Preoperative Albumin-to-Alkaline Phosphatase Ratio as an Independent Predictor of Lymph Node Involvement in Penile Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
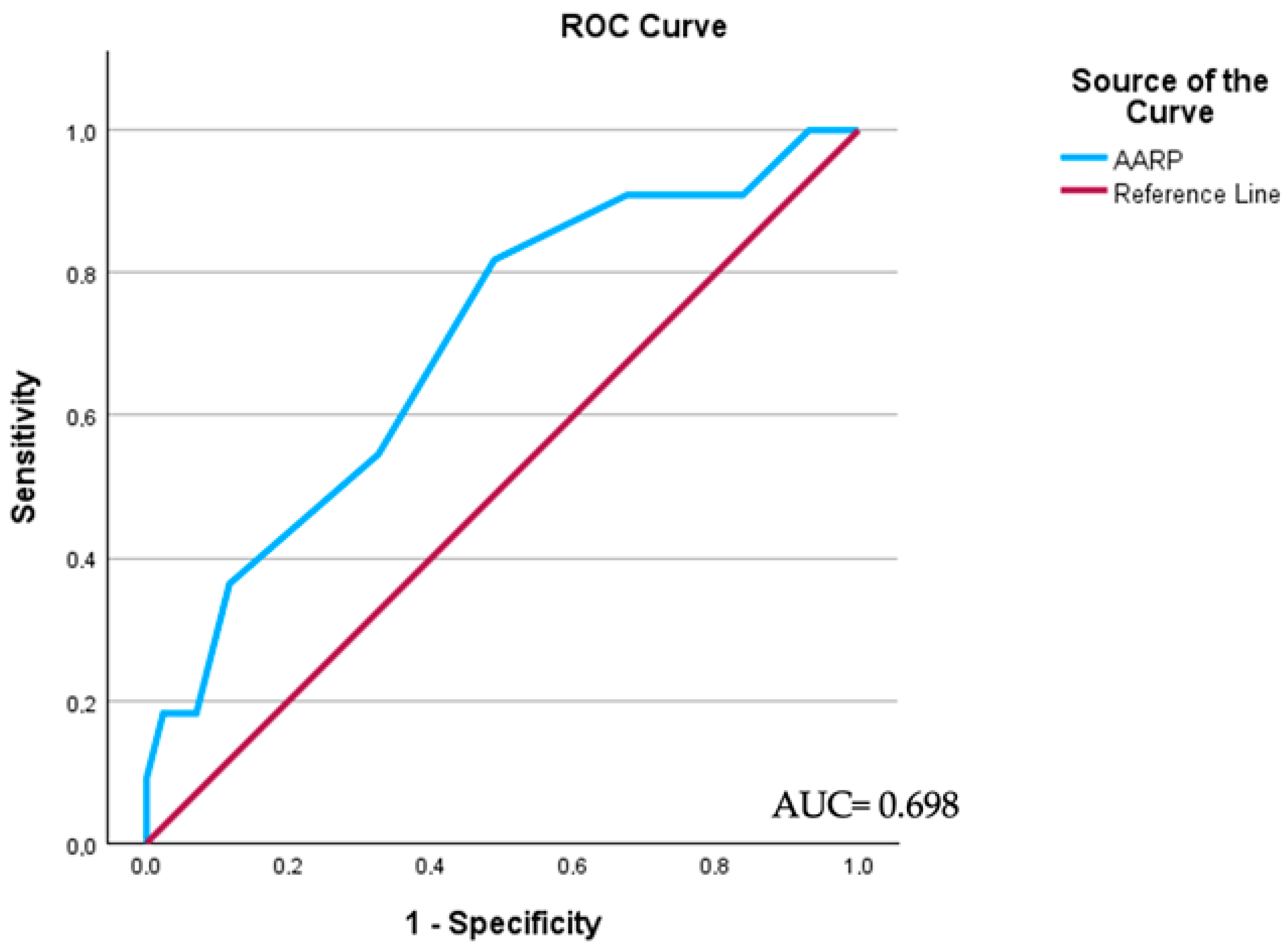
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Chan, S.C.; Pang, W.S.; Liu, X.; Zhang, L.; Lucero-Prisno, D.E., 3rd; Xu, W.; Zheng, Z.J.; Ng, A.C.; Necchi, A.; et al. Global Society of Rare Genitourinary Tumors (GSRGT). Incidence, risk factors, and temporal trends of penile cancer: A global population-based study. BJU Int. 2023, 133, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Douglawi, A.; Masterson, T.A. Updates on the epidemiology and risk factors for penile cancer. Transl. Androl. Urol. 2017, 6, 785–790. [Google Scholar] [CrossRef]
- Cilio, S.; Tufano, A.; Pezone, G.; Alvino, P.; Spena, G.; Pandolfo, S.D.; Del Prete, P.; Amato, C.; Damiano, R.; Salonia, A.; et al. Sexual outcomes after conservative management for patients with localized penile cancer. Curr. Oncol. 2023, 30, 10501–10508. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ye, D.-W. Lymph node metastases and prognosis in penile cancer. Chin. J. Cancer Res. 2012, 24, 90–96. [Google Scholar] [CrossRef][Green Version]
- Hu, C.; Bai, Y.; Li, J.; Zhang, G.; Yang, L.; Bi, C.; Zhao, B.; Yang, Y.; Li, R.; Wu, H.; et al. Prognostic value of systemic inflammatory factors NLR, LMR, PLR and LDH in penile cancer. BMC Urol. 2020, 20, 57. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Dipinto, P.; Passaro, F.; Anceschi, U.; Franco, G.; Flammia, R.S.; Proietti, F.; Antonelli, L.; Di Pierro, G.B.; Prata, F.; et al. The value of fournier’s gangrene scoring systems on admission to predict mortality: A systematic review and meta-analysis. J. Pers. Med. 2023, 13, 1283. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fang, J.; Jiao, D.; Liu, Z. The predictive value of inflammatory markers for pathological response of ipsilateral supraclavicular lymph nodes and for prognosis in breast cancer after neoadjuvant chemotherapy. Gland Surg. 2020, 9, 1354–1362. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, S.X.; Hu, K.S.; Gan, Y.H.; Chen, Y.; Ge, N.L.; Yang, B.W.; Zhang, L.; Chen, R.X.; Ren, Z.G.; et al. Albumin-to-alkaline phosphatase ratio as a predictor of tumor recurrence and prognosis in patients with early-stage hepatocellular carcinoma undergoing radiofrequency ablation as initial therapy. Int. J. Hyperth. 2021, 38, 1–10. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, Y.; Chen, Y.; Zhou, X. Prognostic effect of albumin-to-alkaline phosphatase ratio on patients with hepatocellular carcinoma: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 1808. [Google Scholar] [CrossRef]
- Cindolo, L.; Spiess, P.E.; Bada, M.; Chipollini, J.J.; Nyirády, P.; Chiodini, P.; Varga, J.; Ditonno, P.; Battaglia, M.; De Nunzio, C.; et al. Adherence to EAU guidelines on penile cancer translates into better outcomes: A multicenter international study. World J. Urol. 2019, 37, 1649–1657. [Google Scholar] [CrossRef]
- Lebentrau, S.; Wakileh, G.A.; Schostak, M.; Schmid, H.-P.; Suarez-Ibarrola, R.; Merseburger, A.S.; Hutterer, G.C.; Necknig, U.H.; Rink, M.; Bögemann, M.; et al. Does the identification of a minimum number of cases correlate with better adherence to international guidelines regarding the treatment of penile cancer? Survey results of the European PROspective Penile Cancer Study (E-PROPS). Front. Oncol. 2021, 11, 759362. [Google Scholar] [CrossRef]
- Reyes, M.E.; Borges, H.; Adjao, M.S.; Vijayakumar, N.; Spiess, P.E.; Schabath, M.B. Novel prognostic models for patients with penile carcinoma. Cancer Control. 2020, 27, 924728. [Google Scholar] [CrossRef]
- Flammia, R.S.; Tufano, A.; Antonelli, L.; Bernardotto, A.; Bigalli, A.A.C.; Tian, Z.; Smaldone, M.C.; Karakiewicz, P.I.; Panebianco, V.; Leonardo, C. Diagnostic performance of magnetic resonance imaging for preoperative local staging of penile cancer: A systematic review and meta-analysis. Appl. Sci. 2021, 11, 7090. [Google Scholar] [CrossRef]
- Azizi, M.; Peyton, C.C.; Boulware, D.C.; Chipollini, J.; Juwono, T.; Pow-Sang, J.M.; Spiess, P.E. Prognostic value of neutrophil-to-lymphocyte ratio in penile squamous cell carcinoma patients undergoing inguinal lymph node dissection. Eur. Urol. Focus 2019, 5, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Al Ghazal, A.; Steffens, S.; Steinestel, J.; Lehmann, R.; Schnoeller, T.J.; Schulte-Hostede, A.; Wegener, G.; Jentzmik, F.; Schrader, M.; Kuczyk, M.A.; et al. Elevated C-reactive protein values predict nodal metastasis in patients with penile cancer. BMC Urol. 2013, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Núñez, K.G.; Sandow, T.; Patel, J.; Hibino, M.; Fort, D.; Cohen, A.J.; Thevenot, P. Hypoalbuminemia is a hepatocellular carcinoma independent risk factor for tumor progression in low-risk bridge to transplant candidates. Cancers 2022, 14, 1684. [Google Scholar] [CrossRef]
- Harris, H. The human alkaline phosphatases: What we know and what we don’t know. Clin. Chim. Acta 1990, 186, 133–150. [Google Scholar] [CrossRef]
- Lallès, J.-P. Recent advances in intestinal alkaline phosphatase, inflammation, and nutrition. Nutr. Rev. 2019, 77, 710–724. [Google Scholar] [CrossRef]
- Damera, S.; Raphael, K.L.; Baird, B.C.; Cheung, A.K.; Greene, T.; Beddhu, S. Serum alkaline phosphatase levels associate with elevated serum C-reactive protein in chronic kidney disease. Kidney Int. 2011, 79, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zhang, M.; Wang, Y.; Yang, X.; Teng, X.; Chu, G.; Wang, X.; Niu, H. Prognostic value of preoperative albumin-to-alkaline phosphatase ratio in patients with muscle-invasive bladder cancer after radical cystectomy. OncoTargets Ther. 2020, 13, 13265–13274. [Google Scholar] [CrossRef]
- Tsai, M.H.; Chuang, H.C.; Lin, Y.T.; Yang, K.L.; Lu, H.; Huang, T.L.; Tsai, W.L.; Su, Y.Y.; Fang, F.M. The prognostic value of preoperative albumin-to-alkaline phosphatase ratio on survival outcome for patients with locally advanced oral squamous cell carcinoma. Technol. Cancer Res Treat. 2022, 21, 141254. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, H.; Yang, B.; Zheng, Y.; Ou, Y.; Bao, Y.; Mao, Y.; Feng, Y. Prognostic value of preoperative albumin-to-alkaline phosphatase ratio in patients with surgically treated urological cancer: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1236167. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yang, Z.Q.; Dou, W.C.; Shao, Y.X.; Wang, Y.H.; Lia, T.; Li, X. Validation of the prognostic value of preoperative albumin-to-alkaline phosphatase ratio in patients with surgically treated non-metastatic renal cell carcinoma. Onco Targets Ther. 2020, 13, 8287–8297. [Google Scholar] [CrossRef] [PubMed]
- Won, I.; Shim, S.R.; Kim, S.I.; Kim, S.J.; Cho, D.S. Albumin-to-alkaline phosphatase ratio as a novel prognostic factor in patients undergoing nephrectomy for non-metastatic renal cell carcinoma: Propensity score matching analysis. Clin. Genitourin. Cancer 2022, 20, e253–e262. [Google Scholar] [CrossRef]
- Yoshino, M.; Ishihara, H.; Ishiyama, Y.; Tachibana, H.; Toki, D.; Yamashita, K.; Kobayashi, H.; Fukuda, H.; Yoshida, K.; Takagi, T.; et al. Albumin-to-alkaline phosphatase ratio as a novel prognostic marker of nivolumab monotherapy for previously treated metastatic renal cell carcinoma. In Vivo 2021, 35, 2855–2862. [Google Scholar] [CrossRef]
| Overall | AAPR ≤ 0.53 | AAPR > 0.53 | p Value | |
|---|---|---|---|---|
| n = 42 | n = 18 | n = 24 | ||
| Age, mean (SD) | 63.6 (12.9) | 65.3 (13.7) | 62.1 (11.3) | 0.46 |
| Smoking, n (%) | ||||
| Never | 8 (19.1) | 3 (16.7) | 5 (20.8) | 0.26 |
| Current | 14 (33.3) | 6 (33.3) | 8 (33.3) | |
| Former | 20 (47.6) | 9 (50.0) | 11 (45.9) | |
| HPV, n (%) | ||||
| High Risk | 19 (45.2) | 7 (38.9) | 12 (50.0) | 0.49 |
| Intermediate-Low Risk | 1 (2.4) | 1 (5.6) | 0 (0.0) | |
| No | 4 (9.5) | 2 (11.1) | 2 (8.3) | |
| Unknown | 18 (42.9) | 8 (44.4) | 10 (41.7) | |
| Diabetes, n (%) | 8 (19.0) | 3 (16.6) | 5 (20.8) | 0.38 |
| Hypertension, n (%) | 18 (42.8) | 7 (38.8) | 11 (45.8) | 0.56 |
| ASA score, median (IQR) | 2 (2–2) | 2 (2–2) | 2 (2–2) | 0.78 |
| Charlson Comorbidity Index, median (IQR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.87 |
| Primary tumor surgery, n (%) | ||||
| Total penectomy | 7 (16.7) | 3 (16.7) | 4 (16.6) | 0.23 |
| Partial Penectomy | 21 (50.0) | 8 (44.4) | 13 (54.2) | |
| Penile-sparing surgery | 14 (33.3) | 7 (38.9) | 7 (29.2) | |
| Clinical N stage, n (%) | ||||
| cNx | 1 (2.4) | 1 (5.5) | 0 (0) | 0.03 |
| cN0 | 18 (42.8) | 5 (27.8) | 13 (54.2) | |
| cN+ | 23 (54.8) | 12 (66.7) | 11 (45.8) | |
| Pathologic T stage, n (%) | ||||
| pTa/T1 | 7 (16.7) | 3 (16.7) | 4 (16.7) | 0.08 |
| pT2 | 13 (30.9) | 5 (27.8) | 8 (33.3) | |
| pT3 | 22 (52.4) | 10 (55.5) | 12 (50.0) | |
| pT4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Primary tumor grade, n (%) | ||||
| G1/G2 | 16 (38.1) | 7 (38.9) | 9 (37.5) | 0.88 |
| G3/G4 | 26 (61.9) | 11 (61.1) | 15 (62.5) | |
| Pathologic N stage, n (%) | ||||
| pN0 | 12 (28.6) | 3 (16.7) | 9 (37.5) | 0.02 |
| pN+ | 30 (71.4) | 15 (83.3) | 15 (62.5) | |
| Lymphovascular invasion, n (%) | ||||
| No | 19 (45.2) | 8 (44.4) | 11 (45.8) | 0.67 |
| Yes | 23 (54.8) | 10 (55.6) | 13 (54.2) | |
| Perineural invasion, n (%) | ||||
| No | 11 (26.2) | 5 (27.8) | 6 (25.0) | 0.38 |
| Yes | 13 (30.9) | 6 (33.3) | 7 (29.2) | |
| Unknown | 18 (42.9) | 7 (38.9) | 11 (45.8) | |
| Positive Margins, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Variable | Univariable Analysis | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR * | 95.0% CI * | OR * | 95.0% CI * | |||||
| Lower | Higher | p Value | Lower | Higher | p Value | |||
| Primary tumor grade | ||||||||
| G1/G2 | Ref. | - | - | - | - | - | - | - |
| G3/G4 | 1.73 | 0.78 | 2.1 | 0.42 | ||||
| Lymphovascular Invasion | ||||||||
| No | Ref. | - | - | - | Ref. | - | - | - |
| Yes | 4.88 | 1.97 | 8.90 | 0.003 | 5.38 | 1.47 | 9.93 | 0.022 |
| Clinical N stage | ||||||||
| cN0 | Ref. | - | - | - | Ref. | - | - | - |
| cN+ | 16.76 | 6.89 | 39.02 | <0.001 | 13.68 | 4.37 | 43.90 | 0.009 |
| Albumin-to-Alkaline phosphatase ratio | ||||||||
| >0.53 | Ref. | - | - | - | Ref. | - | - | - |
| ≤0.53 | 3.91 | 1.89 | 9.71 | 0.014 | 3.61 | 1.23 | 12.71 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tufano, A.; Napolitano, L.; Barone, B.; Pezone, G.; Alvino, P.; Cilio, S.; Buonerba, C.; Canciello, G.; Passaro, F.; Perdonà, S. Preoperative Albumin-to-Alkaline Phosphatase Ratio as an Independent Predictor of Lymph Node Involvement in Penile Cancer. Medicina 2024, 60, 414. https://doi.org/10.3390/medicina60030414
Tufano A, Napolitano L, Barone B, Pezone G, Alvino P, Cilio S, Buonerba C, Canciello G, Passaro F, Perdonà S. Preoperative Albumin-to-Alkaline Phosphatase Ratio as an Independent Predictor of Lymph Node Involvement in Penile Cancer. Medicina. 2024; 60(3):414. https://doi.org/10.3390/medicina60030414
Chicago/Turabian StyleTufano, Antonio, Luigi Napolitano, Biagio Barone, Gabriele Pezone, Pierluigi Alvino, Simone Cilio, Carlo Buonerba, Giuseppina Canciello, Francesco Passaro, and Sisto Perdonà. 2024. "Preoperative Albumin-to-Alkaline Phosphatase Ratio as an Independent Predictor of Lymph Node Involvement in Penile Cancer" Medicina 60, no. 3: 414. https://doi.org/10.3390/medicina60030414
APA StyleTufano, A., Napolitano, L., Barone, B., Pezone, G., Alvino, P., Cilio, S., Buonerba, C., Canciello, G., Passaro, F., & Perdonà, S. (2024). Preoperative Albumin-to-Alkaline Phosphatase Ratio as an Independent Predictor of Lymph Node Involvement in Penile Cancer. Medicina, 60(3), 414. https://doi.org/10.3390/medicina60030414









