Acute Heart Failure in the Course of Macrophage Activation Syndrome Due to Newly Diagnosed Systemic Lupus Erythematosus: Case Presentation and Literature Review
Abstract
1. Introduction
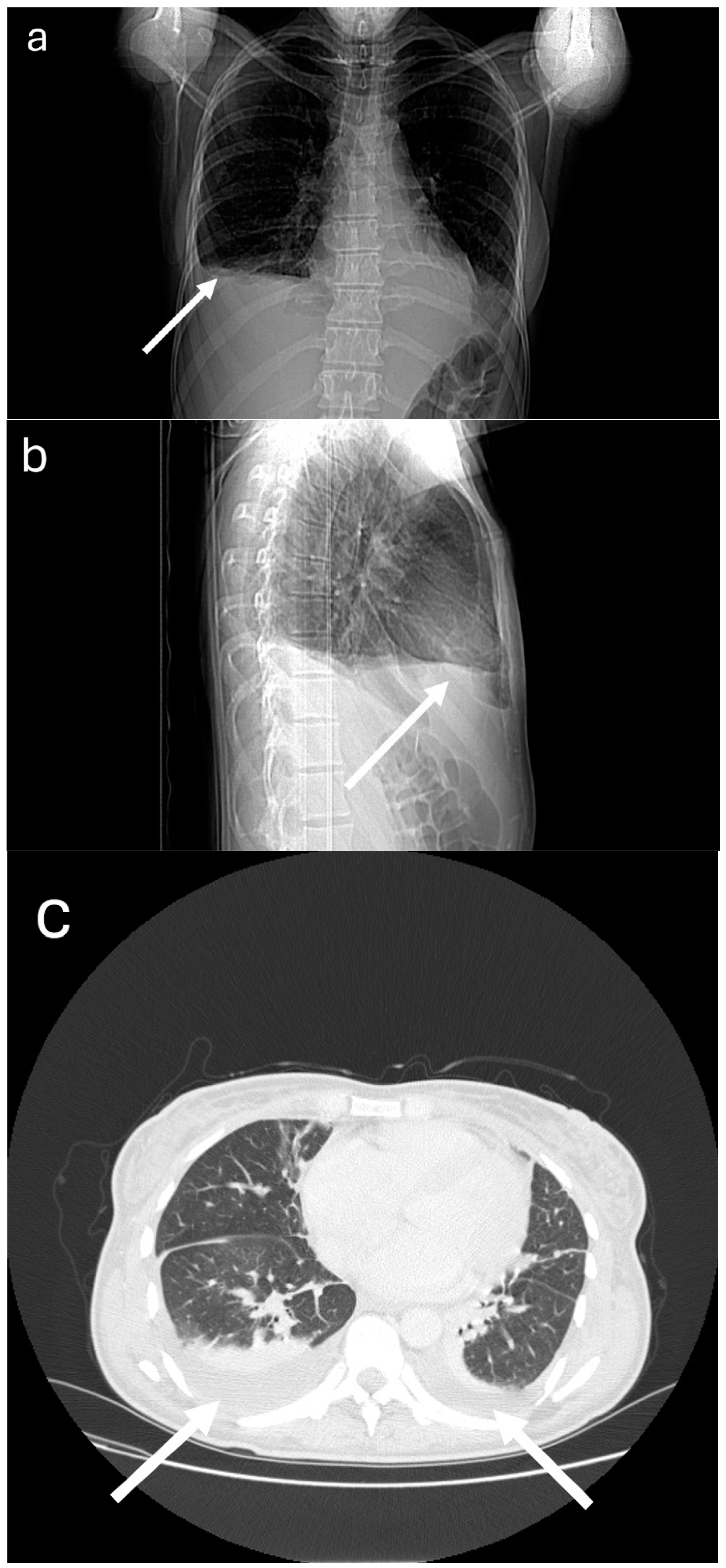
2. Case Presentation
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bojan, A.; Parvu, A.; Zsoldos, I.A.; Torok, T.; Farcas, A.D. Macrophage activation syndrome: A diagnostic challenge (Review). Exp. Ther. Med. 2021, 22, 904. [Google Scholar] [CrossRef] [PubMed]
- Lerkvaleekul, B.; Vilaiyuk, S. Macrophage activation syndrome: Early diagnosis is key. Open Access Rheumatol. Res. Rev. 2018, 10, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef] [PubMed]
- Crayne, C.B.; Albeituni, S.; Nichols, K.E.; Cron, R.Q. The Immunology of Macrophage Activation Syndrome. Front. Immunol. 2019, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Kuna, J.; Żuber, Z.; Chmielewski, G.; Gromadziński, L.; Krajewska-Włodarczyk, M. Role of Distinct Macrophage Populations in the Development of Heart Failure in Macrophage Activation Syndrome. Int. J. Mol. Sci. 2022, 23, 2433. [Google Scholar] [CrossRef]
- Minoia, F.; Davì, S.; Horne, A.; Demirkaya, E.; Bovis, F.; Li, C.; Lehmberg, K.; Weitzman, S.; Insalaco, A.; Wouters, C.; et al. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014, 66, 3160–3169. [Google Scholar] [CrossRef]
- Gavand, P.E.; Serio, I.; Arnaud, L.; Costedoat-Chalumeau, N.; Carvelli, J.; Dossier, A.; Hinschberger, O.; Mouthon, L.; Le Guern, V.; Korganow, A.S.; et al. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: A study of 103 episodes in 89 adult patients. Autoimmun. Rev. 2017, 16, 743–749. [Google Scholar] [CrossRef]
- Chizinga, M.; Kalra, S.S.; Innabi, A.; Rackauskas, M.; Ataya, A.; Emtiazjoo, A. Macrophage activating syndrome causing decompensated right heart failure. Respir. Med. Case Rep. 2021, 33, 101409. [Google Scholar] [CrossRef]
- Seegobin, K.; Moustafa, M.A.; Majeed, U.; Ray, J.C.; Shaikh, M.; Jiang, L.; Tun, H.W. Macrophage Activation Led Acute Heart Failure Managed Successfully with Immunosuppression. J. Blood Med. 2021, 12, 1037–1043. [Google Scholar] [CrossRef]
- Kuna, J.; Chmielewski, G.; Gruchała, M.; Szade, J.; Mikiewicz, M.; Ręcki, P.; Krajewska-Włodarczyk, M. Fatal Acute Heart Failure in the Course of Macrophage Activation Syndrome: Case Report and Literature Review. J. Clin. Med. 2022, 11, 4208. [Google Scholar] [CrossRef]
- Dick, S.A.; Wong, A.; Hamidzada, H.; Nejat, S.; Nechanitzky, R.; Vohra, S.; Mueller, B.; Zaman, R.; Kantores, C.; Aronoff, L.; et al. Three tissue resident macrophage subsets coexist across organs with conserved origins and life cycles. Sci. Immunol. 2022, 7, eabf7777. [Google Scholar] [CrossRef]
- Revelo, X.S.; Parthiban, P.; Chen, C.; Barrow, F.; Fredrickson, G.; Wang, H.; Yücel, D.; Herman, A.; van Berlo, J.H. Cardiac Resident Macrophages Prevent Fibrosis and Stimulate Angiogenesis. Circ. Res. 2021, 129, 1086–1101. [Google Scholar] [CrossRef]
- Wong, N.R.; Mohan, J.; Kopecky, B.J.; Guo, S.; Du, L.; Leid, J.; Feng, G.; Lokshina, I.; Dmytrenko, O.; Luehmann, H.; et al. Resident cardiac macrophages mediate adaptive myocardial remodeling. Immunity 2021, 54, 2072–2088. [Google Scholar] [CrossRef]
- Schulert, G.S.; Grom, A.A. Pathogenesis of Macrophage Activation Syndrome and Potential for Cytokine- Directed Therapies. Annu. Rev. Med. 2015, 66, 145–159. [Google Scholar] [CrossRef]
- Davì, S.; Consolaro, A.; Guseinova, D.; Pistorio, A.; Ruperto, N.; Martini, A.; Cron, R.Q.; Ravelli, A. An International Consensus Survey of Diagnostic Criteria for Macrophage Activation Syndrome in Systemic Juvenile Idiopathic Arthritis. J. Rheumatol. 2011, 38, 764–768. [Google Scholar] [CrossRef]
- Henter, J.; Horne, A.; Aricó, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2006, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Magni-Manzoni, S.; Pistorio, A.; Besana, C.; Foti, T.; Ruperto, N.; Viola, S.; Martini, A. Preliminary diagnostic guidelines for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. J. Pediatr. 2005, 146, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Kyriazopoulou, E.; Leventogiannis, K.; Norrby-Teglund, A.; Dimopoulos, G.; Pantazi, A.; Orfanos, S.E.; Rovina, N.; Tsangaris, I.; Gkavogianni, T.; Botsa, E.; et al. Macrophage activation-like syndrome: An immunological entity associated with rapid progression to death in sepsis. BMC Med. 2017, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Karakike, E.; Giamarellos-Bourboulis, E.J. Macrophage Activation-Like Syndrome: A Distinct Entity Leading to Early Death in Sepsis. Front. Immunol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Retamozo, S.; Brito-Zerón, P.; Sisó-Almirall, A.; Flores-Chávez, A.; Soto-Cárdenas, M.-J.; Ramos-Casals, M. Haemophagocytic syndrome and COVID-19. Clin. Rheumatol. 2021, 40, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Molina, G.; Contreras, R.; Coombes, K.; Walgamage, T.; A Perozo, M.; DesBiens, M.T. Hemophagocytic Lymphohistiocytosis Following COVID-19 Infection. Cureus 2023, 15, e34307. [Google Scholar] [CrossRef] [PubMed]
- Jeyakanthan, T.; Khandpur, B.; Tan, W.Y.; Nasir, S.A.; Ladel, L. Coronavirus Does It Again: Post-COVID-19 Hemophagocytic Lymphohistiocytosis (HLH). Cureus 2023, 15, e35275. [Google Scholar] [CrossRef] [PubMed]
| Hospitalization Day | 1 | 2 | 5 | 6 | 7 | 8 | 12 | 13 | 14 | 15 | 16 | 22 | 23 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC (103/μL) (4–10) | 3.05 | 2.79 | 5.68 | 6.27 | 4.88 | 6.38 | 3.86 | 3.45 | 6.43 | 7.5 | |||
| HGB (g/dL) (13–17) | 7.3 | 6.8 | 6.5 | 6.4 | 6.3 | 6.4 | 8.3 | 8.4 | 11.2 | 10.4 | |||
| HCT (%) (40–52) | 21.9 | 20.3 | 20.1 | 19.8 | 19.5 | 19.6 | 24.9 | 23.5 | 34 | 31.1 | |||
| PLT (103/μL) (150–370) | 117 | 116 | 135 | 110 | 111 | 119 | 215 | 257 | 502 | 492 | |||
| CRP (mg/dL) (<5) | 199 | 169 | 47 | 40 | 39 | 16 | 62 | 80 | 72 | 38 | 28 | ||
| ESR (mm/h) (<15) | 27 | 29 | 35 | ||||||||||
| FERRITIN (ng/mL) (10–200) | 953 | >2000 | 3322 | 1614 | 654 | 605 | 623 | ||||||
| D-DIMER (μg/mL) (0–0.5) | 21 | 1.8 | 6 | 12 | 7 | 5 | |||||||
| FIBRINOGEN (mg/dL) (180–400) | 142 | 132 | 94 | 101 | 271 | 323 | 396 | ||||||
| NT pro-BNP (pg/mL) (<125) | 798 | 4158 | 4601 | 1836 | 1089 | 175 | |||||||
| TRIGLYCERIDES (mg/dL) (40–160) | 268 | 141 | |||||||||||
| TEMPERATURE (°C) | 37.4 | 38.7 | 36.9 | 37 | 36.9 | 37.1 | 38 | 38.7 | 38.7 | 37.2 | 37.2 | 36.8 | 36.7 |
| Methyloprednisolone (i.v.) | 1000 | 1000 | 1000 | 1000 | |||||||||
| Prednisone (p.o.) | 30 | 55 | 55 | 55 | 55 | 55 | 55 | 55 | |||||
| Cyclosporin | 200 | 200 | 200 | 200 | 200 | ||||||||
| Mycofenolate mofetil | 1000 | 1000 | 1000 | 1000 | 1000 | 1000 | |||||||
| Chloroquine | 250 | 250 | 250 | 250 | 250 | 250 | |||||||
| Methotrexate (weekly) | 25 | 25 |
| fHLH Criteria [16] | Ravelli Criteria [17] | MAS Study Group Criteria [15] | H Score for Reactive Hemophagocytic Syndrome [3] |
|---|---|---|---|
| Clinical features: - Fever - Splenomegaly Laboratory features: - Cytopenias (≥2 of 3 lineages in the peripheral blood) - Hypertriglyceridemia and/or hypofibrinogenemia - Hemophagocytosis in bone marrow, spleen, or lymph nodes - Ferritin - Low or absent NK cell activity - Increased serum sIL-2Rα (at least 5 of the 8) Or A molecular diagnosis consistent with HLH (i.e., reported mutations in genes encoding either PRF1 or MUNC13-4, STX11, STXBP2, Rab27a, SH2D1A or BIRC4) | Clinical features: - Central nervous systemic dysfunction - Hemorrhages - Hepatomegaly Laboratory features: - Decreased platelet count - Elevated aspartate aminotransferase - Decreased white blood count - Hypofibrinogenemia Two or more laboratory criteria or any two or more clinical and/or laboratory criteria | Clinical features: - Persistent, continuous fever ≥38 °C Laboratory features: - Platelets - Hyperferritinemia - Liver enzymes - Leukocyte count - Falling ESR - Hypofibrinogenemia - Hypertriglyceridemia - Evidence of macrophage hemophagocytosis in the bone marrow | Clinical features: - Known underlying immunosuppression - Temperature - Organomegaly (liver or spleen) Laboratory features: - No. of cytopenias - Ferritin- Triglycerides - Fibrinogen - Aspartate aminotransferase - Hemophagocytosis features on bone marrow aspirate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuna, J.; Chmielewski, G.; Jaśkiewicz, Ł.; Krajewska-Włodarczyk, M. Acute Heart Failure in the Course of Macrophage Activation Syndrome Due to Newly Diagnosed Systemic Lupus Erythematosus: Case Presentation and Literature Review. Medicina 2024, 60, 392. https://doi.org/10.3390/medicina60030392
Kuna J, Chmielewski G, Jaśkiewicz Ł, Krajewska-Włodarczyk M. Acute Heart Failure in the Course of Macrophage Activation Syndrome Due to Newly Diagnosed Systemic Lupus Erythematosus: Case Presentation and Literature Review. Medicina. 2024; 60(3):392. https://doi.org/10.3390/medicina60030392
Chicago/Turabian StyleKuna, Jakub, Grzegorz Chmielewski, Łukasz Jaśkiewicz, and Magdalena Krajewska-Włodarczyk. 2024. "Acute Heart Failure in the Course of Macrophage Activation Syndrome Due to Newly Diagnosed Systemic Lupus Erythematosus: Case Presentation and Literature Review" Medicina 60, no. 3: 392. https://doi.org/10.3390/medicina60030392
APA StyleKuna, J., Chmielewski, G., Jaśkiewicz, Ł., & Krajewska-Włodarczyk, M. (2024). Acute Heart Failure in the Course of Macrophage Activation Syndrome Due to Newly Diagnosed Systemic Lupus Erythematosus: Case Presentation and Literature Review. Medicina, 60(3), 392. https://doi.org/10.3390/medicina60030392







