Pilot Study of the Total and Phosphorylated Tau Proteins in Early-Stage Multiple Sclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and CSF Collection
2.2. CSF Analysis and Biomarkers Identification
2.3. Statistics
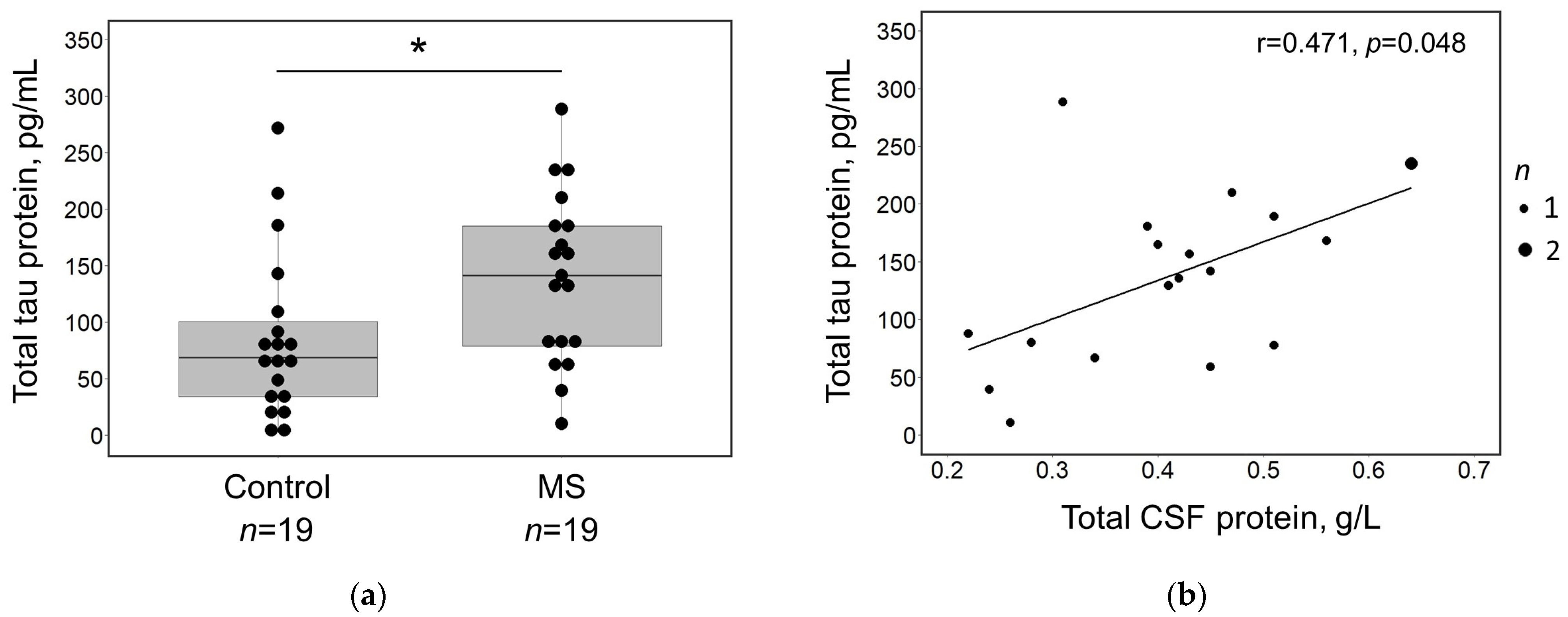
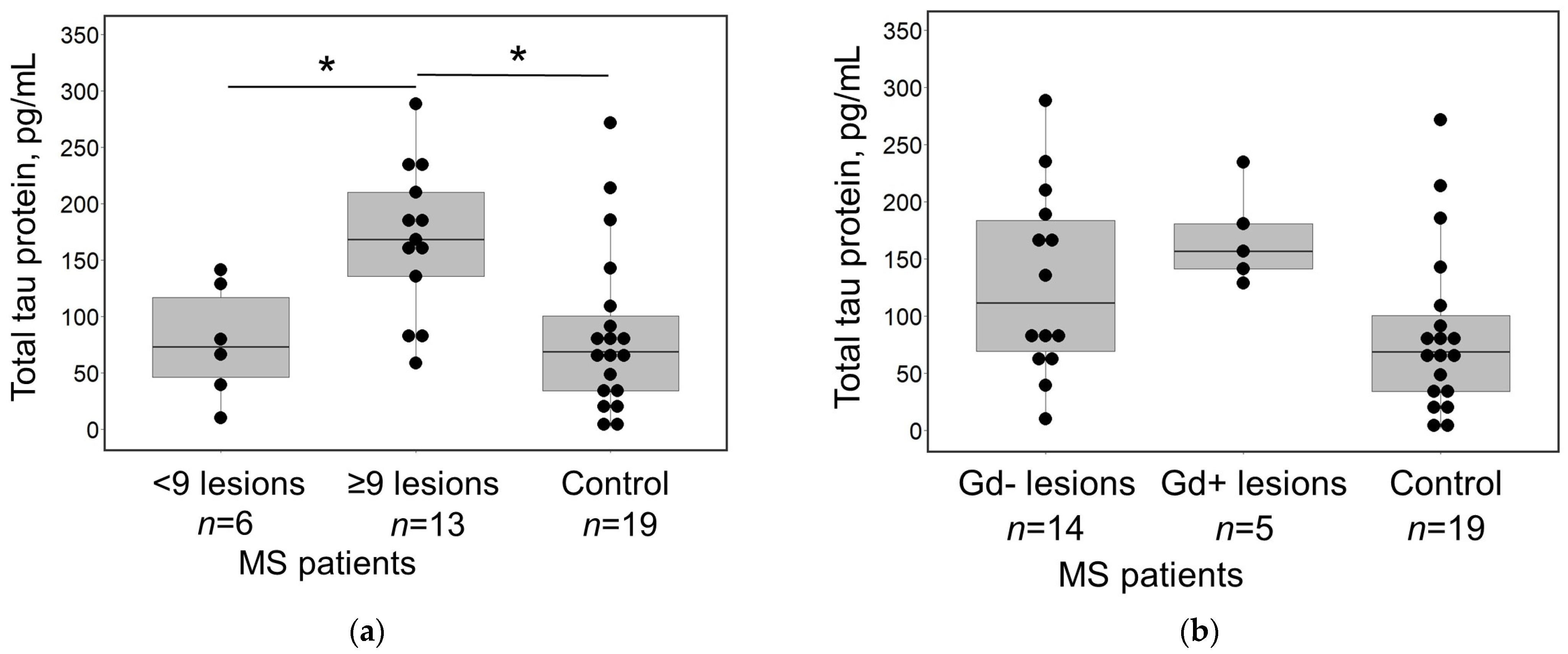
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ward, M.; Goldman, M.D. Epidemiology and Pathophysiology of Multiple Sclerosis. Contin. Lifelong Learn. Neurol. 2022, 28, 988–1005. [Google Scholar] [CrossRef]
- Zhang, Y.; Cofield, S.; Cutter, G.; Krieger, S.; Wolinsky, J.S.; Lublin, F. Predictors of Disease Activity and Worsening in Relapsing-Remitting Multiple Sclerosis. Neurol. Clin. Pract. 2022, 12, e58–e65. [Google Scholar] [CrossRef]
- Eshaghi, A.; Kievit, R.A.; Prados, F.; Sudre, C.H.; Nicholas, J.; Cardoso, M.J.; Chan, D.; Nicholas, R.; Ourselin, S.; Greenwood, J.; et al. Applying causal models to explore the mechanism of action of simvastatin in progressive multiple sclerosis. Proc. Natl. Acad. Sci. USA 2019, 166, 11020–11027. [Google Scholar] [CrossRef]
- Harari, G.; Gurevich, M.; Dolev, M.; Falb, R.Z.; Achiron, A. Faster progression to multiple sclerosis disability is linked to neuronal pathways associated with neurodegeneration: An ethnicity study. PLoS ONE 2023, 18, e0280515. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- Liang, S.Y.; Wang, Z.T.; Tan, L.; Yu, J.T. Tau Toxicity in Neurodegeneration. Mol. Neurobiol. 2022, 59, 3617–3634. [Google Scholar] [CrossRef] [PubMed]
- Didonna, A. Tau at the interface between neurodegeneration and neuroinflammation. Genes Immun. 2020, 21, 288–300. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of tau as a microtubule-associated protein: Structural and functional aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Caamaño-Moreno, M.; Gargini, R. Tauopathies: The Role of Tau in Cellular Crosstalk and Synaptic Dysfunctions. Neuroscience 2023, 518, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Schöll, M.; Vogels, T. Tau seeding mouse models with patient brain-derived aggregates. Int. J. Mol. Sci. 2021, 22, 6132. [Google Scholar] [CrossRef]
- Trushina, N.I.; Bakota, L.; Mulkidjanian, A.Y.; Brandt, R. The Evolution of Tau Phosphorylation and Interactions. Front. Aging Neurosci. 2019, 11, 256. [Google Scholar] [CrossRef]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Schoonenboom, N.; Reesink, F.; Verwey, N.; Kester, M.; Teunissen, C.; van de Ven, P.; Pijnenburg, Y.A.; Blankenstein, M.A.; Rozemuller, A.J.; Scheltens, P.; et al. Cerebrospinal fluid markers for differential dementia diagnosis in a large memory clinic cohort. Neurology 2012, 78, 47–54. [Google Scholar] [CrossRef]
- Urakami, K.; Wada, K.; Arai, H.; Sasaki, H.; Kanai, M.; Shoji, M.; Ishizu, H.; Kashihara, K.; Yamamoto, M.; Tsuchiya-Ikemoto, K.; et al. Diagnostic significance of tau protein in cerebrospinal fluid from patients with corticobasal degeneration or progressive supranuclear palsy. J. Neurol. Sci. 2001, 183, 95–98. [Google Scholar] [CrossRef]
- Brunello, C.A.; Merezhko, M.; Uronen, R.L.; Huttunen, H.J. Mechanisms of secretion and spreading of pathological tau protein. Cell Mol. Life Sci. 2020, 77, 1721–1744. [Google Scholar] [CrossRef]
- Kanmert, D.; Cantlon, A.; Muratore, C.R.; Jin, M.; O’Malley, T.T.; Lee, G.; Young-Pearse, T.L.; Selkoe, D.J.; Walsh, D.M. C-terminally truncated forms of tau, but not full-length tau or its C-terminal fragments, are released from neurons independently of cell death. J. Neurosci. 2015, 35, 10851–10865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Ma, L.; Wei, Y.; Li, H. Possible Mechanisms of Tau Spread and Toxicity in Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 9, 707268. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Z.; Kang, S.G.; Arce, L.; Westaway, D. Prion-like strain effects in tauopathies. Cell Tissue Res. 2023, 392, 179–199. [Google Scholar] [CrossRef]
- LaCroix, M.S.; Mirbaha, H.; Shang, P.; Zandee, S.; Foong, C.; Prat, A.; White, C.L., 3rd; Stuve, O.; Diamond, M.I. Tau seeding in cases of multiple sclerosis. Acta Neuropathol. Commun. 2022, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Torii, T.; Miyamoto, Y.; Nakata, R.; Higashi, Y.; Shinmyo, Y.; Kawasaki, H.; Miyasaka, T.; Misonou, H. Identification of Tau protein as a novel marker for maturation and pathological changes of oligodendrocytes. Glia 2023, 71, 1002–1017. [Google Scholar] [CrossRef] [PubMed]
- LoPresti, P. Inducible Expression of a Truncated Form of Tau in Oligodendrocytes Elicits Gait Abnormalities and a Decrease in Myelin: Implications for Selective CNS Degenerative Diseases. Neurochem. Res. 2015, 40, 2188–2199. [Google Scholar] [CrossRef]
- Torii, T. Abnormal expression of Tau in damaged oligodendrocytes of HLD1 mice. Neural Regen. Res. 2024, 19, 1405–1406. [Google Scholar] [CrossRef]
- Viney, T.J.; Sarkany, B.; Ozdemir, A.T.; Hartwich, K.; Schweimer, J.; Bannerman, D.; Somogyi, P. Spread of pathological human Tau from neurons to oligodendrocytes and loss of high-firing pyramidal neurons in aging mice. Cell Rep. 2022, 41, 111646. [Google Scholar] [CrossRef]
- Mey, G.M.; Mahajan, K.R.; DeSilva, T.M. Neurodegeneration in multiple sclerosis. WIREs Mech. Dis. 2023, 15, e1583. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, T.; Moccia, M.; Coetzee, T.; Cohen, J.A.; Correale, J.; Graves, J.; Marrie, R.A.; Montalban, X.; Yong, V.W.; Thompson, A.J.; et al. Multiple sclerosis progression: Time for a new mechanism-driven framework. Lancet Neurol. 2023, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Paul, F.; Calabresi, P.A.; Barkhof, F.; Green, A.J.; Kardon, R.; Sastre-Garriga, J.; Schippling, S.; Vermersch, P.; Saidha, S.; Gerendas, B.S.; et al. Optical coherence tomography in multiple sclerosis: A 3-year prospective multicenter study. Ann. Clin. Transl. Neurol. 2021, 8, 2235–2251. [Google Scholar] [CrossRef] [PubMed]
- Mey, G.M.; DeSilva, T.M. Utility of the visual system to monitor neurodegeneration in multiple sclerosis. Front. Mol. Neurosci. 2023, 16, 1125115. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Minneboo, A.; Barkhof, F.; Polman, C.H.; Uitdehaag, B.M.J.; Knol, D.L.; Castelijns, J.A. Infratentorial Lesions Predict Long-term Disability in Patients with Initial Findings Suggestive of Multiple Sclerosis. Arch. Neurol. 2004, 61, 217–221. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant graphics for data analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kapaki, E.; Paraskevas, G.P.; Michalopoulou, M.; Kilidireas, K. Increased Cerebrospinal Fluid Tau Protein in Multiple Sclerosis. Eur. Neurol. 2000, 43, 228–232. [Google Scholar] [CrossRef]
- Bartosik-Psujek, H.; Stelmasiak, Z. The CSF levels of total-tau and phosphotau in patients with relapsing-remitting multiple sclerosis. J. Neural Transm. 2006, 113, 339–345. [Google Scholar] [CrossRef]
- Bartosik-Psujek, H.; Psujek, M.; Jaworski, J.; Stelmasiak, Z. Total tau and S100b proteins in different types of multiple sclerosis and during immunosuppressive treatment with mitoxantrone. Acta Neurol. Scand. 2011, 123, 252–256. [Google Scholar] [CrossRef]
- Kosehasanogullari, G.; Ozakbas, S.; Idiman, E. Tau protein levels in the cerebrospinal fluid of the patients with multiple sclerosis in an attack period: Low levels of tau protein may have significance, too. Clin. Neurol. Neurosurg. 2015, 136, 107–109. [Google Scholar] [CrossRef]
- Brettschneider, J.; Maier, M.; Arda, S.; Claus, A.; Süssmuth, S.D.; Kassubek, J.; Tumani, H. Tau protein level in cerebrospinal fluid is increased in patients with early multiple sclerosis. Mult. Scler. 2005, 11, 261–265. [Google Scholar] [CrossRef]
- Brettschneider, J.; Petzold, A.; Junker, A.; Tumani, H. Axonal damage markers in the cerebrospinal fluid of patients with clinically isolated syndrome improve predicting conversion to definite multiple sclerosis. Mult. Scler. 2006, 12, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Terzi, M.; Birinci, A.; Çetinkaya, E.; Onar, M.K. Cerebrospinal fluid total tau protein levels in patients with multiple sclerosis. Acta Neurol. Scand. 2007, 115, 325–330. [Google Scholar] [CrossRef]
- Sladkova, V.; Mareš, J.; Lubenova, B.; Zapletalova, J.; Stejskal, D.; Hlustik, P.; Kanovsky, P. Degenerative and inflammatory markers in the cerebrospinal fluid of multiple sclerosis patients with relapsing-remitting course of disease and after clinical isolated syndrome. Neurol. Res. 2011, 33, 415–420. [Google Scholar] [CrossRef]
- Pietroboni, A.M.; Schiano Di Cola, F.; Scarioni, M.; Fenoglio, C.; Spanò, B.; Arighi, A.; Cioffi, S.M.; Oldoni, E.; De Riz, M.A.; Basilico, P.; et al. CSF β-amyloid as a putative biomarker of disease progression in multiple sclerosis. Mult. Scler. 2017, 23, 1085–1091. [Google Scholar] [CrossRef]
- Jiménez-Jiḿnez, F.J.; Zurdo, J.M.; Hernánz, A.; Medina-Acebrón, S.; de Bustos, F.; Barcenilla, B.; Sayed, Y.; Ayuso-Peralta, L. Tau protein concentrations in cerebrospinal fluid of patients with multiple sclerosis. Acta Neurol. Scand. 2002, 111, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.; Cardoso, M.J.; Sá, M.J. Tau protein seems not to be a useful routine clinical marker of axonal damage in multiple sclerosis. Mult. Scler. 2006, 12, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Iacobaeus, E.; Khademi, M.; Brundin, L.; Norgren, N.; Koel-Simmelink, M.J.A.; Schepens, M.; Bouwman, F.; Twaalfhoven, H.A.; Blom, H.J. Combination of CSF N-acetylaspartate and neurofilaments in multiple sclerosis. Neurology 2009, 72, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Rossi, S.; Sancesario, G.; Codecá, C.; Mataluni, G.; Monteleone, F.; Buttari, F.; Kusayanagi, H.; Castelli, M.; Motta, C.; et al. Cognitive and cortical plasticity deficits correlate with altered amyloid-Β CSF levels in multiple sclerosis. Neuropsychopharmacology 2011, 36, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Szalardy, L.; Zadori, D.; Simu, M.; Bencsik, K.; Vecsei, L.; Klivenyi, P. Evaluating biomarkers of neuronal degeneration and neuroinflammation in CSF of patients with multiple sclerosis—Osteopontin as a potential marker of clinical severity. J. Neurol. Sci. 2013, 331, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Momtazmanesh, S.; Shobeiri, P.; Saghazadeh, A.; Teunissen, C.E.; Burman, J.; Szalardy, L.; Klivenyi, P.; Bartos, A.; Fernandes, A.; Rezaei, N. Neuronal and glial CSF biomarkers in multiple sclerosis: A systematic review and meta-analysis. Rev. Neurosci. 2021, 32, 573–595. [Google Scholar] [CrossRef] [PubMed]
- Colucci, M.; Roccatagliata, L.; Capello, E.; Narciso, E.; Latronico, N.; Tabaton, M.; Mancardi, G.L. The 14-3-3 protein in multiple sclerosis: A marker of disease severity. Mult. Scler. 2004, 10, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Serino, R.; Cantello, R.; Dianzani, U.; Comi, C. Cerebrospinal Tau levels as a predictor of early disability in multiple sclerosis. Mult. Scler. Relat. Disord. 2021, 56, 103231. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, J.; Psujek, M.; Janczarek, M.; Szczerbo-Trojanowska, M.; Bartosik-Psujek, H. Total-tau in cerebrospinal fluid of patients with multiple sclerosis decreases in secondary progressive stage of disease and reflects degree of brain atrophy. Ups. J. Med. Sci. 2012, 117, 284–292. [Google Scholar] [CrossRef][Green Version]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Puricelli, C.; Barbero, P.; Galli, G.; Cantello, R.; Dianzani, U.; Comi, C. Cerebrospinal fluid biomarkers and cognitive functions at multiple sclerosis diagnosis. J. Neurol. 2022, 269, 3249–3257. [Google Scholar] [CrossRef]
- Deisenhammer, F.; Zetterberg, H.; Fitzner, B.; Zettl, U.K. The cerebrospinal fluid in multiple sclerosis. Front. Immunol. 2019, 10, 726. [Google Scholar] [CrossRef]
- Ziemssen, T.; Akgün, K.; Brück, W. Molecular biomarkers in multiple sclerosis. J. Neuroinflammation 2019, 16, 272. [Google Scholar] [CrossRef]
- Oost, W.; Huitema, A.J.; Kats, K.; Giepmans, B.N.G.; Kooistra, S.M.; Eggen, B.J.L.; Baron, W. Pathological ultrastructural alterations of myelinated axons in normal appearing white matter in progressive multiple sclerosis. Acta Neuropathol. Commun. 2023, 11, 100. [Google Scholar] [CrossRef]
- Granberg, T.; Fan, Q.; Treaba, C.A.; Ouellette, R.; Herranz, E.; Mangeat, G.; Louapre, C.; Cohen-Adad, J.; Klawiter, E.C.; Sloane, J.A.; et al. In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis. Brain 2017, 140, 2912–2926. [Google Scholar] [CrossRef]
- Anderson, J.M.; Hampton, D.W.; Patani, R.; Pryce, G.; Crowther, R.A.; Reynolds, R.; Franklin, R.J.; Giovannoni, G.; Compston, D.A.; Baker, D.; et al. Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain 2008, 131, 1736–1748. [Google Scholar] [CrossRef]
- Anderson, J.M.; Patani, R.; Reynolds, R.; Nicholas, R.; Compston, A.; Spillantini, M.G.; Chandran, S. Evidence for abnormal tau phosphorylation in early aggressive multiple sclerosis. Acta Neuropathol. 2009, 117, 583–589. [Google Scholar] [CrossRef]
- Anderson, J.M.; Patani, R.; Reynolds, R.; Nicholas, R.; Compston, A.; Spillantini, M.G.; Chandran, S. Abnormal tau phosphorylation in primary progressive multiple sclerosis. Acta Neuropathol. 2010, 119, 591–600. [Google Scholar] [CrossRef]
- Brown, G.C. Cell death by phagocytosis. Nat. Rev. Immunol. 2023, 24, 91–102. [Google Scholar] [CrossRef]
- Butler, C.A.; Popescu, A.S.; Kitchener, E.J.A.; Allendorf, D.H.; Puigdellívol, M.; Brown, G.C. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J. Neurochem. 2021, 158, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Pampuscenko, K.; Morkuniene, R.; Sneideris, T.; Smirnovas, V.; Budvytyte, R.; Valincius, G.; Brown, G.C.; Borutaite, V. Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. J. Neurochem. 2020, 154, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Pampuscenko, K.; Morkuniene, R.; Krasauskas, L.; Smirnovas, V.; Brown, G.C.; Borutaite, V. Extracellular tau stimulates phagocytosis of living neurons by activated microglia via Toll-like 4 receptor–NLRP3 inflammasome–caspase-1 signalling axis. Sci. Rep. 2023, 13, 10813. [Google Scholar] [CrossRef]
- Puigdellívol, M.; Milde, S.; Vilalta, A.; Cockram, T.O.J.; Allendorf, D.H.; Lee, J.Y.; Dundee, J.M.; Pampuščenko, K.; Borutaite, V.; Nuthall, H.N.; et al. The microglial P2Y6 receptor mediates neuronal loss and memory deficits in neurodegeneration. Cell Rep. 2021, 37, 110148. [Google Scholar] [CrossRef] [PubMed]
- Dejanovic, B.; Huntley, M.A.; De Mazière, A.; Meilandt, W.J.; Wu, T.; Srinivasan, K.; Jiang, Z.; Gandham, V.; Friedman, B.A.; Ngu, H.; et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 2018, 100, 1322–1336.e7. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, T.; Jafari, M.; Kreutzfeldt, M.; Bahn, E.; Brück, W.; Kerschensteiner, M.; Merkler, D. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain 2016, 139, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, K.; Schmitz, F. Synapse Dysfunctions in Multiple Sclerosis. Int. J. Mol. Sci. 2023, 24, 1639. [Google Scholar] [CrossRef] [PubMed]
- Carassiti, D.; Altmann, D.R.; Petrova, N.; Pakkenberg, B.; Scaravilli, F.; Schmierer, K. Neuronal loss, demyelination and volume change in the multiple sclerosis neocortex. Neuropathol. Appl. Neurobiol. 2018, 44, 377–390. [Google Scholar] [CrossRef]
- Werneburg, S.; Jung, J.; Kunjamma, R.; Ha, S.; Luciano, N.; Willis, C.; Gao, G.; Biscola, N.P.; Havton, L.A.; Crocker, S.J.; et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 2020, 52, 167–182.e7. [Google Scholar] [CrossRef]
- Ramaglia, V.; Dubey, M.; Malpede, M.A.; Petersen, N.; de Vries, S.I.; Ahmed, S.M.; Lee, D.S.W.; Schenk, G.J.; Gold, S.M.; Huitinga, I.; et al. Complement-associated loss of CA2 inhibitory synapses in the demyelinated hippocampus impairs memory. Acta Neuropathol. 2021, 142, 643–667. [Google Scholar] [CrossRef]
| Demographic Characteristics | MS Patients (n = 19) |
|---|---|
| Age, median (IQR) | 32 (8–44) |
| Gender (female), n (%) | 12 (63.16%) |
| Symptoms duration (months), median (IQR) | 6 (2–18) |
| Clinical characteristics | |
| Clinical symptoms | |
| Pyramidal, % (n) | 47.36% (9) |
| Sensory, % (n) | 42.10% (8) |
| Brainstem/cerebellum, % (n) | 31.58% (6) |
| Optic neuritis, % (n) | 26.32% (5) |
| Myelitis, % (n) | 5.26% (1) |
| EDSS | |
| At diagnosis, median (range) | 2.5 (1.0–5.5) |
| At six-month follow-up, median (range) | 1.5 (1.0–6.0) |
| The clinical course of MS | |
| Relapsing-remitting MS, % (n) | 63.16% (12) |
| Not yet determined, % (n) | 36.84% (7) |
| MRI characteristics | |
| Lesions load in the brain MRI | |
| <9 lesions, % (n) | 31.58% (6) |
| ≥9 lesions, % (n) | 68.42% (13) |
| Lesions localization | |
| Periventricular, % (n) | 100% (19) |
| Juxta-, subcortical, % (n) | 84.21% (16) |
| Corpus callosum, % (n) | 89.47% (17) |
| Brainstem and cerebellum, % (n) | 94.74% (18) |
| Optic nerve, % (n) | 21.05% (4) |
| Spinal cord, % (n) | 68.42% (13) |
| Gd+ lesions, % (n) | 26.32% (5) |
| Atrophy, % (n) | 15.79% (3) |
| Routine CSF Analytes | MS Patients (n = 19) | Reference Range |
|---|---|---|
| Total WBC count, ×106/L | 4 (1–8) | 0–5 |
| Polymorphonuclear WBC, ×106/L | 0 (0–0) | – |
| Monomorphonuclear WBC, ×106/L | 3 (1–7) | – |
| Total CSF protein, g/L | 0.42 (0.30–0.48) | 0.15–0.45 |
| Glucose, mmol/L | 3.31 (3.13–3.70) | 2.2–3.9 |
| Albumin, mg/L | 234 (183–263) | 139–246 |
| Immunoglobulin G, mg/L | 48.2 (32.7–66.6) | 4.8–58.6 |
| IgG index | 0.81 (0.70–0.94) | – |
| Oligoclonal bands, % (n) | 100% (19) | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masiulienė, I.; Pampuščenko, K.; Žemgulytė, G.; Bilskienė, D.; Borutaitė, V.; Balnytė, R. Pilot Study of the Total and Phosphorylated Tau Proteins in Early-Stage Multiple Sclerosis. Medicina 2024, 60, 416. https://doi.org/10.3390/medicina60030416
Masiulienė I, Pampuščenko K, Žemgulytė G, Bilskienė D, Borutaitė V, Balnytė R. Pilot Study of the Total and Phosphorylated Tau Proteins in Early-Stage Multiple Sclerosis. Medicina. 2024; 60(3):416. https://doi.org/10.3390/medicina60030416
Chicago/Turabian StyleMasiulienė, Ieva, Katryna Pampuščenko, Gintarė Žemgulytė, Diana Bilskienė, Vilmantė Borutaitė, and Renata Balnytė. 2024. "Pilot Study of the Total and Phosphorylated Tau Proteins in Early-Stage Multiple Sclerosis" Medicina 60, no. 3: 416. https://doi.org/10.3390/medicina60030416
APA StyleMasiulienė, I., Pampuščenko, K., Žemgulytė, G., Bilskienė, D., Borutaitė, V., & Balnytė, R. (2024). Pilot Study of the Total and Phosphorylated Tau Proteins in Early-Stage Multiple Sclerosis. Medicina, 60(3), 416. https://doi.org/10.3390/medicina60030416






