Nephroprotective and Anti-Diabetic Potential of Beta vulgaris L. Root (Beetroot) Methanolic Extract in a Rat Model of Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Diets
2.3. Preparation of Beetroot Stems Methanol Extract
2.4. GC–MS analysis of Beta vulgaris (L.) Pulp Extract
2.5. Establishment of T2DM
2.6. Experimental groups
- (1)
- Control rats: fed the standard diets and administered 5% carboxymethyl cellulose (CMC) as a vehicle.
- (2)
- Control + BE (250 mg/kg)-treated groups: fed the standard diet and orally administered BE extract (250 mg/kg/day).
- (3)
- Control + BE (500 mg/kg)-treated groups: fed a standard diet and orally treated with BE extract alone (500 mg/kg/day).
- (4)
- T2DM model group: rats with pre-established T2DM which fed HFD and orally administered 5% CMC.
- (5)
- T2DM + BE (250 mg)-treated group: rats with pre-established T2DM that continued on HFD and co-treated with BE extract (250 mg/kg/day).
- (6)
- T2DM + BE (500 mg/kg)-treated groups: rats with pre-established T2DM that were fed HFD and co-treated with BE extract (500 mg/kg/day).
2.7. Collection of Urine, Blood, and Kidneys
2.8. Biochemical Measurements in the Urine and Serum
2.9. Biochemical Analysis in the Renal Homogenates
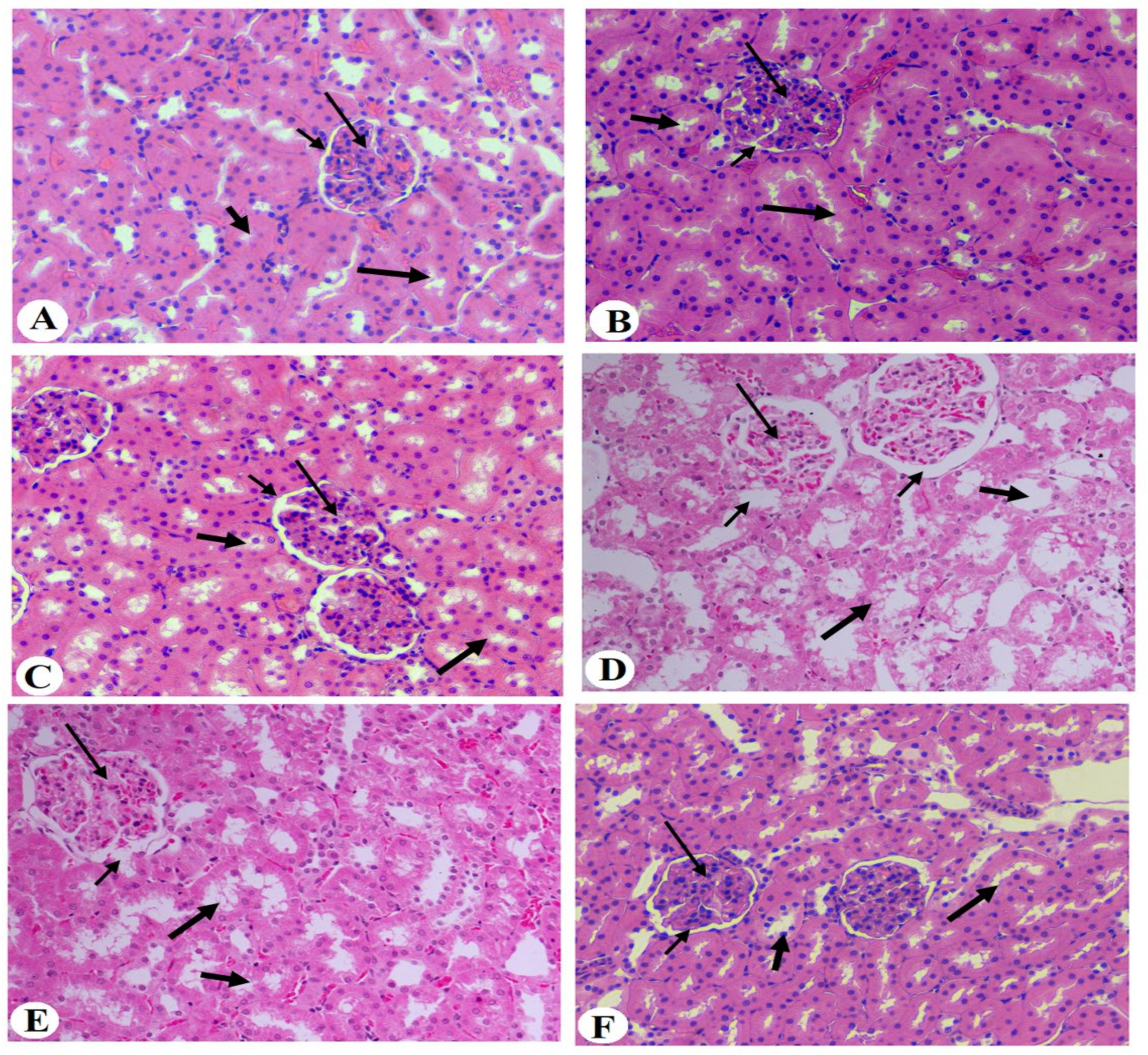
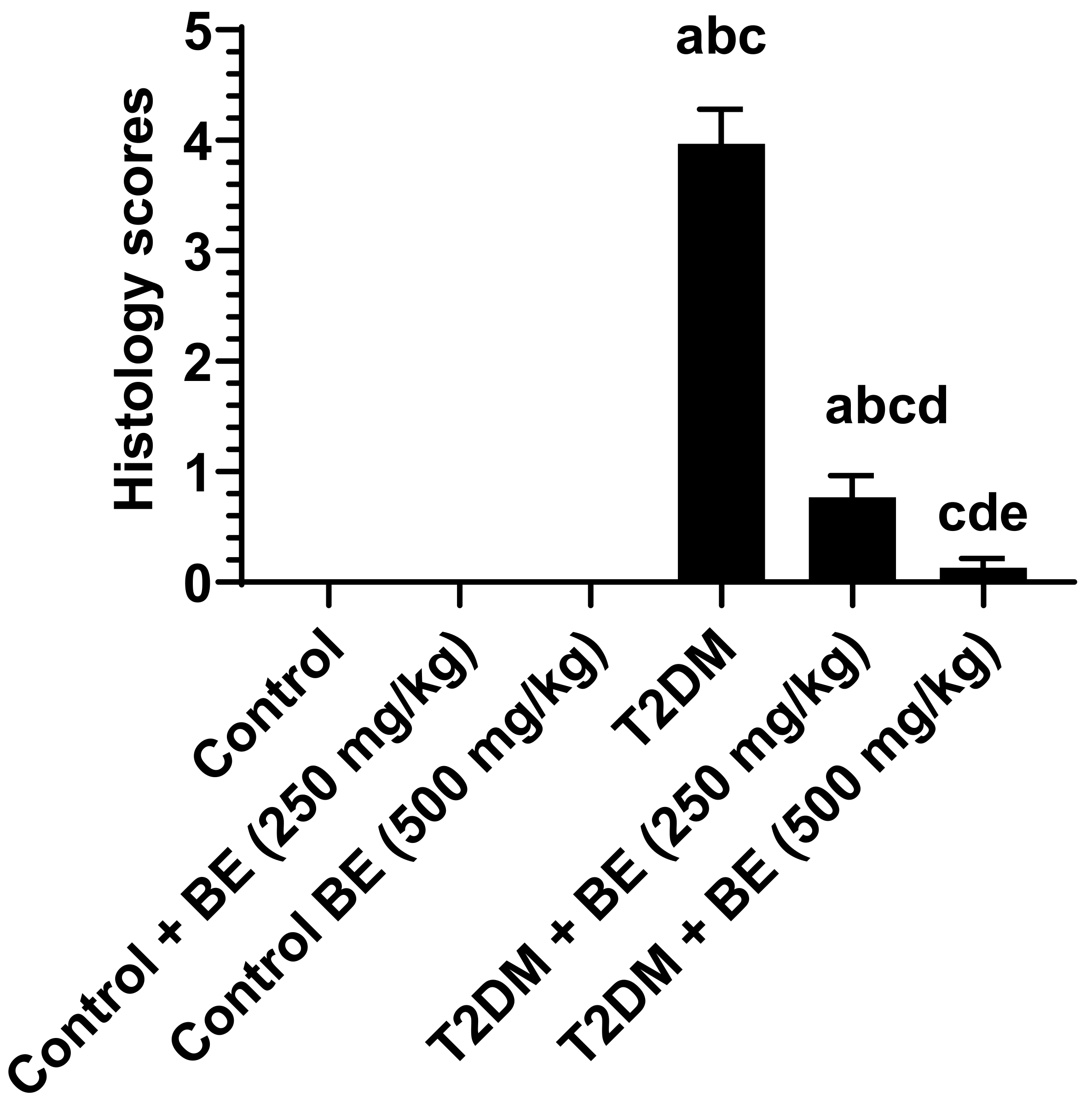
2.10. Histopathological Evaluation
2.11. Statistical Analysis
3. Results
3.1. Identification of Chemical Components from BE
3.2. Final Body Weights and Fasting Plasma Levels of Glucose and Insulin in All Groups of Rats
3.3. Serum Levels of the Lipid Profile of All Experimented Groups of Rats
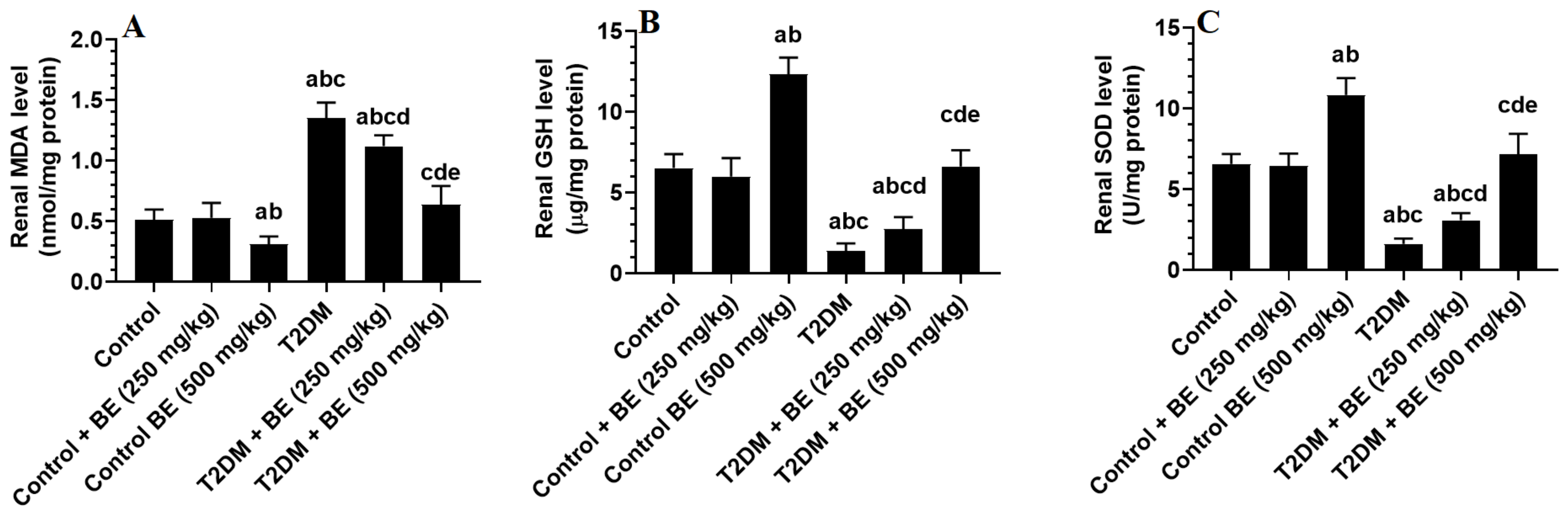
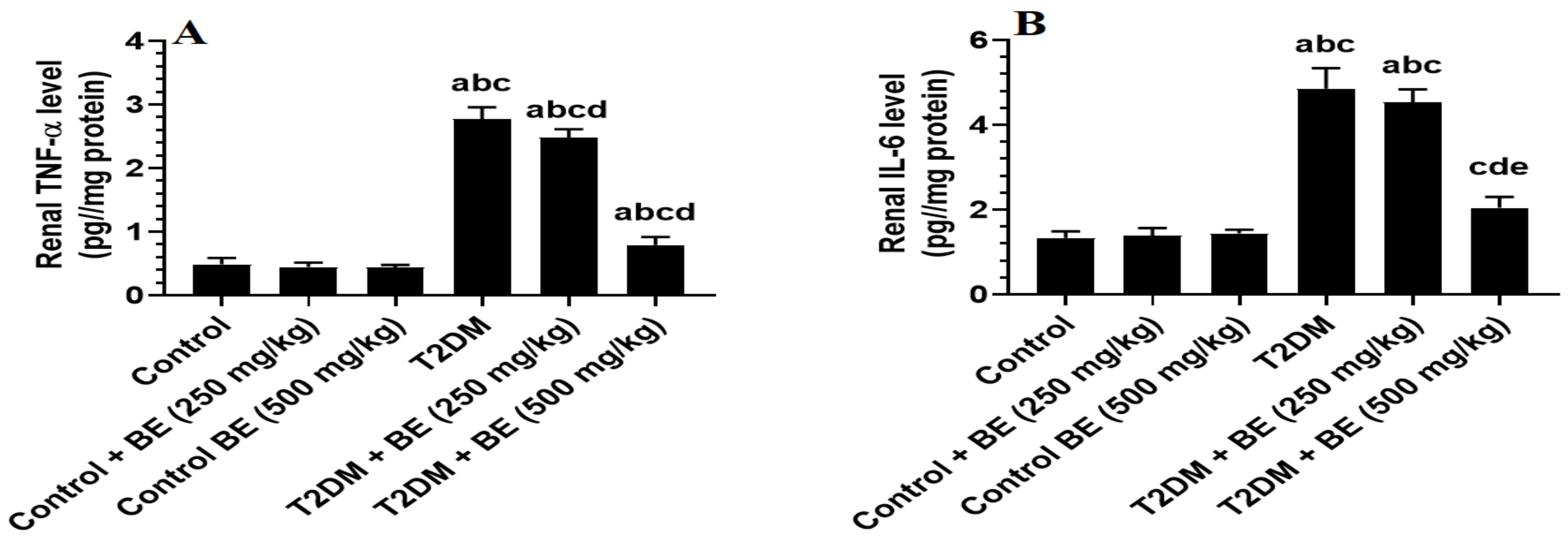
3.4. The Renal Levels of Oxidative Stress (GSH, SOD, and MDA) and Inflammatory Markers (TNF-α and IL-6) in All Groups of Rats
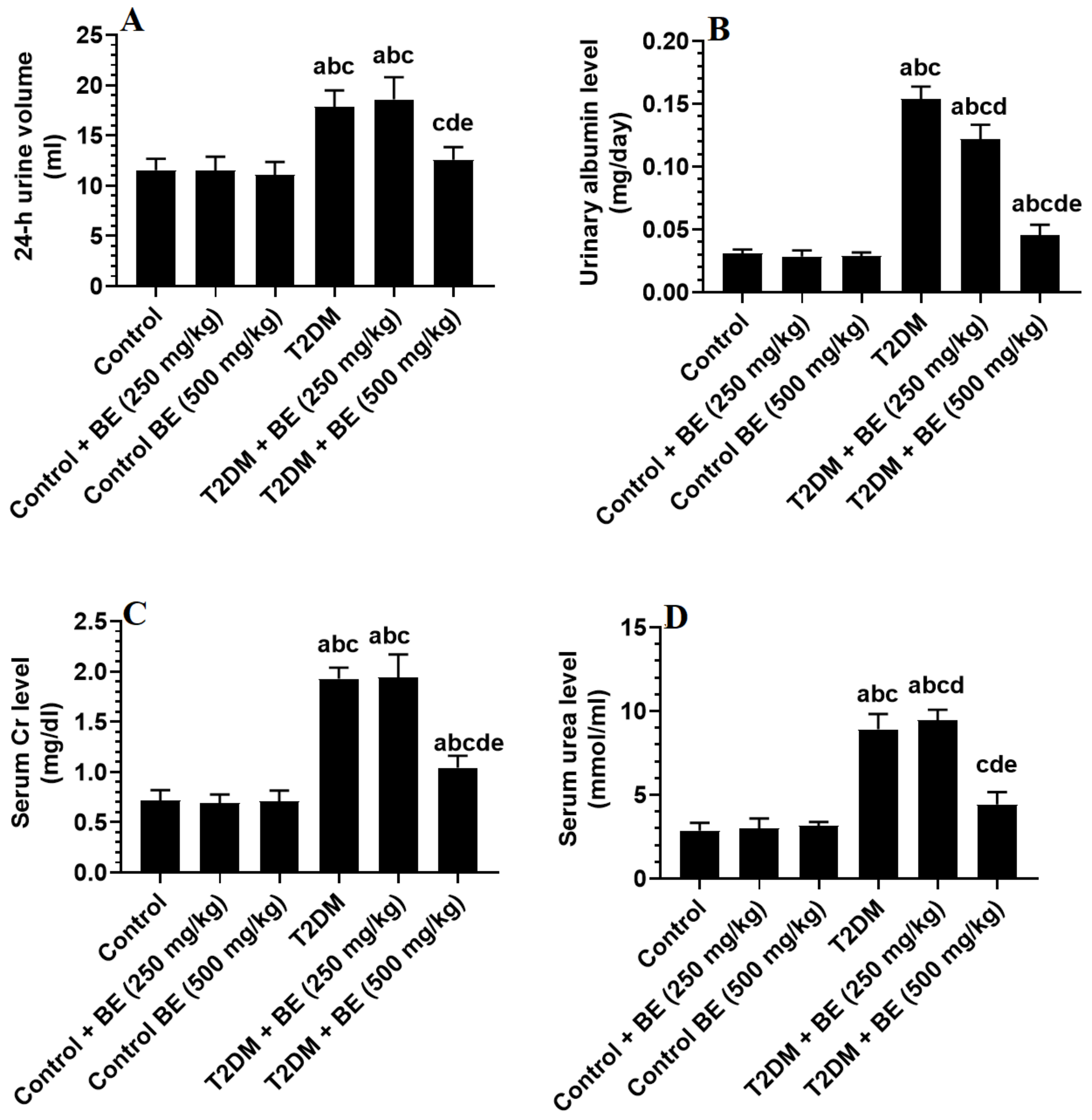
3.5. Urinary Albumin and Serum Levels of Creatinine (Cr) and Urea
3.6. Histological Results of the Kidneys of All Groups of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef]
- Heredia-Martine, A.; Rosa-Diez, G.; Ferraris, J.R.; Sohlenius-Sternbeck, A.K.; Nihlen, C.; Olsson, A.; Lundberg, J.O.; Weitzberg, E.; Carlström, M.; Krmar, R.T. Plasma nitrate and nitrite kinetics after single intake of beetroot juice in adult patients on chronic hemodialysis and in healthy volunteers: A randomized, single-blind, placebo-controlled, crossover study. Nutrients 2022, 14, 2480. [Google Scholar] [CrossRef] [PubMed]
- Rajendra, A.; Bondonno, N.P.; Rainey-Smith, S.R.; Gardener, S.L.; Hodgson, J.M.; Bondonno, C.P. Potential role of dietary nitrate in relation to cardiovascular and cerebrovascular health, cognition, cognitive decline and dementia: A review. Food Funct. 2022, 13, 12572–12589. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Singh, A.; Chaudhary, V.; Sharma, N.; Lorenzo, J.M. Beetroot as a novel ingredient for its versatile food applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 8403–8427. [Google Scholar] [CrossRef] [PubMed]
- Holy, B.; Isaac, N.N.; Ngoye, B.O. Post-prandial effect of beetroot (Beta vulgaris) juice on glucose and lipids levels of apparently healthy subjects. Eur. J. Pharm. Med. Res. 2017, 4, 60–62. [Google Scholar]
- Albasher, G.; Almeer, R.; Al-Otibi, F.O.; Al-Kubaisi, N.; Mahmoud, A.M. Ameliorative Effect of Beta vulgaris Root Extract on Chlorpyrifos-Induced Oxidative Stress, Inflammation and Liver Injury in Rats. Biomolecules 2019, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumari, S.; Dubey, N.K.; Usha, D. Anti-Diabetic and Haematinic Effects of Beetroot Juice (Beta vulgaris L.) in Alloxan Induced Type-1 Diabetic Albino Rats. J. Diabetes Res. Ther. 2020, 6, 1–3. [Google Scholar]
- Al-Harbi, L.; Alshammari, G.M.; Al-Dossari, A.M.; Subash-Babu, P.; Binobead, M.A.; Alhussain, M.H.; AlSedairy, S.A.; Al-Nouri, D.M.; Shamlan, G. Beta vulgaris L. (Beetroot) methanolic extract prevents hepatic steatosis and liver damage in T2DM Rats by hypoglycemic, insulin-sensitizing, antioxidant effects, and upregulation of PPARα. Biology 2021, 10, 1306. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.S.G.; Fanton, S.; Cardozo, L.; Borges, N.A.; Combet, E.; Shiels, P.G.; Stenvinkel, P.; Mafra, D. Pink pressure: Beetroot (Beta vulgaris rubra) as a possible novel medical therapy for chronic kidney disease. Nutr. Rev. 2022, 80, 1041–1061. [Google Scholar] [CrossRef]
- Albrahim, T. Silver nanoparticles-induced nephrotoxicity in rats: The protective role of red beetroot (Beta vulgaris) juice. Environ. Sci. Pollut. Res. Int. 2020, 27, 38871–38880. [Google Scholar] [CrossRef]
- Albasher, G.; Almeer, R.; Alarifi, S.; Alkhtani, S.; Farhood, M.; Al-Otibi, F.O.; Alkubaisi, N.; Rizwana, H. Nephroprotective role of Beta vulgaris L. root extract against chlorpyrifos-induced renal injury in rats. Evid. Based Complement. Alternat Med. 2019, 2019, 3595761. [Google Scholar] [CrossRef]
- Iahtisham-Ul-Haq; Butt, M.S.; Randhawa, M.A.; Shahid, M. Nephroprotective effects of red beetroot-based beverages against gentamicin-induced renal stress. J. Food Biochem. 2019, 43, e12873. [Google Scholar] [CrossRef]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediators Inflamm. 2014, 2014, 983952. [Google Scholar] [CrossRef]
- Wang, Y.; Sui, Z.; Wang, M.; Liu, P. Natural products in attenuating renal inflammation via inhibiting the NLRP3 inflammasome in diabetic kidney disease. Front. Immunol. 2023, 14, 1196016. [Google Scholar] [CrossRef]
- Gross, J.L.; De Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention, and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 7th ed.; IDF: Brussels, Belgium, 2015. [Google Scholar]
- Su, W.; Cao, R.; He, Y.C.; Guan, Y.F.; Ruan, X.Z. Crosstalk of Hyperglycemia and Dyslipidemia in Diabetic Kidney Disease. Kidney Dis. 2017, 3, 171–180. [Google Scholar] [CrossRef]
- Weir, M.R.; Agarwal, R.; Rossing, P.; Usman, M.S.; Khan, M.S.; Butler, J.; Norris, K.C.; Dagogo-Jack, S.; Naaman, S.C.; Bakris, G.L. Chronic Kidney Disease and Type 2 Diabetes; American Diabetes Association: Arlington, VA, USA, 2021. [Google Scholar]
- Lin, Y.-C.; Chang, Y.-H.; Yang, S.-Y.; Wu, K.-D.; Chu, T.-S. Update of pathophysiology and management of diabetic kidney disease. J. Formos. Med. Assoc. 2018, 117, 662–675. [Google Scholar] [CrossRef]
- Alsawaf, S.; Alnuaimi, F.; Afzal, S.; Thomas, R.M.; Chelakkot, A.L.; Ramadan, W.S.; Hodeify, R.; Matar, R.; Merheb, M.; Siddiqui, S.S.; et al. Plant Flavonoids on Oxidative Stress-Mediated Kidney Inflammation. Biology 2022, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.; Jena, G.B. NLRP3 inhibitor glibenclamide attenuates high-fat diet and streptozotocin-induced non-alcoholic fatty liver disease in rat: Studies on oxidative stress, inflammation, DNA damage and insulin signalling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Wongmekiat, O.; Lailerd, N.; Kobroob, A.; Peerapanyasut, W. Protective Effects of Purple Rice Husk against Diabetic Nephropathy by Modulating PGC-1α/SIRT3/SOD2 Signaling and Maintaining Mitochondrial Redox Equilibrium in Rats. Biomolecules 2021, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- El Amin Ali, A.M.; Osman, H.M.; Zaki, A.M.; Shaker, O.; Elsayed, A.M.; Abdelwahed, M.Y.; Mohammed, R.A. Reno-protective effects of GLP-1 receptor agonist and anti-platelets in experimentally induced diabetic kidney disease in male albino rats. Iran. J. Basic Med. Sci. 2022, 25, 1487–1497. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Dyun, M.A.S.; Pivonka, E. Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: Selected literature. J. Am. Diet. Assoc. 2000, 200, 1511–1521. [Google Scholar]
- Lee, J.H.; Son, C.W.; Kim, M.Y.; Kim, M.H.; Kim, H.R.; Kwak, E.S.; Kim, S.; Kim, M.R. Red beet (Beta vulgaris L.) leaf supplementation improves antioxidant status in C57BL/6J mice fed high fat high cholesterol diet. Nutr. Res. Pract. 2009, 3, 114–121. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Khalifi, S.; Rahimipour, A.; Jeddi, S.; Ghanbari, M.; Kazerouni, F.; Ghasemi, A. Dietary nitrate improves glucose tolerance and lipid profile in an animal model of hyperglycemia. Nitric Oxide 2015, 44, 24–30. [Google Scholar] [CrossRef]
- Rabeh, M.N.; Ibrahim, E.M. Antihypercholesterolemic effects of beet (Beta vulgaris L.) root waste extract on hypercholesterolemic rats and its antioxidant potential properties. Pak. J. Nutr. 2014, 13, 500. [Google Scholar] [CrossRef][Green Version]
- Singh, A.; Verma, S.; Singh, V.; Jappa, C.; Roopa, N.; Raju, P.; Singh, S. Beetroot Juice Supplementation Increases High Density Lipoprotein-Cholesterol and Reduces Oxidative Stress in Physically Active Individuals. J. Pharm. Nutr. Sci. 2015, 5, 179–185. [Google Scholar] [CrossRef]
- Lotfi, M.; Azizi, M.; Tahmasebi, W.; Bashiri, P. Efficacy of Beetroot Juice Consumption on the Lipid Profile of Female Soccer Players. Med. Lab. J. 2020, 14, 26–30. [Google Scholar] [CrossRef]
- Rashid, K.; Chowdhury, S.; Ghosh, S.; Sil, P.C. Curcumin attenuates oxidative stress induced nfκb mediated inflammation and endoplasmic reticulum dependent apoptosis of splenocytes in diabetes. Biochem. Pharmacol. 2017, 143, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.S.; Syed, I.; Gray, J.P.; Ray, S.D. Curcumin, hesperidin, and rutin selectively interfere with apoptosis signaling and attenuate streptozotocin-induced oxidative stress-mediated hyperglycemia. Curr. Neurovasc. Res. 2015, 12, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Peng, L.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar] [CrossRef]
- Mima, A. Inflammation and oxidative stress in diabetic nephropathy: New insights on its inhibition as new therapeutic targets. J. Diabetes Res. 2013, 2013, 248563. [Google Scholar] [CrossRef]
- Kawanami, D.; Matoba, K.; Utsunomiya, K. Dyslipidemia in diabetic nephropathy. Ren. Replace. Ther. 2016, 2, 16. [Google Scholar] [CrossRef]
- Amorim, R.G.; Guedes, G.D.S.; Vasconcelos, S.M.L.; Santos, J.C.F. Kidney Disease in Diabetes Mellitus: Cross-Linking between Hyperglycemia, Redox Imbalance and Inflammation. Arq. Bras. Cardiol. 2019, 112, 577–587. [Google Scholar] [CrossRef]
- Sahakyan, G.; Vejux, A.; Sahakyan, N. The Role of Oxidative Stress-Mediated Inflammation in the Development of T2DM-Induced Diabetic Nephropathy: Possible Preventive Action of Tannins and Other Oligomeric Polyphenols. Molecules 2022, 27, 9035. [Google Scholar] [CrossRef]
- Aliahmadi, M.; Amiri, F.; Bahrami, L.S.; Hosseini, A.F.; Abiri, B.; Vafa, M. Effects of raw red beetroot consumption on metabolic markers and cognitive function in type 2 diabetes patients. J. Diabetes Metab. Disord. 2021, 20, 673–682. [Google Scholar] [CrossRef]
- Vidal, P.J.; López-Nicolás, J.M.; Gandía-Herrero, F.; García-Carmona, F. Inactivation of lipoxygenase and cyclooxygenase by natural betalains and semi-synthetic analogues. Food Chem. 2014, 154, 246–254. [Google Scholar] [CrossRef]
- Lytvyn, Y.; Bjornstad, P.; van Raalte, D.H.; Heerspink, H.L.; Cherney, D.Z.I. The New Biology of Diabetic Kidney Disease-Mechanisms and Therapeutic Implications. Endocr. Rev. 2020, 41, 202–231. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sato, E.; Mishima, E.; Miyazaki, M.; Tanaka, T. What’s New in the Molecular Mechanisms of Diabetic Kidney Disease: Recent Advances. Int. J. Mol. Sci. 2022, 24, 570. [Google Scholar] [CrossRef] [PubMed]
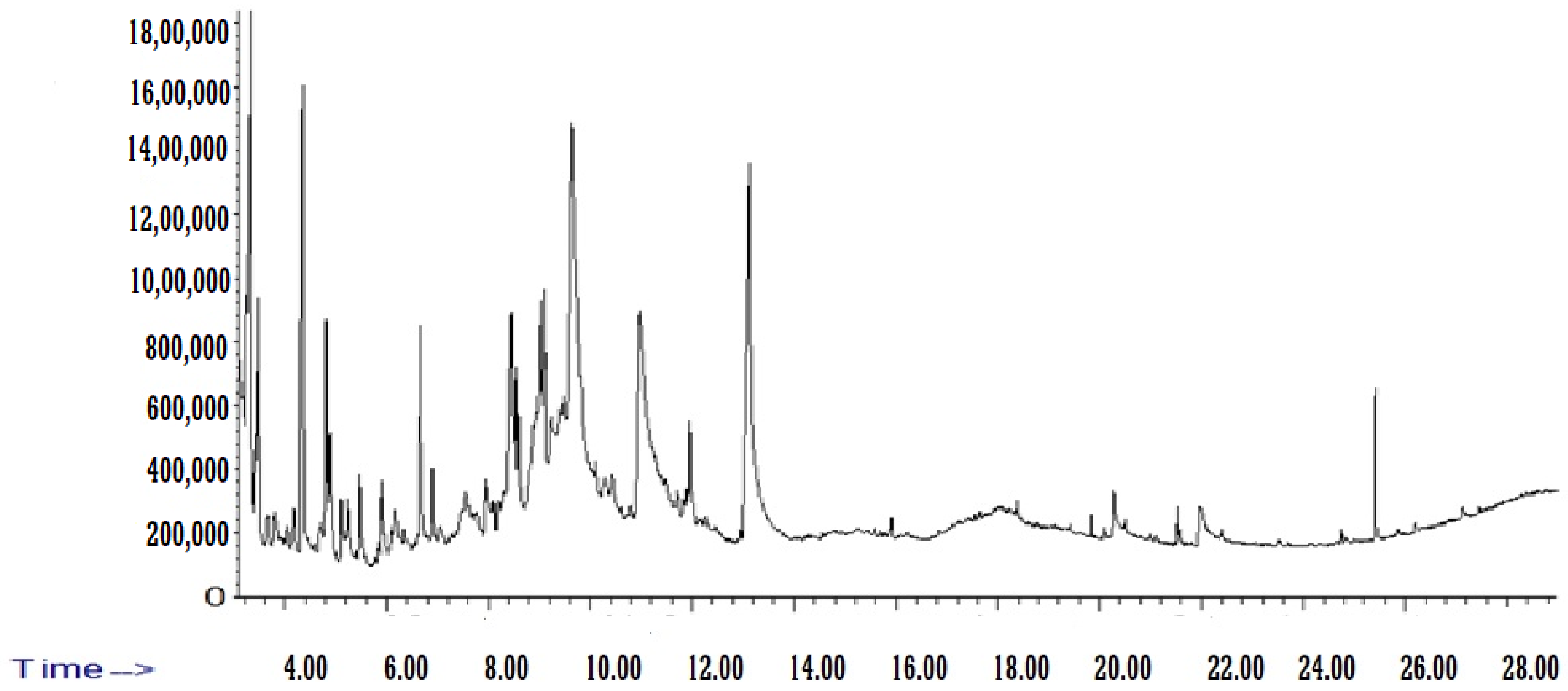
| Name of Compound | Chemical Formula | Molecular Weight (g/mol) | RT (min) | Area% |
|---|---|---|---|---|
| Acetic acid | CH3COOH | 60.05 | 3.322 | 5.853792 |
| 2-Pentanone | C5H10O | 86.13 | 3.418 | 0.328596 |
| Hydroxyacetone | C3H6O2 | 74.08 | 3.481 | 2.542776 |
| N-(1,1,3,3-Tetramethylbutyl)formamide | C9H19NO | 157.25 | 3.653 | 0.475798 |
| N-nitroso-n-methylurethane | C4H8N2O3 | 132.12 | 3.818 | 0.726619 |
| N-Formyl-beta-alanine | C4H7NO3 | 117.1 | 4.054 | 0.324584 |
| 2,2,6-Trimethyl-4H-1,3-dioxin-4-one | C7H10O3 | 142.15 | 4.181 | 0.921803 |
| Methyl pyruvate | C4H6O3 | 102.09 | 4.334 | 9.822988 |
| 2-Methylpyrazine | C5H6N2 | 94.11 | 4.696 | 0.535542 |
| Furfural | C5H4O2 | 96.08 | 4.817 | 2.79617 |
| Pholedrine | C10H15NO | 165.23 | 4.894 | 1.169538 |
| Butyl 2-methoxyethyl phthalate | C15H20O5 | 280.31 | 5.123 | 0.680656 |
| METHYL PYRIDINE-4 | C6H7N | 93.13 | 5.237 | 0.75844 |
| 2-Cyclopentene-1,4-dione | C5H4O2 | 96.08 | 5.492 | 1.648608 |
| Hydroxybutanoic Acid-4 | C4H8O3 | 104.1 | 5.924 | 1.400053 |
| Methylcyclopentanone-2 | C6H10O | 98.14 | 6.179 | 0.661933 |
| 1,6;3,4-Dianhydro-2-O-Acetyl-Beta-D-Galactopyranose | C8H10O5 | 186 | 6.389 | 0.425374 |
| METHYLFURFURAL-5 | C6H6O2 | 110.11 | 6.669 | 3.096278 |
| 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one | C6H8O4 | 144.12 | 6.898 | 1.074236 |
| Octamethylcyclotetrasiloxane | C8H24O4Si4 | 296.61 | 7.063 | 0.540393 |
| 4-(Dimethylamino)butanenitrile | C6H12N2 | 112.17 | 7.47 | 0.643909 |
| Methylpiperazine-1 | C5H12N2 | 100.16 | 7.559 | 0.278528 |
| (Ethenyloxy)pentane-1 | C7H14O | 114.19 | 7.966 | 1.093734 |
| Ethanol, 2-(1-methylethoxy)-, acetate | C7H14O3 | 146.18 | 8.196 | 0.343234 |
| p-Cymene | C10H14 | 134.22 | 8.431 | 3.925166 |
| Ethyl-1,2-dimethylbenzen-4 | C10H14 | 134.22 | 8.539 | 0.926115 |
| Furan-3-carboxaldehyde, 2-methoxy-2,3-dihydro- | C6H8O3 | 128.13 | 8.596 | 0.7491 |
| 1,2,3,4-Tetramethylfulvene | C10H14 | 134.22 | 8.857 | 2.575367 |
| Tetramethylbenzene-1,2,4,5 | C10H14 | 134.22 | 9.093 | 1.615564 |
| N,N-Dimethylglycine | C4H9NO2 | 103.12 | 9.239 | 0.577166 |
| 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | C6H8O4 | 144.12 | 9.659 | 11.97173 |
| 5-Hydroxymethylfurfural | C6H6O3 | 126.11 | 11.02 | 0.479261 |
| Glycine, N-2-pyrrolidinylidene- | C6H10N2O2 | 142.16 | 11.707 | 11.84368 |
| 2-Methoxy-4-vinylphenol | C9H10O2 | 150.17 | 11.962 | 0.311455 |
| methyl 5-oxopyrrolidine-2-carboxylate | C6H9NO3 | 143.14 | 13.164 | 1.356614 |
| Methyl Palmitate | C17H34O2 | 270.5 | 19.838 | 20.14024 |
| Palmitic acid | C16H32O2 | 256.42 | 20.309 | 0.258838 |
| Methyl linoleate | C19H34O2 | 294.5 | 21.524 | 1.107171 |
| 9,12-Octadecadienoic acid | C18H32O2 | 280.4 | 21.95 | 0.376314 |
| Control | Control + BE (250 mg/kg) | Control + BE (500 mg) | T2DM | T2DM + BE (250 mg/kg) | T2DM + BE (500 mg/kg) | |
|---|---|---|---|---|---|---|
| Final body weight (g) | 437.2 ± 29.5 | 446.6 ± 33.5 | 449.2 ± 31.6 | 626.8 ± 25.6 abc | 565.4 ± 33.2 abcd | 428.7 ± 28.6 de |
| Glucose (mg/dL) | 101.5 ± 8.6 | 96.5 ± 7.8 | 65.4 ± 6.1 ab | 153.7 ± 7.1 abc | 136.6 ± 11.7 abcd | 104.9 ± 10.4 bcde |
| Insulin (ng/mL) | 4.8 ± 0.7 | 4.6 ± 0.6 | 4.7 ± 0.6 | 9.3 ± 0.8 abc | 6.9 ± 0.4 abcd | 5.1 ± 0.5 de |
| HOMA-IR | 1.19 ± 0.24 | 1.11 ± 0.13 | 0.75 ± 0.11 ab | 3.545 ± 0.45 abc | 2.36 ± 0.32 abcd | 1.32 ± 0. 24 cde |
| Lipids (mg/dL) | Control | Control + BE (250 mg/kg) | Control + BE (500 mg) | T2DM | T2DM + BE (250 mg/kg) | T2DM + BE (500 mg/kg) | |
|---|---|---|---|---|---|---|---|
| Liver | TGs | 0.44 ± 0.6 | 0.38 ± 0.8 | 0.24 ± 0.3 ab | 0.88 ± 0.13 abc | 0.69 ± 0.06 abcd | 0.48 ± 0.05 abcde |
| CHOL | 2.71 ± 4.1 | 2.57 ± 0.14 | 2.0 ± 0.17 ab | 6.3 ± 0.52 abc | 4.9 ± 0.3 abcd | 3.1 ± 0.3 bcde | |
| Serum | TGs | 51.17 ± 4.8 | 40.6 ± 3.5 a | 30.1 ± 2.9 ab | 120.8 ± 8.3 abc | 85.0 ± 6.8 abcd | 55.3 ± 5.1 abcde |
| CHOL | 79.8 ± 6.2 | 68.1 ± 6.9 a | 55.3 ± 6.2 ab | 162.3 ± 11.8 abc | 136.1 ± 11.2 abcd | 95.0 ± 8.7 abcde | |
| LDL-C | 42.4 ± 4.1 | 38.7 ± 3.8 | 26.5 ± 2.7 ab | 96.3 ± 8.3 abc | 74.5 ± 6.7 abcd | 54.3 ± 4.6 abcde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Harbi, L.N.; Alshammari, G.M.; Shamlan, G.; Binobead, M.A.; AlSedairy, S.A.; Al-Nouri, D.M.; Arzoo, S.; Yahya, M.A. Nephroprotective and Anti-Diabetic Potential of Beta vulgaris L. Root (Beetroot) Methanolic Extract in a Rat Model of Type 2 Diabetes Mellitus. Medicina 2024, 60, 394. https://doi.org/10.3390/medicina60030394
Al-Harbi LN, Alshammari GM, Shamlan G, Binobead MA, AlSedairy SA, Al-Nouri DM, Arzoo S, Yahya MA. Nephroprotective and Anti-Diabetic Potential of Beta vulgaris L. Root (Beetroot) Methanolic Extract in a Rat Model of Type 2 Diabetes Mellitus. Medicina. 2024; 60(3):394. https://doi.org/10.3390/medicina60030394
Chicago/Turabian StyleAl-Harbi, Laila Naif, Ghedeir M. Alshammari, Ghalia Shamlan, Manal Abdulaziz Binobead, Sahar Abdulaziz AlSedairy, Doha M. Al-Nouri, Shaista Arzoo, and Mohammed Abdo Yahya. 2024. "Nephroprotective and Anti-Diabetic Potential of Beta vulgaris L. Root (Beetroot) Methanolic Extract in a Rat Model of Type 2 Diabetes Mellitus" Medicina 60, no. 3: 394. https://doi.org/10.3390/medicina60030394
APA StyleAl-Harbi, L. N., Alshammari, G. M., Shamlan, G., Binobead, M. A., AlSedairy, S. A., Al-Nouri, D. M., Arzoo, S., & Yahya, M. A. (2024). Nephroprotective and Anti-Diabetic Potential of Beta vulgaris L. Root (Beetroot) Methanolic Extract in a Rat Model of Type 2 Diabetes Mellitus. Medicina, 60(3), 394. https://doi.org/10.3390/medicina60030394





