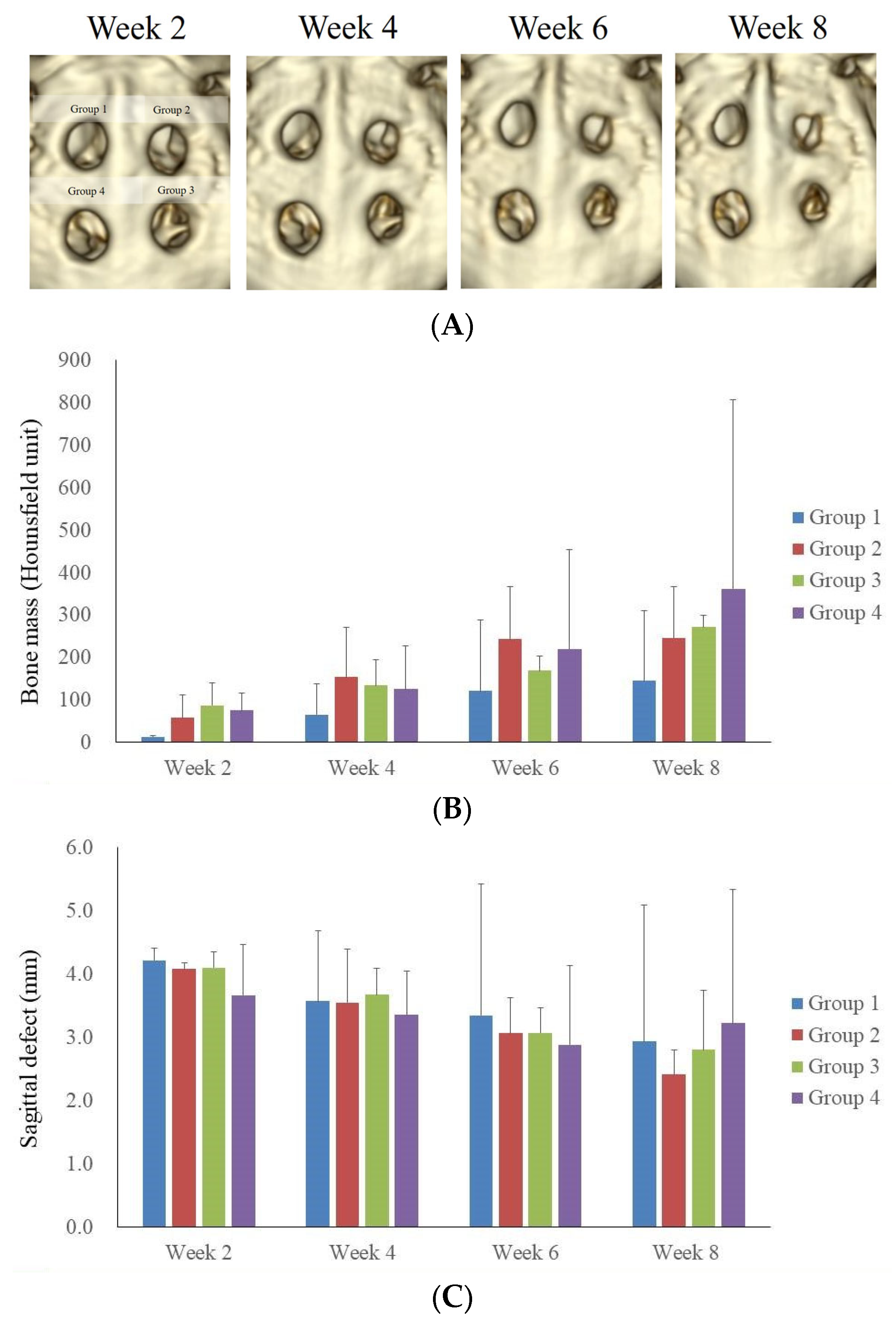
3.1. Computed Tomographic Evaluation
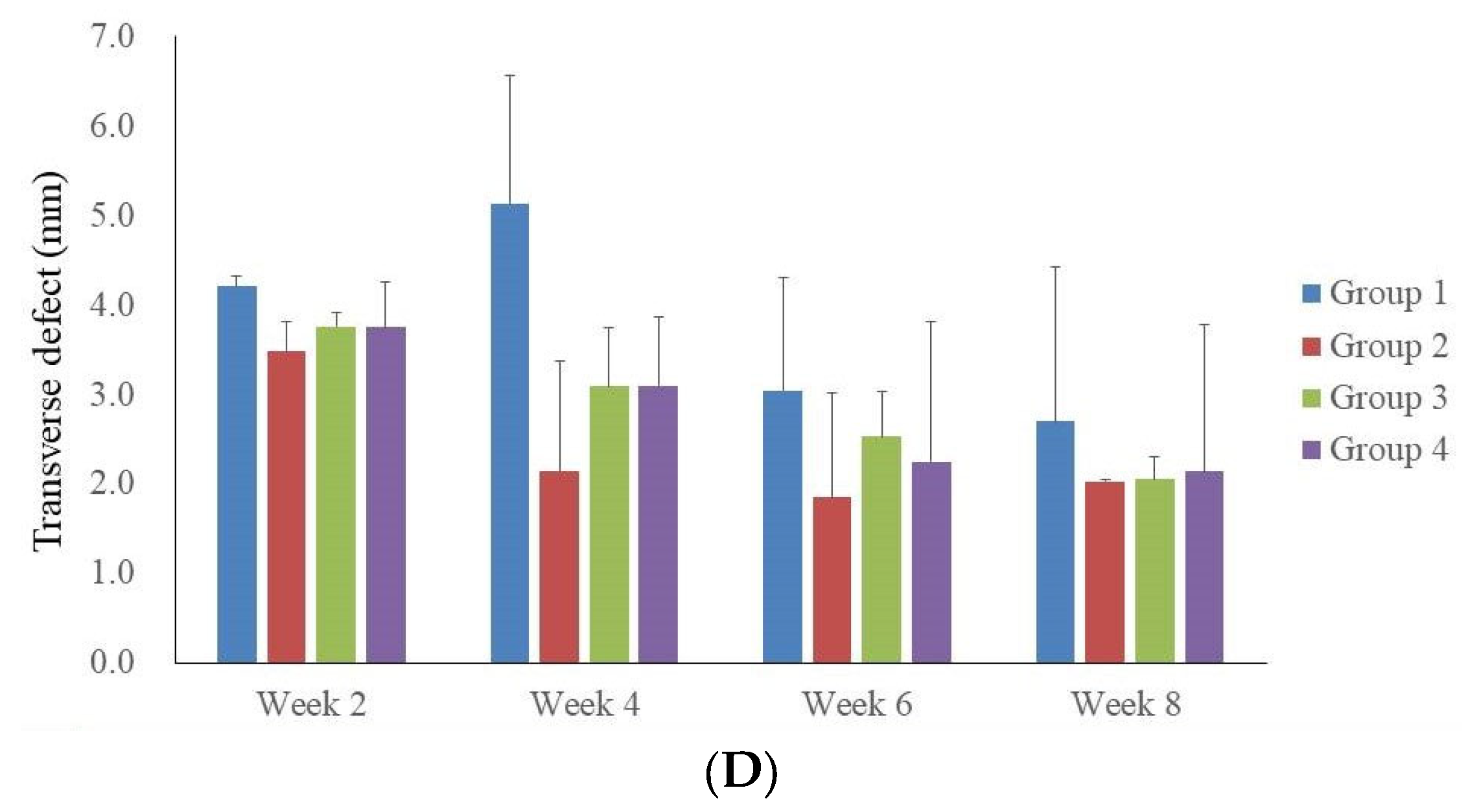
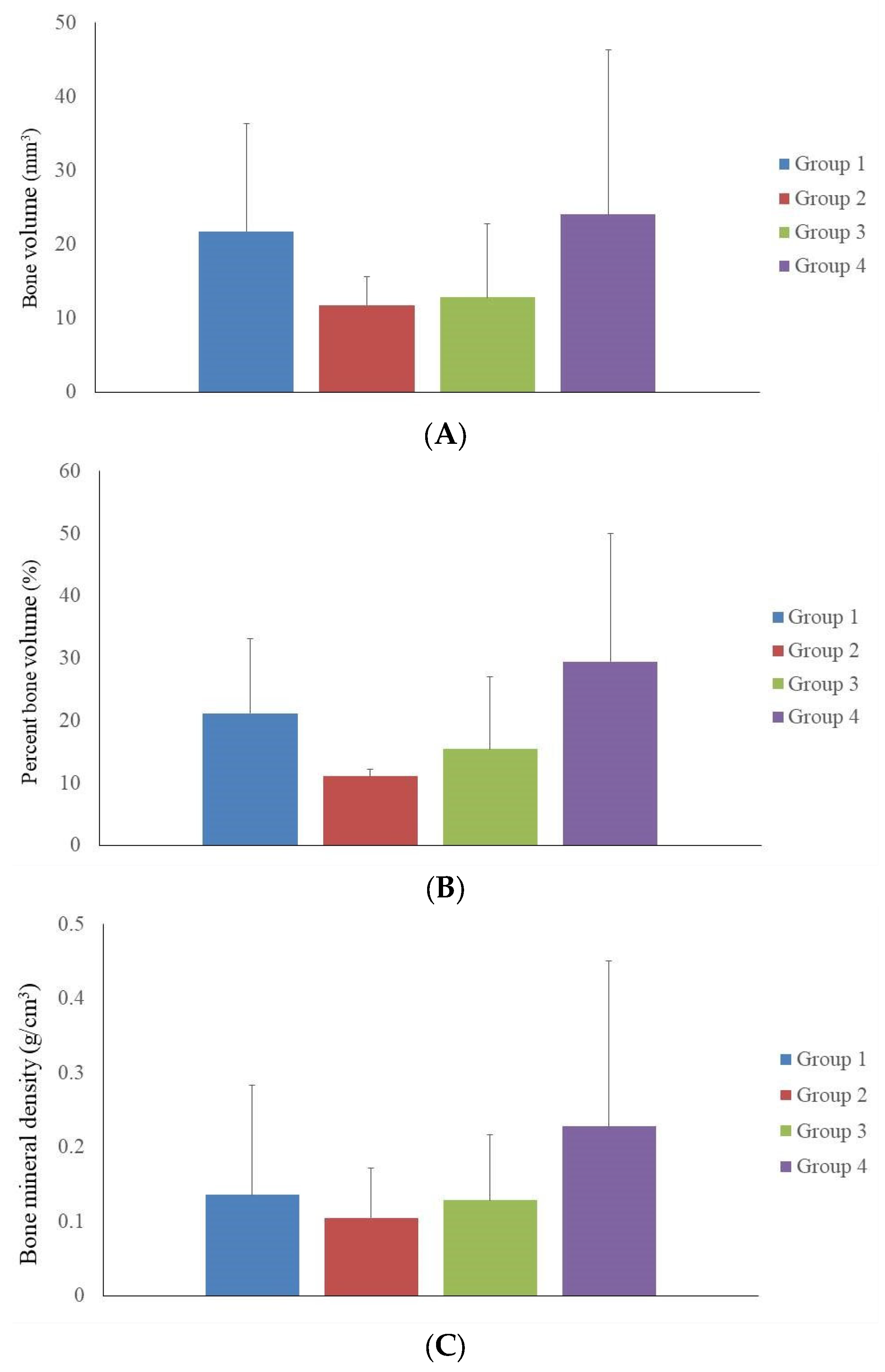
3.2. Microcomputed Tomographic Measurement
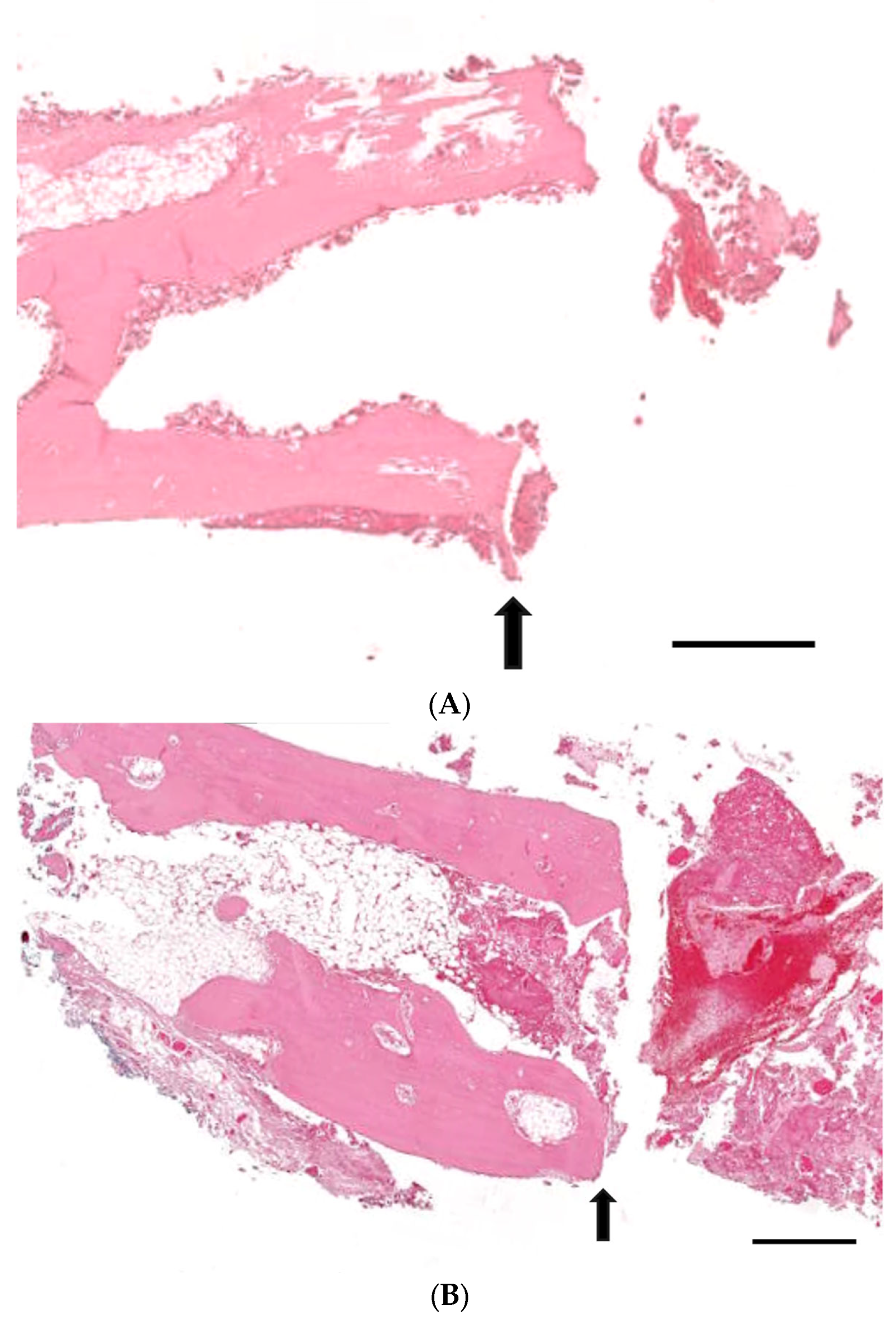
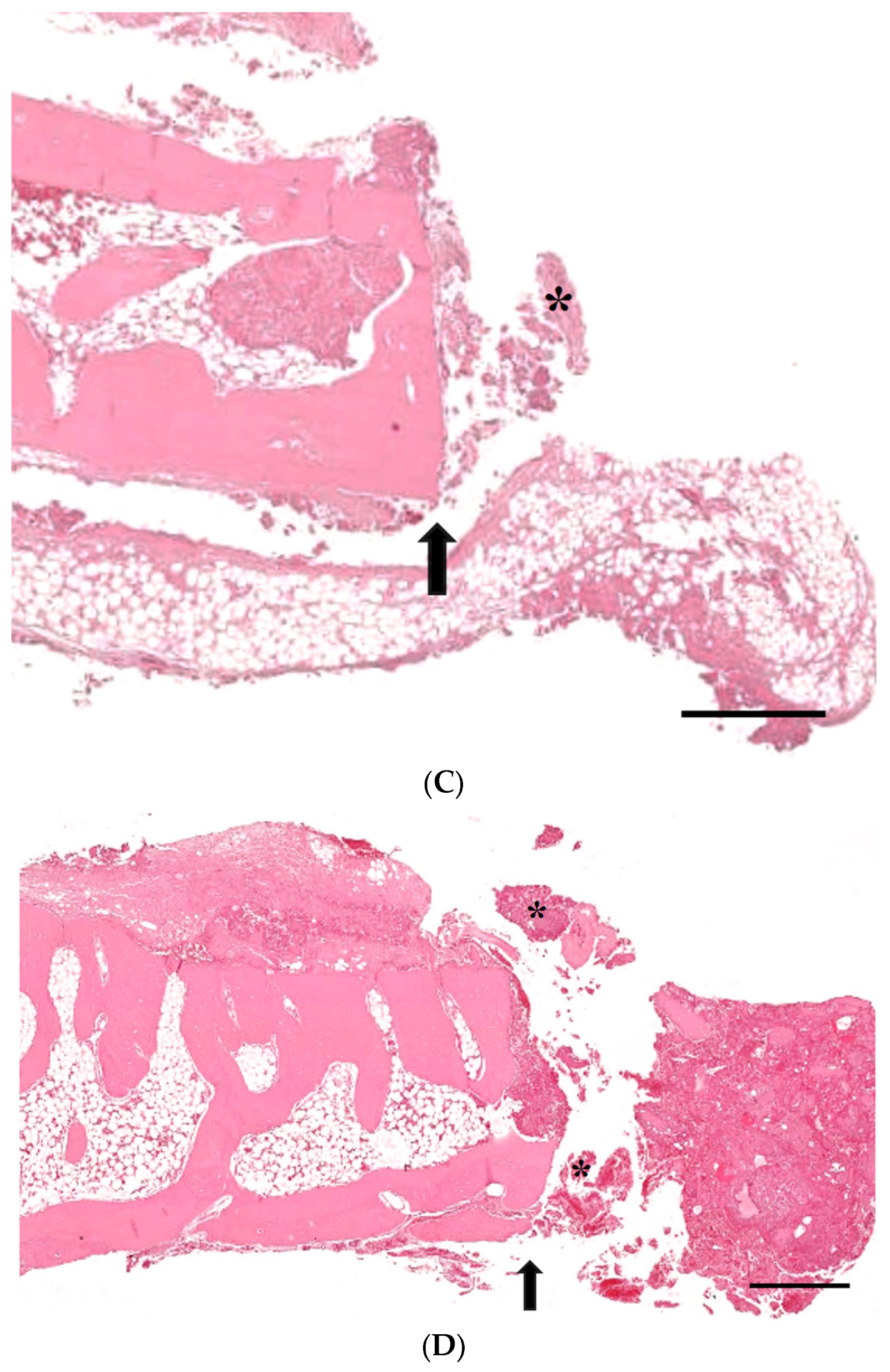
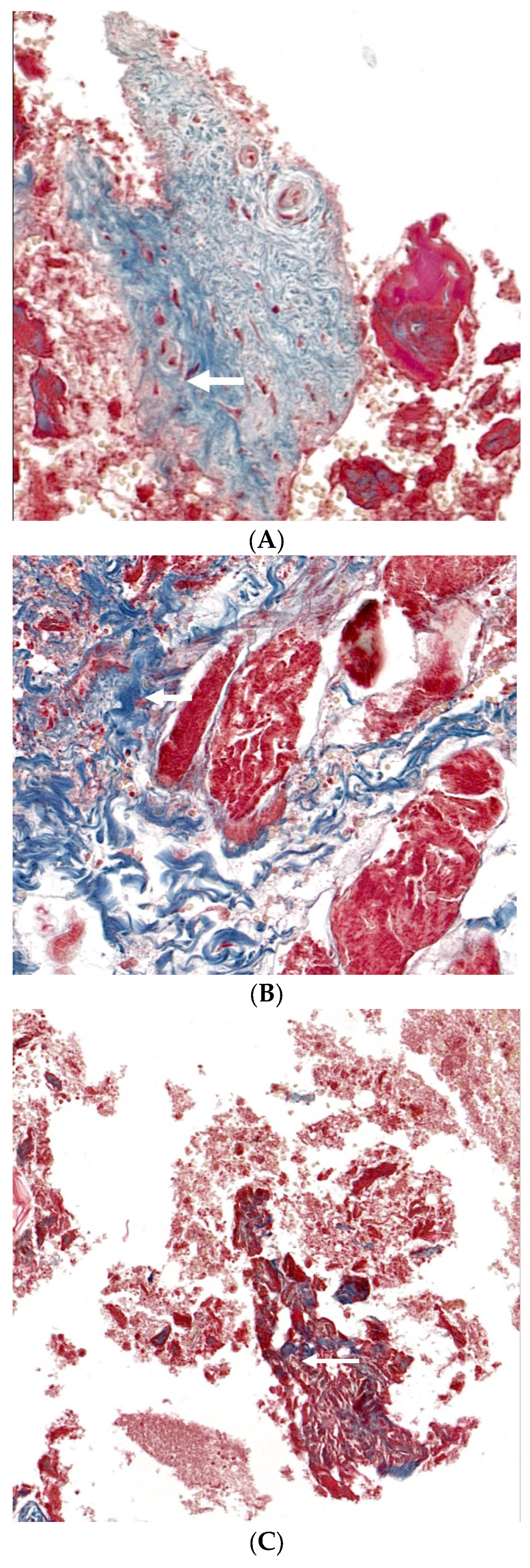
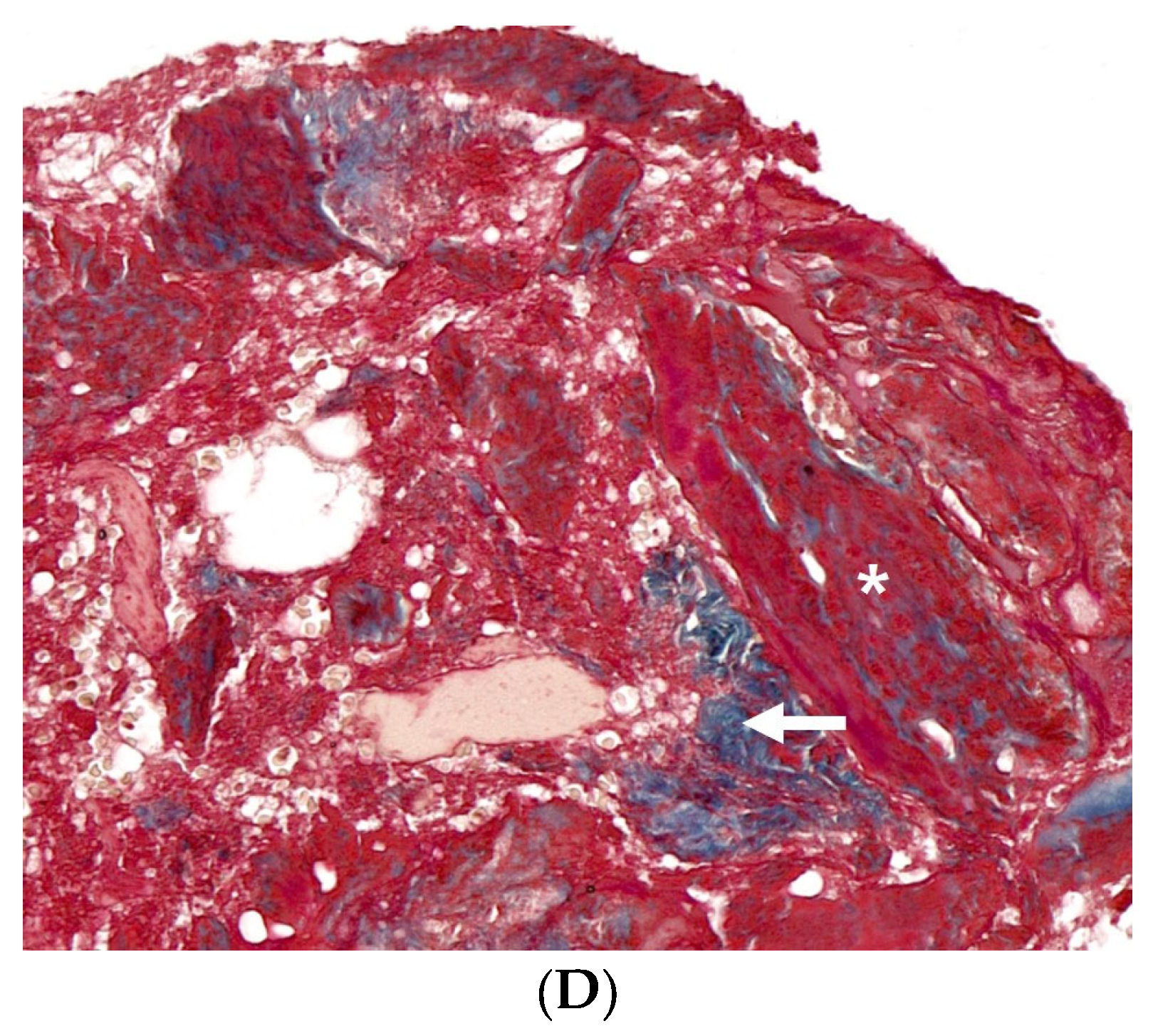
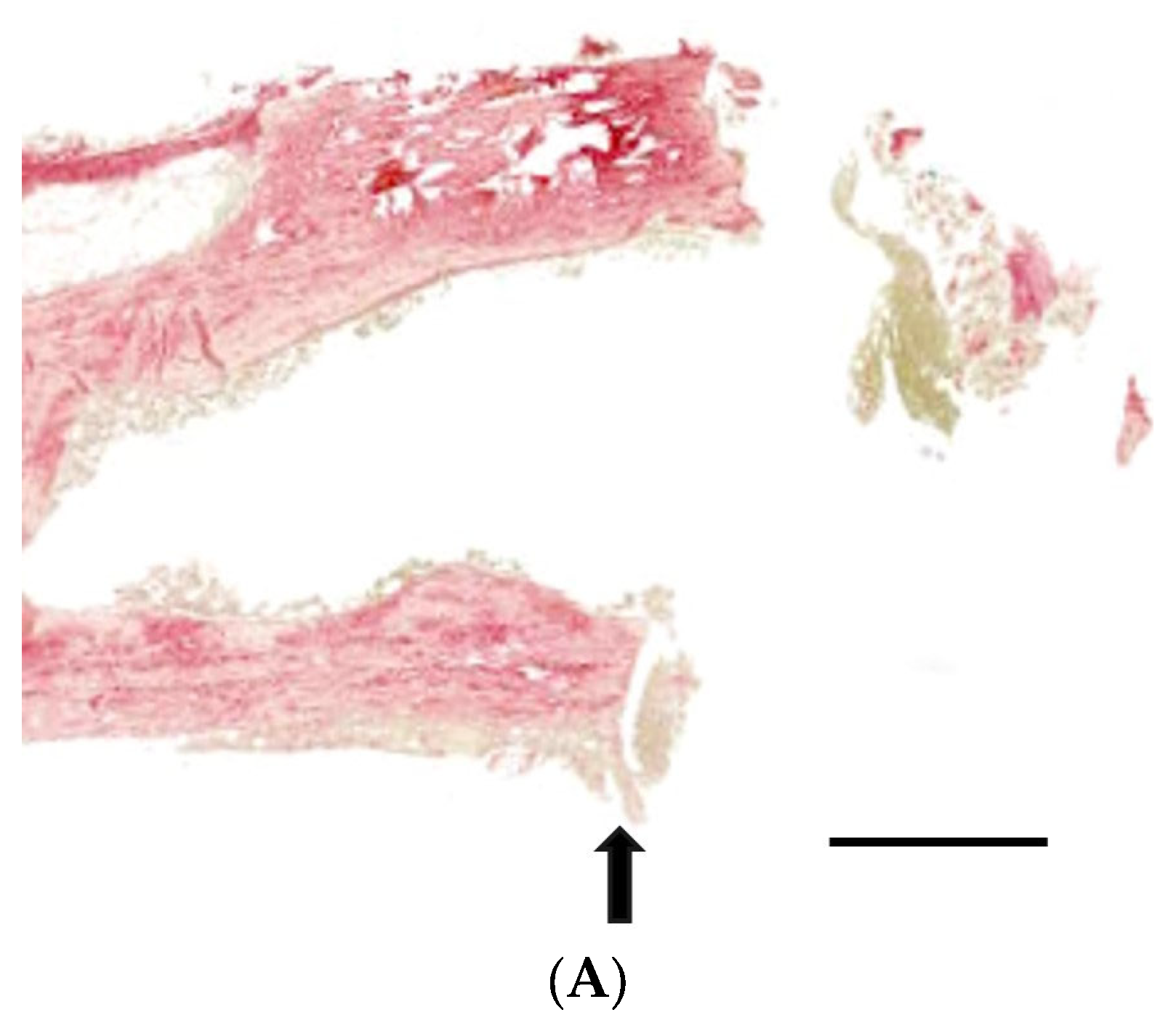
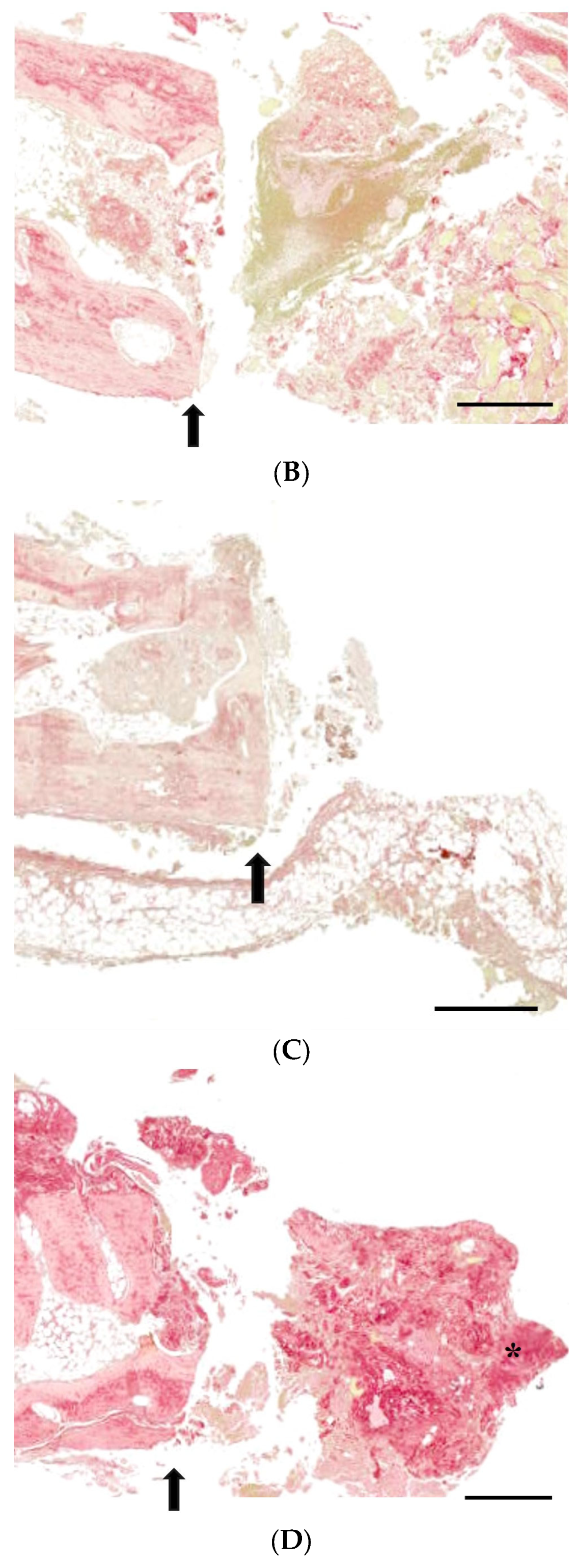
3.3. Histological Analysis with Hematoxylin and Eosin, Masson’s Trinchrome, and Picro-Sirius Red Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zeng, A.; Li, H.; Liu, J.; Wu, M. The Progress of Decellularized Scaffold in Stomatology. Tissue Eng. Regen. Med. 2022, 19, 451–461. [Google Scholar] [CrossRef]
- Grawish, M.E.; Grawish, L.M.; Grawish, H.M.; Grawish, M.M.; Holiel, A.A.; Sultan, N.; El-Negoly, S.A. Demineralized Dentin Matrix for Dental and Alveolar Bone Tissues Regeneration: An Innovative Scope Review. Tissue Eng. Regen. Med. 2022, 19, 687–701. [Google Scholar] [CrossRef]
- Alrayyes, Y.; Al-Jasser, R. Regenerative Potential of Platelet Rich Fibrin (PRF) in Socket Preservation in Comparison with Conventional Treatment Modalities: A Systematic Review and Meta-Analysis. Tissue Eng. Regen. Med. 2022, 19, 463–475. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Li, Y.; Mao, X.; Liu, R.; Qi, Y.; Lee, E.-S.; Jiang, H.B. Clinical Reference Strategy for the Selection of Treatment Materials for Maxillofacial Bone Transplantation: A Systematic Review and Network Meta-Analysis. Tissue Eng. Regen. Med. 2022, 19, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: A paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Laubach, M.; Hildebrand, F.; Suresh, S.; Wagels, M.; Kobbe, P.; Gilbert, F.; Kneser, U.; Holzapfel, B.M.; Hutmacher, D.W. The Concept of Scaffold-Guided Bone Regeneration for the Treatment of Long Bone Defects: Current Clinical Application and Future Perspective. J. Funct. Biomater. 2023, 14, 341. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Khatun, M.R.; Narmatha, S.; Nagarajan, R.; Noh, I. Modulation of 3D Bioprintability in Polysaccharide Bioink by Bioglass Nanoparticles and Multiple Metal Ions for Tissue Engineering. Tissue Eng. Regen. Med. 2023, 21, 261–275. [Google Scholar] [CrossRef]
- Park, J.B.; Kim, I.; Lee, W.; Kim, H. Evaluation of the regenerative capacity of stem cells combined with bone graft material and collagen matrix using a rabbit calvarial defect model. J. Periodontal Implant Sci. 2023, 53, 467–477. [Google Scholar] [CrossRef]
- Lee, H.J.; Na, K.H.; Uddin, M.S.; Park, J.B. Assessment of the Impacts of Centipeda minima (L.) on Cell Viability, and Osteogenic Differentiation of Mesenchymal Stem Cell Spheroids. Medicina 2022, 59, 43. [Google Scholar] [CrossRef]
- Park, J.B. Application of enamel matrix derivative and deproteinized bovine bone for the treatment of peri-implantitis after decontamination with an ultrasonic scaler: A case report. Medicine 2018, 97, e13461. [Google Scholar] [CrossRef]
- Park, J.B. The use of enamel matrix derivative for the treatment of the apically involved tooth: A case report. Medicine 2019, 98, e18115. [Google Scholar] [CrossRef]
- Hwa, S.; Lee, H.J.; Ko, Y.; Park, J.B. Effects of Enamel Matrix Derivative on Cell Spheroids Made of Stem Cells Obtained from the Gingiva on Osteogenic Differentiation. Medicina 2023, 59, 377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Ko, Y.; Park, J.B. Evaluation of the osteogenic differentiation of gingiva-derived stem cells grown on culture plates or in stem cell spheroids: Comparison of two- and three-dimensional cultures. Exp. Ther. Med. 2017, 14, 2434–2438. [Google Scholar] [CrossRef]
- Lee, D.H.; Bhang, S.H. Development of Hetero-Cell Type Spheroids Via Core-Shell Strategy for Enhanced Wound Healing Effect of Human Adipose-Derived Stem Cells. Tissue Eng. Regen. Med. 2023, 20, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, H.; Na, C.B.; Song, I.S.; Ryu, J.J.; Park, J.B. Evaluation of the Age- and Sex-Related Changes of the Osteogenic Differentiation Potentials of Healthy Bone Marrow-Derived Mesenchymal Stem Cells. Medicina 2021, 57, 520. [Google Scholar] [CrossRef]
- Kang, S.H.; Park, J.B.; Kim, I.; Lee, W.; Kim, H. Assessment of stem cell viability in the initial healing period in rabbits with a cranial bone defect according to the type and form of scaffold. J. Periodontal Implant Sci. 2019, 49, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Yoo, J.-H.; Choi, S.-H.; Lee, M.-H.; Lee, S.-J.; Song, S.U.; Oh, N.-S. Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration of calvarial defects in rabbits. Tissue Eng. Regen. Med. 2012, 9, 17–23. [Google Scholar] [CrossRef]
- Fazeli, N.; Arefian, E.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Accelerated reconstruction of rat calvaria bone defect using 3D-printed scaffolds coated with hydroxyapatite/bioglass. Sci. Rep. 2023, 13, 12145. [Google Scholar] [CrossRef]
- Kouroupis, D.; Correa, D. Increased Mesenchymal Stem Cell Functionalization in Three-Dimensional Manufacturing Settings for Enhanced Therapeutic Applications. Front. Bioeng. Biotechnol. 2021, 9, 621748. [Google Scholar] [CrossRef]
- Bou-Ghannam, S.; Kim, K.; Grainger, D.W.; Okano, T. 3D cell sheet structure augments mesenchymal stem cell cytokine production. Sci. Rep. 2021, 11, 8170. [Google Scholar] [CrossRef] [PubMed]
- Lyngstadaas, S.P.; Wohlfahrt, J.C.; Brookes, S.J.; Paine, M.L.; Snead, M.L.; Reseland, J.E. Enamel matrix proteins; old molecules for new applications. Orthod. Craniofacial Res. 2009, 12, 243–253. [Google Scholar] [CrossRef]
- Bajaj, V.A.; Kolte, A.P.; Kolte, R.A. Efficacy of bone graft as monotherapy and in combination with platelet concentrates in grade II furcation defect—A systematic review and meta-analysis. Saudi Dent. J. 2022, 34, 637–646. [Google Scholar] [CrossRef]
- Ivanovski, S. Periodontal regeneration. Aust. Dent. J. 2009, 54 (Suppl. 1), S118–S128. [Google Scholar] [CrossRef]
- Abtahi, S.; Chen, X.; Shahabi, S.; Nasiri, N. Resorbable Membranes for Guided Bone Regeneration: Critical Features, Potentials, and Limitations. ACS Mater. Au. 2023, 3, 394–417. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, P.; Wang, H.L. Collagen membranes: A review. J. Periodontol. 2001, 72, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Verna, C.; Dalstra, M.; Wikesjö, U.M.; Trombelli, L. Healing patterns in calvarial bone defects following guided bone regeneration in rats. A micro-CT scan analysis. J. Clin. Periodontol. 2002, 29, 865–870. [Google Scholar] [CrossRef]
- Alqahtani, A.M. Guided Tissue and Bone Regeneration Membranes: A Review of Biomaterials and Techniques for Periodontal Treatments. Polymers 2023, 15, 3355. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Li, J.; Jin, F.; Wang, R.; Shang, X.; Yang, P.; Zhu, Y.; Tsoi, J.K.H.; Chan, K.; Wang, S. Guided Bone Regeneration in a Periodontally Compromised Individual with Autogenous Tooth Bone Graft: A Radiomics Analysis. J. Funct. Biomater. 2023, 14, 220. [Google Scholar] [CrossRef]
- Dogan, N.; Okcu, K.M.; Ortakoglu, K.; Dalkiz, M.; Gunaydin, Y. Barrier membrane and bone graft treatments of dehiscence-type defect at existing implant: A case report. Implant Dent. 2003, 12, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Rodella, L.F.; Favero, G.; Labanca, M. Biomaterials in maxillofacial surgery: Membranes and grafts. Int. J. Biomed. Sci. IJBS 2011, 7, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hürzeler, M.B.; Kohal, R.J.; Naghshbandl, J.; Mota, L.F.; Conradt, J.; Hutmacher, D.; Caffesse, R.G. Evaluation of a new bioresorbable barrier to facilitate guided bone regeneration around exposed implant threads. An experimental study in the monkey. Int. J. Oral Maxillofac. Surg. 1998, 27, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Undale, A.H.; Westendorf, J.J.; Yaszemski, M.J.; Khosla, S. Mesenchymal stem cells for bone repair and metabolic bone diseases. Mayo Clin. Proc. 2009, 84, 893–902. [Google Scholar] [CrossRef] [PubMed]
- El-Husseiny, H.M.; Mady, E.A.; Hamabe, L.; Abugomaa, A.; Shimada, K.; Yoshida, T.; Tanaka, T.; Yokoi, A.; Elbadawy, M.; Tanaka, R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio 2022, 13, 100186. [Google Scholar] [CrossRef]
- Manzini, B.M.; Machado, L.M.R.; Noritomi, P.Y.; da Silva, J.V.L. Advances in Bone tissue engineering: A fundamental review. J. Biosci. 2021, 46, 17. [Google Scholar] [CrossRef]
- Schmitz, J.P.; Hollinger, J.O. The critical size defect as an experimental model for craniomandibulofacial nonunions. Clin. Orthop. Relat. Res. 1986, 299–308. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Critical size defects for bone regeneration experiments in rabbit calvariae: Systematic review and quality evaluation using ARRIVE guidelines. Clin. Oral Implant Res. 2015, 26, 915–930. [Google Scholar] [CrossRef]
- Schlund, M.; Depeyre, A.; Kotagudda Ranganath, S.; Marchandise, P.; Ferri, J.; Chai, F. Rabbit calvarial and mandibular critical-sized bone defects as an experimental model for the evaluation of craniofacial bone tissue regeneration. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 601–609. [Google Scholar] [CrossRef]
- Gil, L.F.; Nayak, V.V.; Benalcázar Jalkh, E.B.; Tovar, N.; Chiu, K.J.; Salas, J.C.; Marin, C.; Bowers, M.; Freitas, G.; Mbe Fokam, D.C.; et al. Laddec® versus Bio-Oss®: The effect on the healing of critical-sized defect–Calvaria rabbit model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2744–2750. [Google Scholar] [CrossRef]
- Sohn, J.-Y.; Park, J.-C.; Um, Y.-J.; Jung, U.-W.; Kim, C.-S.; Cho, K.-S.; Choi, S.-H. Spontaneous healing capacity of rabbit cranial defects of various sizes. J. Periodontal Implant Sci. 2010, 40, 180–187. [Google Scholar] [CrossRef]
- Umoh, J.U.; Sampaio, A.V.; Welch, I.; Pitelka, V.; Goldberg, H.A.; Underhill, T.M.; Holdsworth, D.W. In vivo micro-CT analysis of bone remodeling in a rat calvarial defect model. Phys. Med. Biol. 2009, 54, 2147–2161. [Google Scholar] [CrossRef] [PubMed]
- Cengiz, I.F.; Oliveira, J.M.; Reis, R.L. Micro-CT–A digital 3D microstructural voyage into scaffolds: A systematic review of the reported methods and results. Biomater. Res. 2018, 22, 26. [Google Scholar] [CrossRef] [PubMed]
- Ezzahmouly, M.; Elmoutaouakkil, A.; Ed-Dhahraouy, M.; Khallok, H.; Elouahli, A.; Mazurier, A.; El Albani, A.; Hatim, Z. Micro-computed tomographic and SEM study of porous bioceramics using an adaptive method based on the mathematical morphological operations. Heliyon 2019, 5, e02557. [Google Scholar] [CrossRef]
- Keklikoglou, K.; Arvanitidis, C.; Chatzigeorgiou, G.; Chatzinikolaou, E.; Karagiannidis, E.; Koletsa, T.; Magoulas, A.; Makris, K.; Mavrothalassitis, G.; Papanagnou, E.-D.; et al. Micro-CT for Biological and Biomedical Studies: A Comparison of Imaging Techniques. J. Imaging 2021, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Kessler, H.P. Masson trichrome stain helps differentiate myofibroma from smooth muscle lesions in the head and neck region. J. Formos. Med. Assoc. 2008, 107, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Yuan, X.; Li, X.; Liu, Y.; Zhou, J. Bone regeneration of mouse critical-sized calvarial defects with human mesenchymal stem cell sheets co-expressing BMP2 and VEGF. J. Dent. Sci. 2023, 18, 135–144. [Google Scholar] [CrossRef]
- Kim, T.-Y.; Park, J.-K.; Aryal, Y.P.; Lee, E.-S.; Neupane, S.; Sung, S.; Pokharel, E.; Yeon, C.-Y.; Kim, J.-Y.; Jung, J.-K.; et al. Facilitation of Bone Healing Processes Based on the Developmental Function of Meox2 in Tooth Loss Lesion. Int. J. Mol. Sci. 2020, 21, 8701. [Google Scholar] [CrossRef] [PubMed]
- Hiraki, T.; Kunimatsu, R.; Nakajima, K.; Abe, T.; Yamada, S.; Rikitake, K.; Tanimoto, K. Stem cell-derived conditioned media from human exfoliated deciduous teeth promote bone regeneration. Oral Dis. 2020, 26, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Keshav Narayan, A.; Logith Kumar, R.; Viji Chandran, S.; Vairamani, M.; Selvamurugan, N. Proliferation and differentiation of mesenchymal stem cells on scaffolds containing chitosan, calcium polyphosphate and pigeonite for bone tissue engineering. Cell Prolif. 2018, 51, e12408. [Google Scholar] [CrossRef] [PubMed]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2014, 62, 751–758. [Google Scholar] [CrossRef] [PubMed]
- De Padilla, C.M.L.; Coenen, M.J.; Tovar, A.; De la Vega, R.E.; Evans, C.H.; Müller, S.A. Picrosirius Red Staining: Revisiting Its Application to the Qualitative and Quantitative Assessment of Collagen Type I and Type III in Tendon. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2021, 69, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Greiner, C.; Grainger, S.; Farrow, S.; Davis, A.; Su, J.L.; Saybolt, M.D.; Wilensky, R.; Madden, S.; Sum, S.T. Robust quantitative assessment of collagen fibers with picrosirius red stain and linearly polarized light as demonstrated on atherosclerotic plaque samples. PLoS ONE 2021, 16, e0248068. [Google Scholar] [CrossRef]
- Rittié, L. Method for Picrosirius Red-Polarization Detection of Collagen Fibers in Tissue Sections. Methods Mol. Biol. 2017, 1627, 395–407. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, K.-H.; Lee, H.-J.; Lee, J.-E.; Park, J.-B. Regeneration of Rabbit Calvarial Defects with Combination of Stem Cells and Enamel Matrix Derivative: A Microcomputed Tomography and Histological Evaluation Comparing Two- and Three-Dimensional Cell Constructs. Medicina 2024, 60, 451. https://doi.org/10.3390/medicina60030451
Na K-H, Lee H-J, Lee J-E, Park J-B. Regeneration of Rabbit Calvarial Defects with Combination of Stem Cells and Enamel Matrix Derivative: A Microcomputed Tomography and Histological Evaluation Comparing Two- and Three-Dimensional Cell Constructs. Medicina. 2024; 60(3):451. https://doi.org/10.3390/medicina60030451
Chicago/Turabian StyleNa, Kyung-Hwan, Hyun-Jin Lee, Ji-Eun Lee, and Jun-Beom Park. 2024. "Regeneration of Rabbit Calvarial Defects with Combination of Stem Cells and Enamel Matrix Derivative: A Microcomputed Tomography and Histological Evaluation Comparing Two- and Three-Dimensional Cell Constructs" Medicina 60, no. 3: 451. https://doi.org/10.3390/medicina60030451
APA StyleNa, K.-H., Lee, H.-J., Lee, J.-E., & Park, J.-B. (2024). Regeneration of Rabbit Calvarial Defects with Combination of Stem Cells and Enamel Matrix Derivative: A Microcomputed Tomography and Histological Evaluation Comparing Two- and Three-Dimensional Cell Constructs. Medicina, 60(3), 451. https://doi.org/10.3390/medicina60030451








