Analysis of Factors Contributing to Adverse Events and Evaluation of Their Impact on Prognosis in Metastatic Renal Cell Carcinoma Patients—Real-World Experience in a Single-Center Retrospective Study and Narrative Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Collection
2.2. Data Collection and Inclusion Criteria
2.3. Assessment of Treatment Response and Adverse Events
2.4. Search Strategy and Selection Criteria
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Interpretation of Results Based on Analysis of Existing Literature
4.2. Mini Review of Cabozantinib-Related AEs in Randomized Clinical Trials and Real-World Experiences
4.3. Efficacy of Cabozantinib and the Search for Synergistic Combines
4.4. The Synergism Phenomenon of Combining VEGFR Inhibitors with Immune Checkpoint Inhibitors
4.5. Difficult Treatment Choice after Progression
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Cancer Research Fund. Kidney Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/kidney-cancer-statistics/ (accessed on 20 December 2023).
- Didkowska, J.; Wojciechowska, U.; Michalek, I.M.; Caetano dos Santos, F.L. Cancer Incidence and Mortality in Poland in 2019. Sci. Rep. 2022, 12, 10875. [Google Scholar] [CrossRef]
- Klatte, T.; Pantuck, A.J. Molecular Biology of Renal Cortical Tumors. Urol. Clin. N. Am. 2008, 35, 573–580. [Google Scholar] [CrossRef]
- Zbar, B.; Brauch, H.; Talmadge, C.; Linehan, M. Loss of Alleles of Loci on the Short Arm of Chromosome 3 in Renal Cell Carcinoma. Nature 1987, 327, 721–724. [Google Scholar] [CrossRef]
- Aron, M.; Nguyen, M.M.; Stein, R.J.; Gill, I.S. Impact of Gender in Renal Cell Carcinoma: An Analysis of the SEER Database. Eur. Urol. 2008, 54, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.P.; Hobisch, A. Treatment Options in Localised and Metastatic Renal Cell Carcinoma. Memo—Mag. Eur. Med. Oncol. 2008, 1, 167–170. [Google Scholar] [CrossRef]
- Bedke, J.; Albiges, L.; Capitanio, U.; Giles, R.H.; Hora, M.; Lam, T.B.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A.; et al. Updated European Association of Urology Guidelines on Renal Cell Carcinoma: Nivolumab plus Cabozantinib Joins Immune Checkpoint Inhibition Combination Therapies for Treatment-naïve Metastatic Clear-Cell Renal Cell Carcinoma. Eur. Urol. 2021, 79, 339–342. [Google Scholar] [CrossRef]
- Rathmell, W.K.; Rumble, R.B.; Van Veldhuizen, P.J.; Al-Ahmadie, H.; Emamekhoo, H.; Hauke, R.J.; Louie, A.V.; Milowsky, M.I.; Molina, A.M.; Rose, T.L.; et al. Management of Metastatic Clear Cell Renal Cell Carcinoma: ASCO Guideline. J. Clin. Oncol. 2022, 40, 2957–2995. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab Plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Apolo, A.B.; Nadal, R.; Tomita, Y.; Davarpanah, N.N.; Cordes, L.M.; Steinberg, S.M.; Cao, L.; Parnes, H.L.; Costello, R.; Merino, M.J.; et al. Cabozantinib in Patients with Platinum-Refractory Metastatic Urothelial Carcinoma: An Open-Label, Single-Centre, Phase 2 Trial. Lancet Oncol. 2020, 21, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hodi, F.S.; Buchbinder, E.I. Inhibition of Immune Checkpoints and Vascular Endothelial Growth Factor as Combination Therapy for Metastatic Melanoma: An Overview of Rationale, Preclinical Evidence, and Initial Clinical Data. Front. Oncol. 2015, 5, 202. [Google Scholar] [CrossRef]
- Kwilas, A.R.; Ardiani, A.; Donahue, R.N.; Aftab, D.T.; Hodge, J.W. Dual Effects of a Targeted Small-Molecule Inhibitor (Cabozantinib) on Immune-Mediated Killing of Tumor Cells and Immune Tumor Microenvironment Permissiveness When Combined with a Cancer Vaccine. J. Transl. Med. 2014, 12, 294. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Jancewicz, I.; Szarkowska, J.; Konopinski, R.; Stachowiak, M.; Swiatek, M.; Blachnio, K.; Kubala, S.; Oksinska, P.; Cwiek, P.; Rusetska, N.; et al. PD-L1 Overexpression, SWI/SNF Complex Deregulation, and Profound Transcriptomic Changes Characterize Cancer-Dependent Exhaustion of Persistently Activated CD4(+) T Cells. Cancers 2021, 13, 4148. [Google Scholar] [CrossRef]
- Bodnar, L.; Kopczyńska, A.; Żołnierek, J.; Wieczorek-Rutkowska, M.; Chrom, P.; Tomczak, P. Real-world Experience of Cabozantinib as Second- or Subsequent Line Treatment in Patients With Metastatic Renal Cell Carcinoma: Data From the Polish Managed Access Program. Clin. Genitourin. Cancer 2019, 17, e556–e564. [Google Scholar] [CrossRef]
- Buda-Nowak, A.; Kucharz, J.; Dumnicka, P.; Kuzniewski, M.; Herman, R.M.; Zygulska, A.L.; Kusnierz-Cabala, B. Sunitinib-Induced Hypothyroidism Predicts Progression-Free Survival in Metastatic Renal Cell Carcinoma Patients. Med. Oncol. 2017, 34, 68. [Google Scholar] [CrossRef]
- Kucharz, J.; Budnik, M.; Dumnicka, P.; Pastuszczak, M.; Kuśnierz-Cabala, B.; Demkow, T.; Popko, K.; Wiechno, P. Hand-Foot Syndrome and Progression-Free Survival in Patients Treated with Sunitinib for Metastatic Clear Cell Renal Cell Carcinoma. Adv. Exp. Med. Biol. 2019, 1133, 35–40. [Google Scholar] [CrossRef]
- Kucharz, J.; Dumnicka, P.; Kusnierz-Cabala, B.; Demkow, T.; Wiechno, P. The Correlation between the Incidence of Adverse Events and Progression-Free Survival in Patients Treated with Cabozantinib for Metastatic Renal Cell Carcinoma (mRCC). Med. Oncol. 2019, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.; Brenner, B.M. The Aging Kidney: Structure, Function, Mechanisms, and Therapeutic Implications. J. Am. Geriatr. Soc. 1987, 35, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Heng, D.Y.; Xie, W.; Regan, M.M.; Warren, M.A.; Golshayan, A.R.; Sahi, C.; Eigl, B.J.; Ruether, J.D.; Cheng, T.; North, S.; et al. Prognostic Factors for Overall Survival in Patients with Metastatic Renal Cell Carcinoma Treated with Vascular Endothelial Growth Factor-Targeted Agents: Results from a Large, Multicenter Study. J. Clin. Oncol. 2009, 27, 5794–5799. [Google Scholar] [CrossRef] [PubMed]
- Agency, European Medicines. Summmary of Product Characteristics Cabozantinib. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/cabometyx-epar-product-information_en.pdf (accessed on 21 February 2024).
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (Ctcae) Version 5.0. 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 21 February 2024).
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Martinez Chanza, N.; Farah, S.; Xie, W.; Flippot, R.; Braun, D.A.; Rathi, N.; Thouvenin, J.; Collier, K.A.; Seront, E.; et al. Clinical Activity and Safety of Cabozantinib for Brain Metastases in Patients With Renal Cell Carcinoma. JAMA Oncol. 2021, 7, 1815–1823. [Google Scholar] [CrossRef]
- Richter, I.; Poprach, A.; Zemankova, A.; Buchler, T.; Bartos, J.; Samal, V.; Studentova, H.; Rozsypalova, A.; Dvorak, J.; Brom, O.; et al. Patients with Metastatic Renal Cell Carcinoma Treated with Cabozantinib in the Czech Republic: Analysis of Four Cancer Centers. Biomed. Pap. Med. Fac. Palacky Univ. Olomouc 2022, 166, 97–104. [Google Scholar] [CrossRef]
- McElwee, J.H.; Gourdin, T.S.; Mikoll, J.; Weeda, E.; Sion, A.M. Cabozantinib Use in Metastatic Renal Cell Carcinoma Patients in Clinical Practice: Evaluation of Dosing Patterns, Tolerability, and Outcomes Compared to Clinical Trials. J. Oncol. Pharm. Pract. 2020, 26, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Martínez Chanzá, N.; Xie, W.; Asim Bilen, M.; Dzimitrowicz, H.; Burkart, J.; Geynisman, D.M.; Balakrishnan, A.; Bowman, I.A.; Jain, R.; Stadler, W.; et al. Cabozantinib in Advanced Non-Clear-Cell Renal Cell Carcinoma: A Multicentre, Retrospective, Cohort Study. Lancet Oncol. 2019, 20, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.T.; Bilen, M.A.; Shah, A.Y.; Lemke, E.; Jonasch, E.; Venkatesan, A.M.; Altinmakas, E.; Duran, C.; Msaouel, P.; Tannir, N.M. Cabozantinib for the Treatment of Patients with Metastatic Non-Clear Cell Renal Cell Carcinoma: A Retrospective Analysis. Eur. J. Cancer 2018, 104, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Prisciandaro, M.; Ratta, R.; Massari, F.; Fornarini, G.; Caponnetto, S.; Iacovelli, R.; De Giorgi, U.; Facchini, G.; Scagliarini, S.; Sabbatini, R.; et al. Safety and Efficacy of Cabozantinib for Metastatic Nonclear Renal Cell Carcinoma: Real-world Data From an Italian Managed Access Program. Am. J. Clin. Oncol. 2019, 42, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Procopio, G.; Prisciandaro, M.; Iacovelli, R.; Cortesi, E.; Fornarini, G.; Facchini, G.; Cartenì, G.; Sabbatini, R.; Del Bene, G.; Galli, L.; et al. Safety and Efficacy of Cabozantinib in Metastatic Renal-Cell Carcinoma: Real-World Data From an Italian Managed Access Program. Clin. Genitourin. Cancer 2018, 16, e945–e951. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.R.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.L.; Dudani, S.; Wells, J.C.; Donskov, F.; Pal, S.K.; Dizman, N.; Rathi, N.; Beuselinck, B.; Yan, F.; Lalani, A.A.; et al. Cabozantinib Real-World Effectiveness in the First-Through Fourth-Line Settings for the Treatment of Metastatic Renal Cell Carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Cancer Med. 2021, 10, 1212–1221. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Tannir, N.M.; Mainwaring, P.N.; Rini, B.I.; Hammers, H.J.; Donskov, F.; Roth, B.J.; Peltola, K.; et al. Cabozantinib versus Everolimus in Advanced Renal Cell Carcinoma (METEOR): Final Results from a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2016, 17, 917–927. [Google Scholar] [CrossRef]
- Nakaigawa, N.; Tomita, Y.; Tamada, S.; Tatsugami, K.; Osawa, T.; Oya, M.; Kanayama, H.; Miura, Y.; Sassa, N.; Nishimura, K.; et al. Final Efficacy and Safety Results and Biomarker Analysis of a Phase 2 Study of Cabozantinib in Japanese Patients with Advanced Renal Cell Carcinoma. Int. J. Clin. Oncol. 2023, 28, 416–426. [Google Scholar] [CrossRef]
- Iinuma, K.; Tomioka-Inagawa, R.; Kameyama, K.; Taniguchi, T.; Kawada, K.; Ishida, T.; Nagai, S.; Enomoto, T.; Ueda, S.; Kawase, M.; et al. Efficacy and Safety of Cabozantinib in Patients with Advanced or Metastatic Renal Cell Carcinoma: A Multicenter Retrospective Cohort Study. Biomedicines 2022, 10, 3172. [Google Scholar] [CrossRef]
- Thouvenin, J.; Alhalabi, O.; Carlo, M.; Carril-Ajuria, L.; Hirsch, L.; Martinez-Chanza, N.; Négrier, S.; Campedel, L.; Martini, D.; Borchiellini, D.; et al. Efficacy of Cabozantinib in Metastatic Mit Family Translocation Renal Cell Carcinomas. Oncologist 2022, 27, 1041–1047. [Google Scholar] [CrossRef]
- Procopio, G.; Sepe, P.; Claps, M.; Buti, S.; Colecchia, M.; Giannatempo, P.; Guadalupi, V.; Mariani, L.; Lalli, L.; Fucà, G.; et al. Cabozantinib as First-line Treatment in Patients With Metastatic Collecting Duct Renal Cell Carcinoma: Results of the Bonsai Trial for the Italian Network for Research in Urologic-Oncology (Meet-URO 2 Study). JAMA Oncol. 2022, 8, 910–913. [Google Scholar] [CrossRef]
- Hahn, A.W.; Surasi, D.S.; Viscuse, P.V.; Bathala, T.K.; Wiele, A.J.; Campbell, M.T.; Zurita, A.J.; Shah, A.Y.; Jonasch, E.; Gao, J.; et al. Treatment Outcomes in Patients With Metastatic Renal Cell Carcinoma With Sarcomatoid and/or Rhabdoid Dedifferentiation After Progression on Immune Checkpoint Therapy. Oncologist 2023, oyad302. [Google Scholar] [CrossRef]
- Baudry, E.; Naoun, N.; Auclin, E.; Saldana, C.; Barthelemy, P.; Geoffrois, L.; Thibault, C.; de Vries-Brilland, M.; Borchiellini, D.; Maillet, D.; et al. Efficacy and Safety of Cabozantinib Rechallenge in Metastatic Renal Cell Carcinoma: A Retrospective Multicentric Study. Eur. J. Cancer 2023, 193, 113292. [Google Scholar] [CrossRef]
- Maráz, A.; Nagyiványi, K.; Balogh, I.; Bodoky, G.; Mangel, L.; Küronya, Z.; Géczi, L.; Torday, L.; Dudás, S.; Szűcs, M.; et al. Multicentric Hungarian Results of Cabozantinib Therapy in Patients with Metastatic Kidney Cancer Based on Real-World Data. Magy Onkol. 2023, 67, 73–83. [Google Scholar]
- Santoni, M.; Massari, F.; Grande, E.; Procopio, G.; Matrana, M.R.; Rizzo, M.; De Giorgi, U.; Basso, U.; Milella, M.; Iacovelli, R.; et al. Cabozantinib in Pretreated Patients with Metastatic Renal Cell Carcinoma with Sarcomatoid Differentiation: A Real-World Study. Target. Oncol. 2021, 16, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.K.; Tangen, C.; Thompson, I.M., Jr.; Balzer-Haas, N.; George, D.J.; Heng, D.Y.C.; Shuch, B.; Stein, M.; Tretiakova, M.; Humphrey, P.; et al. A Comparison of Sunitinib with Cabozantinib, Crizotinib, and Savolitinib for Treatment of Advanced Papillary Renal Cell Carcinoma: A Randomised, Open-Label, Phase 2 Trial. Lancet 2021, 397, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Fogli, S.; Porta, C.; Del Re, M.; Crucitta, S.; Gianfilippo, G.; Danesi, R.; Rini, B.I.; Schmidinger, M. Optimizing Treatment of Renal Cell Carcinoma with VEGFR-TKIs: A Comparison of Clinical Pharmacology and Drug-Drug Interactions of Anti-Angiogenic Drugs. Cancer Treat. Rev. 2020, 84, 101966. [Google Scholar] [CrossRef] [PubMed]
- Lemke, E.A.; Shah, A.Y.; Campbell, M.; Tannir, N.M. Real-World Experience With Cabozantinib in Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Analysis. J. Adv. Pract. Oncol. 2019, 10, 333–339. [Google Scholar] [CrossRef]
- Proskorovsky, I.; Benedict, A.; Negrier, S.; Bargo, D.; Sandin, R.; Ramaswamy, K.; Desai, J.; Cappelleri, J.C.; Larkin, J. Axitinib, Cabozantinib, or Everolimus in the Treatment of Prior Sunitinib-Treated Patients with Metastatic Renal Cell Carcinoma: Results of Matching-Adjusted Indirect Comparison Analyses. BMC Cancer 2018, 18, 1271. [Google Scholar] [CrossRef]
- Mennitto, A.; Zattarin, E.; Di Maio, M.; Bimbatti, D.; De Giorgi, U.; Buti, S.; Santini, D.; Casadei, C.; Sorarù, M.; Messina, C.; et al. Cabozantinib beyond Progression Improves Survival in Advanced Renal Cell Carcinoma Patients: The CABEYOND Study (Meet-URO 21). Expert Rev. Anticancer Ther. 2022, 22, 115–121. [Google Scholar] [CrossRef]
- Venugopal, B.; Pillai, M.; Powles, T.; Savage, P.; Michael, A.; Fife, K.; Klair, B.; Perrot, V.; Szabados, B. Early Clinical Experience with Cabozantinib for Advanced Renal Cell Carcinoma in the UK: Real-World Treatment Pathways and Clinical Outcomes. Clin. Genitourin. Cancer 2022, 20, 94–94.e10. [Google Scholar] [CrossRef]
- Martini, D.J.; Evans, S.T.; Liu, Y.; Shabto, J.M.; Uner, O.E.; Olsen, T.A.; Brown, J.T.; Russler, G.A.; Yantorni, L.; Caulfield, S.; et al. Analysis of Toxicity and Clinical Outcomes in Full Versus Reduced Starting Dose Cabozantinib in Metastatic Renal Cell Carcinoma Patients. Clin. Genitourin. Cancer 2022, 20, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Procopio, G.; Claps, M.; Pircher, C.; Porcu, L.; Sepe, P.; Guadalupi, V.; De Giorgi, U.; Bimbatti, D.; Nolè, F.; Carrozza, F.; et al. A Multicenter Phase 2 Single Arm Study of Cabozantinib in Patients with Advanced or Unresectable Renal Cell Carcinoma Pre-Treated with One Immune-Checkpoint Inhibitor: The BREAKPOINT Trial (Meet-Uro Trial 03). Tumori 2023, 109, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Albiges, L.; Rodriguez, C.; Liu, B.; Doss, J.; Khurana, S.; Scheffold, C.; Voss, M.; Choueiri, T. CONTACT-03: Randomized, Open-Label Phase III Study of Atezolizumab Plus Cabozantinib versus Cabozantinib Monotherapy Following Progression on/after Immune Checkpoint Inhibitor (ICI) Treatment in Patients with Advanced/Metastatic Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, TPS370. [Google Scholar] [CrossRef]
- Gomez de Liaño Lista, A.; Venugopal, B.; Fife, K.; Symeonides, S.; Vasudev, N.; Rudman, S.; Vohra, S.; Khasati, L.; Pettinger, C.; Szabados, B.; et al. 893PCabozantinib in Metastatic Renal Cell Carcinoma (mRCC): Data from UK Expanded Access Program (EAP). Ann. Oncol. 2018, 29, viii317. [Google Scholar] [CrossRef]
- McGregor, B.A.; Lalani, A.K.A.; Xie, W.; Steinharter, J.A.; Bakouny, Z.E.; Martini, D.J.; Fleischer, J.H.; Abou-Alaiwi, S.; Nassar, A.; Nuzzo, P.V.; et al. Activity of Cabozantinib after Immune Checkpoint Blockade in Metastatic Clear-Cell Renal Cell Carcinoma. Eur. J. Cancer 2020, 135, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Domański, P.; Jarosińska, J.; Kruczyk, B.; Piętak, M.; Mydlak, A.; Demkow, T.; Kuncman, Ł.; Darewicz, M.; Sikora-Kupis, B.; Dumnicka, P.; et al. Activity of Cabozantinib in Further Line Treatment of Metastatic Clear Cell Renal Cell Carcinoma. Real-World Experience in a Single-Center Retrospective Study. Contemp. Oncol./Współczesna Onkol. 2023, 27, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Park, B.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Choi, H.Y.; Adami, H.O.; Lee, J.E.; Lee, H.M. Body Mass Index and Survival in Patients with Renal Cell Carcinoma: A Clinical-Based Cohort and Meta-Analysis. Int. J. Cancer 2013, 132, 625–634. [Google Scholar] [CrossRef]
- Nishihara, R.; VanderWeele, T.J.; Shibuya, K.; Mittleman, M.A.; Wang, M.; Field, A.E.; Giovannucci, E.; Lochhead, P.; Ogino, S. Molecular Pathological Epidemiology Gives Clues to Paradoxical Findings. Eur. J. Epidemiol. 2015, 30, 1129–1135. [Google Scholar] [CrossRef]
- Renfro, L.A.; Loupakis, F.; Adams, R.A.; Seymour, M.T.; Heinemann, V.; Schmoll, H.J.; Douillard, J.Y.; Hurwitz, H.; Fuchs, C.S.; Diaz-Rubio, E.; et al. Body Mass Index Is Prognostic in Metastatic Colorectal Cancer: Pooled Analysis of Patients From First-Line Clinical Trials in the ARCAD Database. J. Clin. Oncol. 2016, 34, 144–150. [Google Scholar] [CrossRef]
- Waalkes, S.; Merseburger, A.S.; Kramer, M.W.; Herrmann, T.R.; Wegener, G.; Rustemeier, J.; Hofmann, R.; Schrader, M.; Kuczyk, M.A.; Schrader, A.J. Obesity Is Associated with Improved Survival in Patients with Organ-Confined Clear-Cell Kidney Cancer. Cancer Causes Control. 2010, 21, 1905–1910. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Thompson, R.H.; Yee, D.S.; Kaag, M.; Donat, S.M.; Russo, P. Obesity Is Associated with a Higher Risk of Clear-Cell Renal Cell Carcinoma Than with Other Histologies. BJU Int. 2010, 105, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Domański, P.; Jarosińska, J.; Kruczyk, B.; Piętak, M.; Mydlak, A.; Demkow, T.; Kuncman, Ł.; Darewicz, M.; Sikora-Kupis, B.; Michalski, W.; et al. Prognostic Value of Pan-Immune-Inflammation Value and Body Mass Index in Geriatric Patients with Metastatic Renal Cell Carcinoma Treated with Tyrosine Kinase Inhibitors as First Line Treatment. A Single-Center Retrospective Study. Contemp. Oncol./Współczesna Onkol. 2024, 27. [Google Scholar] [CrossRef]
- Poprach, A.; Pavlik, T.; Melichar, B.; Puzanov, I.; Dusek, L.; Bortlicek, Z.; Vyzula, R.; Abrahamova, J.; Buchler, T. Skin Toxicity and Efficacy of Sunitinib and Sorafenib in Metastatic Renal Cell Carcinoma: A National Registry-Based Study. Ann. Oncol. 2012, 23, 3137–3143. [Google Scholar] [CrossRef] [PubMed]
- Bono, P.; Rautiola, J.; Utriainen, T.; Joensuu, H. Hypertension as Predictor of Sunitinib Treatment Outcome in Metastatic Renal Cell Carcinoma. Acta Oncol. 2011, 50, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Q.; Xu, Y.; Zhao, J.; Zhang, L.; Wei, L.; Zhong, W.; Wang, M. Immunotherapy as Second-Line Treatment and beyond for Non-Small Cell Lung Cancer in a Single Center of China: Outcomes, Toxicities, and Clinical Predictive Factors from a Real-World Retrospective Analysis. Thorac. Cancer 2020, 11, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yao, Z.; Yang, H.; Liang, N.; Zhang, X.; Zhang, F. Are Immune-Related Adverse Events Associated with the Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer? A Systematic Review and Meta-Analysis. BMC Med. 2020, 18, 87. [Google Scholar] [CrossRef] [PubMed]
- Verzoni, E.; Cartenì, G.; Cortesi, E.; Giannarelli, D.; De Giglio, A.; Sabbatini, R.; Buti, S.; Rossetti, S.; Cognetti, F.; Rastelli, F.; et al. Real-World Efficacy and Safety of Nivolumab in Previously-Treated Metastatic Renal Cell Carcinoma, and Association between Immune-Related Adverse Events and Survival: The Italian expanded access program. J. Immunother. Cancer 2019, 7, 99. [Google Scholar] [CrossRef]
- Iinuma, K.; Enomoto, T.; Kawada, K.; Fujimoto, S.; Ishida, T.; Takagi, K.; Nagai, S.; Ito, H.; Kawase, M.; Nakai, C.; et al. Utility of Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Systemic Immune Inflammation Index as Prognostic, Predictive Biomarkers in Patients with Metastatic Renal Cell Carcinoma Treated with Nivolumab and Ipilimumab. J. Clin. Med. 2021, 10, 5325. [Google Scholar] [CrossRef]
- Yano, Y.; Ohno, T.; Komura, K.; Fukuokaya, W.; Uchimoto, T.; Adachi, T.; Hirasawa, Y.; Hashimoto, T.; Yoshizawa, A.; Yamazaki, S.; et al. Serum C-reactive Protein Level Predicts Overall Survival for Clear Cell and Non-Clear Cell Renal Cell Carcinoma Treated with Ipilimumab plus Nivolumab. Cancers 2022, 14, 5659. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, J.; Li, Y.; Jiao, X.; Wang, Y.; Zhuo, N.; Gao, M.; Gong, J.; Li, J.; Zhang, X.; et al. Serological Biomarkers Predict Immune-Related Adverse Events and Clinical Benefit in Patients with Advanced Gastrointestinal Cancers. Front. Immunol. 2022, 13, 987568. [Google Scholar] [CrossRef]
- Escudier, B.; Powles, T.; Motzer, R.J.; Olencki, T.; Arén Frontera, O.; Oudard, S.; Rolland, F.; Tomczak, P.; Castellano, D.; Appleman, L.J.; et al. Cabozantinib, a New Standard of Care for Patients with Advanced Renal Cell Carcinoma and Bone Metastases? Subgroup Analysis of the METEOR Trial. J. Clin. Oncol. 2018, 36, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Niewada, M.; Macioch, T.; Konarska, M.; Mela, A.; Goszczynski, A.; Przekopinska, B.; Rajkiewicz, K.; Wysocki, P.; Krzakowski, M. Immune Checkpoint Inhibitors Combined with Tyrosine Kinase Inhibitors or Immunotherapy for Treatment-Naive Metastatic Clear-Cell Renal Cell Carcinoma-A Network Meta-Analysis. Focus on Cabozantinib Combined with Nivolumab. Front. Pharmacol. 2022, 13, 1063178. [Google Scholar] [CrossRef]
- Tannir, N.M.; Agarwal, N.; Porta, C.; Lawrence, N.J.; Motzer, R.; McGregor, B.; Lee, R.J.; Jain, R.K.; Davis, N.; Appleman, L.J.; et al. Efficacy and Safety of Telaglenastat Plus Cabozantinib vs Placebo Plus Cabozantinib in Patients With Advanced Renal Cell Carcinoma: The CANTATA Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Escudier, B.; Powles, T.; Mainwaring, P.N.; Rini, B.I.; Donskov, F.; Hammers, H.; Hutson, T.E.; Lee, J.L.; Peltola, K.; et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1814–1823. [Google Scholar] [CrossRef] [PubMed]
- Libertino, J.; Gee, J. Renal Cancer Contemporary Management: Contemporary Management; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Dirkx, A.E.; Oude Egbrink, M.G.; Kuijpers, M.J.; van der Niet, S.T.; Heijnen, V.V.; Bouma-ter Steege, J.C.; Wagstaff, J.; Griffioen, A.W. Tumor Angiogenesis Modulates Leukocyte-Vessel Wall Interactions In Vivo by Reducing Endothelial Adhesion Molecule Expression. Cancer Res. 2003, 63, 2322–2329. [Google Scholar] [PubMed]
- Motz, G.T.; Santoro, S.P.; Wang, L.P.; Garrabrant, T.; Lastra, R.R.; Hagemann, I.S.; Lal, P.; Feldman, M.D.; Benencia, F.; Coukos, G. Tumor Endothelium FasL Establishes a Selective Immune Barrier Promoting Tolerance in Tumors. Nat. Med. 2014, 20, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Shibata, M.; Gonda, K.; Yazawa, T.; Shimura, T.; Anazawa, T.; Suzuki, S.; Sakurai, K.; Koyama, Y.; Ohto, H.; et al. Serum Levels of Vascular Endothelial Growth Factor Are Increased and Correlate with Malnutrition, Immunosuppression Involving MDSCs and Systemic Inflammation in Patients with Cancer of the Digestive System. Oncol. Lett. 2013, 5, 1682–1686. [Google Scholar] [CrossRef]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular Endothelial Growth Factor Inhibits the Development of Dendritic Cells and Dramatically Affects the Differentiation of Multiple Hematopoietic Lineages In Vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [CrossRef] [PubMed]
- Dikov, M.M.; Ohm, J.E.; Ray, N.; Tchekneva, E.E.; Burlison, J.; Moghanaki, D.; Nadaf, S.; Carbone, D.P. Differential Roles of Vascular Endothelial Growth Factor Receptors 1 and 2 in Dendritic Cell Differentiation. J. Immunol. 2005, 174, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Overwijk, W.W. Breaking Tolerance in Cancer Immunotherapy: Time to ACT. Curr. Opin. Immunol. 2005, 17, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ Tumor-Infiltrating Lymphocytes and a High CD8+/Regulatory T Cell Ratio Are Associated with Favorable Prognosis in Ovarian Cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, C.; Suarez, N.; Gonzalez, A.; Solano, S.; Erro, L.; Dubrot, J.; Palazon, A.; Hervas-Stubbs, S.; Gurpide, A.; Lopez-Picazo, J.M.; et al. Influence of Bevacizumab, Sunitinib and Sorafenib as Single Agents or in Combination on the Inhibitory Effects of VEGF on Human Dendritic Cell Differentiation from Monocytes. Br. J. Cancer 2009, 100, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.; Mier y Teran, E.; Waaga-Gasser, A.M.; Gasser, M.; Choueiri, T.K.; Freeman, G.; Pal, S. Novel Roles of c-Met in the Survival of Renal Cancer Cells through the Regulation of HO-1 and PD-L1 Expression. J. Biol. Chem. 2015, 290, 8110–8120. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Oudard, S.; Grünwald, V.; Calvo, E.; Michaelson, M.D.; Burotto, M.; Melichar, B.; Tyagi, R.; Hilmi, F.; Gaur, A. 718P A Phase II Study of Patients with Advanced or Metastatic Renal Cell Carcinoma (mRCC) Receiving Pazopanib after Previous Checkpoint Inhibitor Treatment. Ann. Oncol. 2020, 31, S564. [Google Scholar] [CrossRef]
- Grande, E.; Gordoa, T.A.; Torras, O.R.; Esteban, E.; Castellano, D.; Muro, X.G.d.; Vidal, M.J.M.; García-Donas, J.; Arranz, J.A.; Rodriguez, C.S. INMUNOSUN-SOGUG Trial: A Prospective Phase II Study to Assess the Efficacy and Safety of Sunitinib as Second-Line (2L) Treatment in Patients (pts) with Metastatic Renal Cell Cancer (RCC) Who Received Immunotherapy-Based Combination Upfront. J. Clin. Oncol. 2020, 38, 5060. [Google Scholar] [CrossRef]
- Choueiri, T.K.; McDermott, D.F.; Merchan, J.; Bauer, T.M.; Figlin, R.; Heath, E.I.; Michaelson, M.D.; Arrowsmith, E.; D’Souza, A.; Zhao, S.; et al. Belzutifan plus Cabozantinib for Patients with Advanced Clear Cell Renal Cell Carcinoma Previously Treated with Immunotherapy: An Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2023, 24, 553–562. [Google Scholar] [CrossRef] [PubMed]
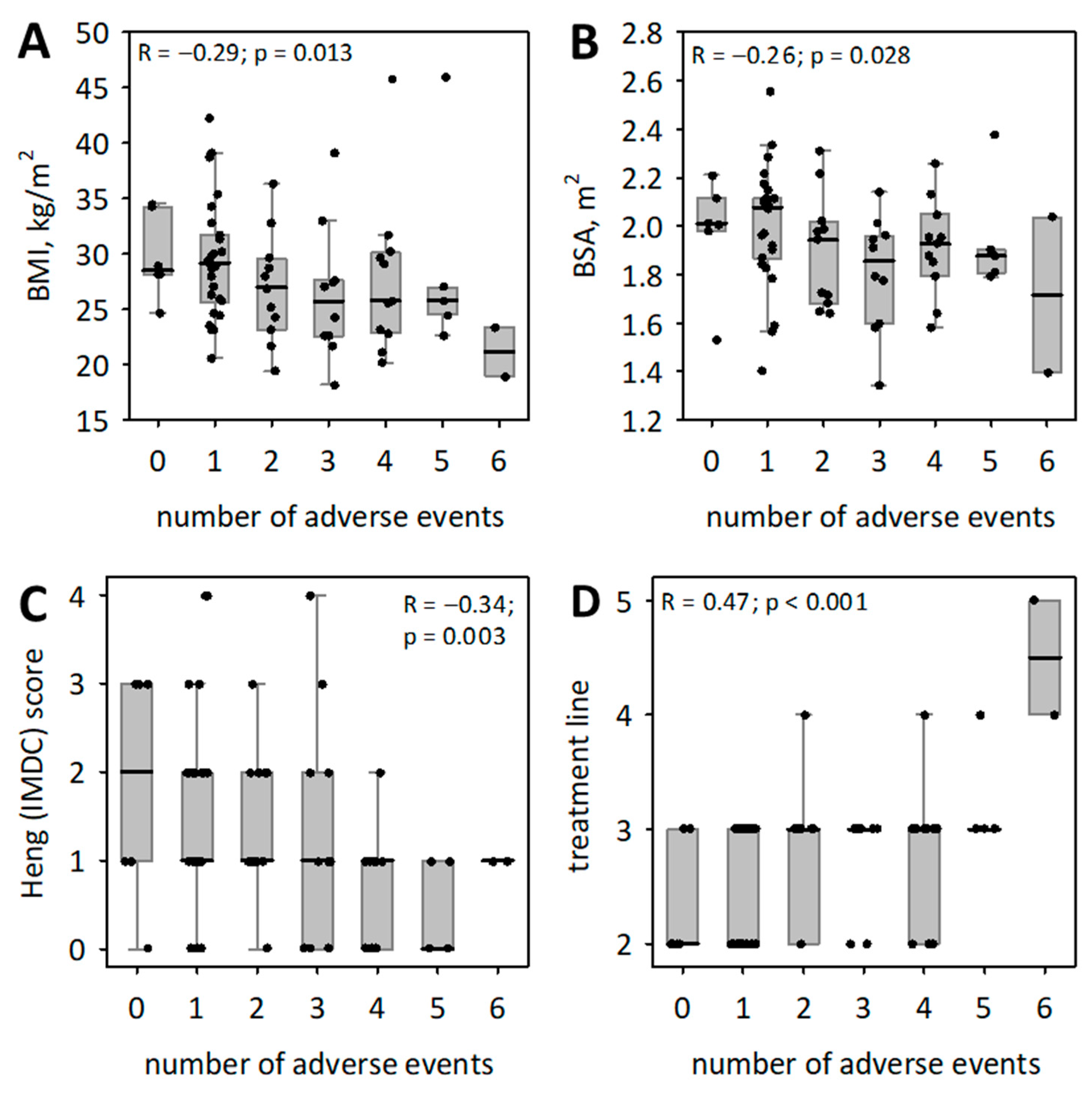
| Characteristic | Values Observed in mRCC Patients (n = 71) |
|---|---|
| Male sex, n (%) | 46 (65) |
| Mean age (SD), years | 63 (9) |
| Median time from RCC diagnosis (Q1; Q3), years | 4.3 (2.0; 8.2) |
| Mean body weight (SD), kg | 82.1 (19.3) |
| Mean BMI (SD), kg/m2 | 28.1 (5.9) |
| Mean body surface area (BSA) (SD), m2 | 1.93 (0.24) |
| Morphology | |
| Clear cell, n (%) | 69 (97) |
| Non-clear cell, n (%) | 6 (8) |
| Sarcomatoid differentiation, n (%) | 11 (14) |
| Nephrectomy, n (%) | 69 (97) |
| Fuhrman grade | |
| 1, n (%) | 6 (8) |
| 2, n (%) | 33 (46) |
| 3, n (%) | 21 (30) |
| 4, n (%) | 11 (15) |
| MSKCC score | |
| 0, n (%) | 19 (27) |
| 1, n (%) | 36 (51) |
| 2, n (%) | 15 (21) |
| 3, n (%) | 1 (1) |
| IMDC prognostic score | |
| 0, n (%) | 16 (23) |
| 1, n (%) | 30 (42) |
| 2, n (%) | 15 (21) |
| 3, n (%) | 7 (10) |
| 4, n (%) | 3 (4) |
| Metastases | |
| Lungs, n (%) | 53 (75) |
| Bone, n (%) | 24 (34) |
| Liver, n (%) | 12 (17) |
| Pancreas, n (%) | 6 (8) |
| Other sites, n (%) | 31 (44) |
| Median number of sites (Q1; Q3) | 2 (2; 3) |
| ECOG performance score | |
| 0, n (%) | 19 (27) |
| 1, n (%) | 42 (59) |
| 2, n (%) | 9 (13) |
| 3, n (%) | 1 (1) |
| Karnofsky performance scale | |
| 100, n (%) | 10 (14) |
| 90, n (%) | 21 (30) |
| 80, n (%) | 37 (52) |
| <80, n (%) | 3 (4) |
| Cabozantinib as second-line treatment, n (%) | 30 (42) |
| Cabozantinib as third-line treatment, n (%) | 36 (50) |
| Cabozantinib as fourth- or fifth-line treatment, n (%) | 5 (7) |
| n (%) | |
|---|---|
| First-line treatment | |
| TKI (sunitinib, pazopanib, sorafenib), n (%) | 65 (92) |
| Other (immunotherapy), n (%) | 6 (8) |
| Second-line treatment | |
| TKI (axitinib, sunitinib, pazopanib, sorafenib), n (%) | 20 (28) |
| Everolimus, temsirolimus, n (%) | 18 (25) |
| Nivolumab, n (%) | 3 (4) |
| Third-line treatment | |
| TKI (sorafenib, pazopanib), n (%) | 4 (6) |
| Nivolumab, n (%) | 1 (1) |
| Fourth-line treatment (nivolumab), n (%) | 1 (1) |
| Variable | Values Observed in mRCC Patients (n = 71) |
|---|---|
| Any adverse event, n (%) | 65 (92) |
| Hypothyroidism, n (%) | 35 (49) |
| Hand-foot syndrome, n (%) | 33 (46) |
| Hypertension, n (%) | 28 (39) |
| Diarrhea, n (%) | 28 (39) |
| Asthenia, n (%) | 24 (34) |
| Liver toxicity, n (%) | 11 (15) |
| >1 reported adverse event, n (%) | 39 (55) |
| Median number of adverse events (Q1, Q3) | 2 (1–4) |
| Dose reduction | 35 (49) |
| Author (Year) | Patients, n Male n (%) Female n (%) | Mean Age (SD), Years | ECOG Performance Score, n (%) | IMDC Score, n (%) | CTAE All Stages, n (%) | CTAE ≥ 3, n (%) | Dose Reduction, n (%) |
|---|---|---|---|---|---|---|---|
| Domański et al. (2024) [59] | 71 | 63 (9) | 0, n = 19 (27) 1, n = 42 (59) 2, n = 9 (13) 3, n = 1 (1) | 0, n = 16 (23) 1, n = 30 (42) 2, n = 15 (21) 3, n = 7 (10) 4, n = 3 (4) | Hypothyroidism, n = 35 (49) Diarrhea, n = 28 (39) HFS, n = 33 (46) Hypertension, n = 28 (39) Any AEs: n = 65 (92) | N/D | 35 (49) |
| Patients treated in clinical trials | |||||||
| METEOR Choueiri et al. (2016) [33] | 330, male, n = 253 (77) female, n = 77 (23) | 63 | 0, n = 226 (68) 1, n = 104 (32) | Favorable, n = 66 (20) Intermediate, n = 210 (64) Poor, n = 54 (16) | Hypothyroidism, n = 76 (23) Diarrhea, n = 249 (75) HFS, n = 142 (43) Hypertension, n = 122 (37) Any AEs: n = 331 (100) | Hypothyroidism, n = 0 Diarrhea, n = 43 (13) HFS, n = 27 (8) Hypertension, n = 49 (15) Any AEs: n = 235 (71) | 206 (62) |
| CABOSUN Choueiri et al. (2017) [31] | 79, male, n = 66 (84) female, n = 13 (16) | 63 | 0, n = 36 (46) 1, n = 33 (42) 2, n = 10 (13) | Intermediate, n = 64 (81) Poor, n = 15 (19) | Hypothyroidism, n = 18 (23) Diarrhea, n = 57 (73) HFS, n = 33 (43) Hypertension, n = 52 (67) Any AEs: n = 75 (96) | Hypothyroidism, n = 0 Diarrhea, n = 8 (10) HFS, n = 6 (8) Hypertension, n = 22 (28) Any AEs: n = 53 (68) | 36 (46) |
| Retrospective cohort and real-world experience studies | |||||||
| Prisciandaro et al. (2019) [29] | 17 male, n = 11 (65) female, n = 6 (35) | 66 | 0, n = 3 (18) 1, n = 12 (70) 2, n = 2 (12) | Favorable, n = 9 (53) Intermediate, n = 8 (47) Poor, n = 0 | Hypothyroidism, n = 4 (24) Diarrhea, n = 6 (35) HFS, n = 4 (24) Hypertension, n = 3 (18) Any AEs: n = 16 (94) | Hypothyroidism, n = 1 (6) Diarrhea, n = 2 (11) HFS, n = 0 Hypertension, n = 3 (18) Any AEs: n = 7 (41) | 8 (47) |
| Lemke et al. (2019) [44] | 38 male, n = 25 (66) female, n = 13 (34) | 58 | 0, n = 2 (5) 1, n = 21 (55) 2, n = 15 (40) | Favorable, n = 7 (18) Intermediate, n = 20 (56) Poor, n = 9 (26) | Hypothyroidism, n = 13 (34) Diarrhea, n = 11 (29) HFS, n = 13 (34) Hypertension, n = 9 (24) Any AEs: N/D | Hypothyroidism, n = 4 (24) Diarrhea, n = 0 HFS, n = 1 (3) Hypertension, n = 0 Any AEs: N/D | 22 (58) |
| McElwee et al. (2019) [26] | 35 male, n = 30 (86) female, n = 5 (14) | 64 | 0, n = 8 (23) 1, n = 20 (57) 2, n = 7 (20) | N/D | Hypothyroidism, no data Diarrhea, N/D HFS, n = 9 (26) Hypertension, n = 8 (23) Any AEs: N/D | N/D | 8 (23) |
| Gomez de Liano Lista et al. (2018) [51] | 128 male, n = 87 (68) female, n = 41 (32) | 62 | 0, n = 20 (16) 1, n = 85 (66) 2, n = 23 (18) | Favorable, n = 35 (27) Intermediate, n = 62 (49) Poor, n = 27 (21) Unknown, n = 4 (3) | N/D | Hypothyroidism, N/D Diarrhea, n = 12 (9) HFS, n = 6 (5) Hypertension, N/D Any AEs: 48 (37) | N/D |
| Bodnar et al. (2019) [15] | 115 male n = 84 (73) female, n = 31 (27) | 64 | 0, n = 19 (16) 1, n = 79 (69) 2, n = 17 (15) | Favorable, n = 14 (12) Intermediate, n = 79 (69) Poor, n = 22 (19) | Hypothyroidism, n = 77 (67) Diarrhea, n = 70 (61) HFS, n = 52 (45) Hypertension, n = 51 (44) Any AEs: n = 115 (100) | Hypothyroidism, n = 0 Diarrhea, n = 11 (10) HFS, n = 14 (12) Hypertension, n = 6 (5) Any AEs: n = 56 (49) | 79 (69) |
| McGregor et al. (2020) [52] | 86 male n = 61 (71) female, n = 25 (29) | 63 | N/D | Favorable, n = 4 (5) Intermediate, n = 57 (66) Poor, n = 24 (28) | Hypothyroidism, N/D Diarrhea, n = 9 (10) HFS, n = 14 (16) Hypertension, n = 3 (3) Any AEs: n = 81 (94) | Hypothyroidism, N/D Diarrhea, n = 1 (1) HFS, n = 2 (2) Hypertension, n = 2 (2) Any AEs: n = 20 (23) | 39 (45) |
| Sumanta K. Pal et al. (2021) [42] | 44 male n = 36 (82) female n = 8 (18) | 65 | N/D | Favorable, n = 10 (23) Intermediate, n = 28 (64) Poor, n = 6 (14) | Hypothyroidism, N/D Diarrhea, n = 24 (55) HFS, n = 21 (48) Hypertension, n = 28 (65) Any AEs: n = 42 (97) | Hypothyroidism, N/D Diarrhea, n = 2 (4) HFS, n = 9 (20) Hypertension, n = 14 (32) Any AEs: n = 32 (74) | 10 (23) * |
| Matthew T. Campbell et al. (2018) [28] | 30 male n = 26 (87) female n = 4 (13) | 58 | N/D | Favorable, n = 2 (7) Intermediate, n = 23 (77) Poor, n = 5 (17) | Hypothyroidism, N/D Diarrhea, n = 17 (57) HFS, n = 11 (37) Hypertension, N/D Any AEs: N/D | Hypothyroidism, N/D Diarrhea, n = 0 HFS, n = 2 (7) Hypertension, N/D Any AEs: N/D | 17 (57) |
| Nieves Martínez Chanzá et al. (2019) [27] | 112 male n = 85 (76) female n = 27 (24) | 60 | 0, n = 23 (20) 1, n = 59 (53) 2–3, n = 11 (10) | Favorable, n = 9 (8) Intermediate, n = 71 (63) Poor, n = 29 (26) unknown, n = 3 (3) | Hypothyroidism, n = 17 (15) Diarrhea, n = 38 (34) HFS, n = 35 (31) Hypertension, n = 31 (28) Any AEs: N/D | Hypothyroidism **, n = 0 Diarrhea **, n = 3 (3) HFS **, n = 5 (4) Hypertension **, n = 4 (4) Any AEs **: N/D | 51 (46) |
| Noboru Nakaigawa et al. (2023) [34] | 35 male, N/D female, N/D | 63 | N/D | Favorable, n = 6 (17) Intermediate, n = 22 (63) Poor, n = 7 (20) | Hypothyroidism, n = 6 (17) Diarrhea, n = 24 (67) HFS, n = 23 (66) Hypertension, n = 15 (43) Any AEs: 35 (100) | Hypothyroidism, n = 0 Diarrhea, n = 3 (9) HFS, n = 3 (9) Hypertension, n = 5 (14) Any AEs: 29 (83) | 33 (94) |
| Koji Iinuma et al. (2022) [35] | 53 male n = 44 (83) female n = 9 (17) | 72 | 0, n = 18 (34) 1, n = 17 (32) 2, n = 9 (17) 3, n = 8 (15) 4, n = 1 (2) | Favorable, n = 12 (23) Intermediate, n = 17 (51) Poor, n = 14 (26) | Hypothyroidism, n = 9 (17)) Diarrhea, n = 14 (26) HFS, n = 9 (17) Hypertension, n = 11 (21) Any AEs: 42 (79) | Hypothyroidism, n = 0 Diarrhea, n = 2 (4) HFS, n = 3 (6) Hypertension, n = 1 (2) Any AEs: 10 (19) | 4 (8) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domański, P.; Piętak, M.; Staneta, S.; Fortuniak, W.; Kruczyk, B.; Kobiernik, A.; Bakuła, P.; Mydlak, A.; Demkow, T.; Sikora-Kupis, B.; et al. Analysis of Factors Contributing to Adverse Events and Evaluation of Their Impact on Prognosis in Metastatic Renal Cell Carcinoma Patients—Real-World Experience in a Single-Center Retrospective Study and Narrative Review. Medicina 2024, 60, 398. https://doi.org/10.3390/medicina60030398
Domański P, Piętak M, Staneta S, Fortuniak W, Kruczyk B, Kobiernik A, Bakuła P, Mydlak A, Demkow T, Sikora-Kupis B, et al. Analysis of Factors Contributing to Adverse Events and Evaluation of Their Impact on Prognosis in Metastatic Renal Cell Carcinoma Patients—Real-World Experience in a Single-Center Retrospective Study and Narrative Review. Medicina. 2024; 60(3):398. https://doi.org/10.3390/medicina60030398
Chicago/Turabian StyleDomański, Piotr, Mateusz Piętak, Szymon Staneta, Weronika Fortuniak, Barbara Kruczyk, Adam Kobiernik, Piotr Bakuła, Anna Mydlak, Tomasz Demkow, Bożena Sikora-Kupis, and et al. 2024. "Analysis of Factors Contributing to Adverse Events and Evaluation of Their Impact on Prognosis in Metastatic Renal Cell Carcinoma Patients—Real-World Experience in a Single-Center Retrospective Study and Narrative Review" Medicina 60, no. 3: 398. https://doi.org/10.3390/medicina60030398
APA StyleDomański, P., Piętak, M., Staneta, S., Fortuniak, W., Kruczyk, B., Kobiernik, A., Bakuła, P., Mydlak, A., Demkow, T., Sikora-Kupis, B., Dumnicka, P., & Kucharz, J. (2024). Analysis of Factors Contributing to Adverse Events and Evaluation of Their Impact on Prognosis in Metastatic Renal Cell Carcinoma Patients—Real-World Experience in a Single-Center Retrospective Study and Narrative Review. Medicina, 60(3), 398. https://doi.org/10.3390/medicina60030398







