Red Cell Distribution Width as a Predictor of Survival in Patients with Hepatocellular Carcinoma
Abstract
1. Introduction
2. Materials and Methods
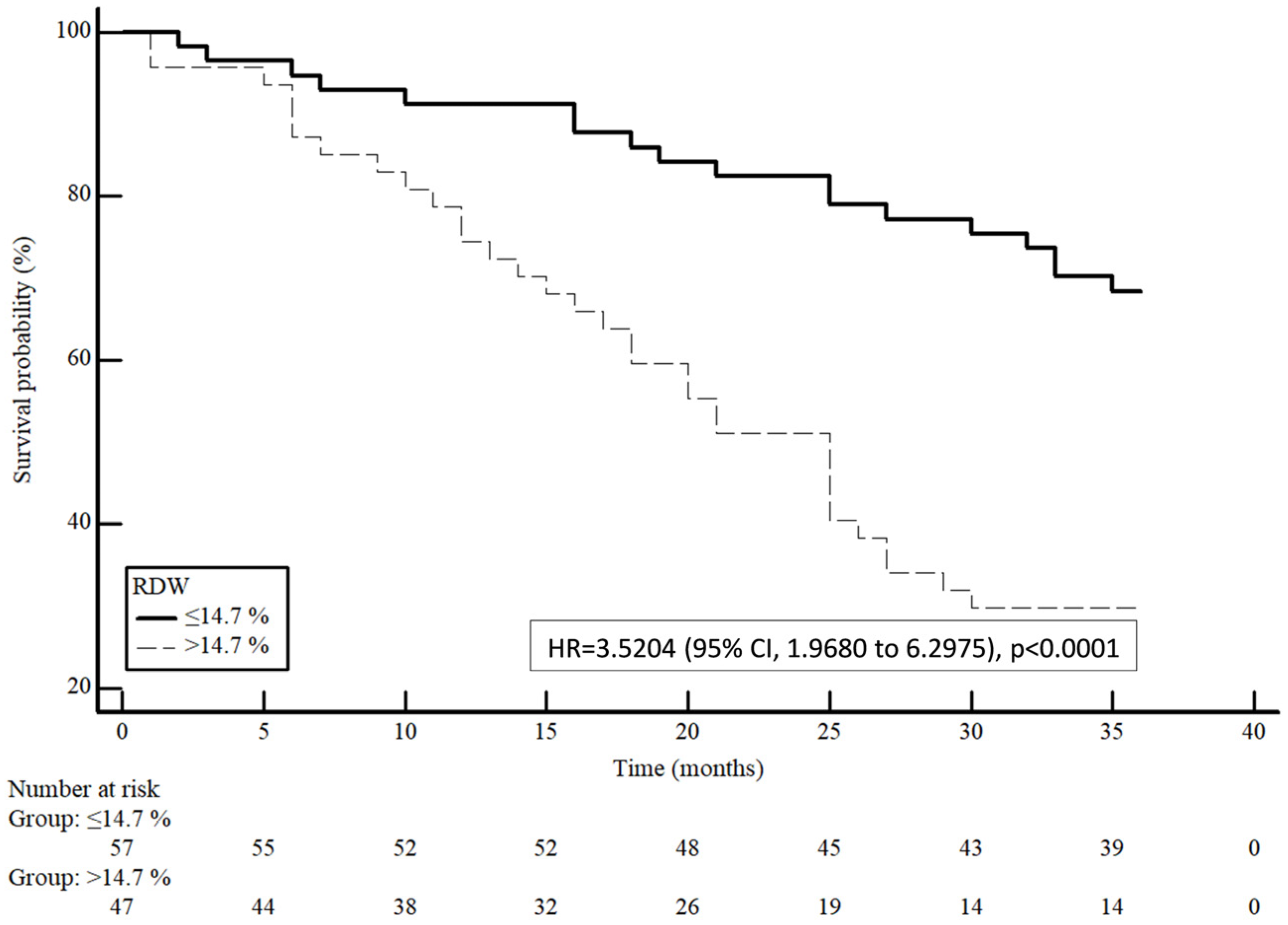
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (accessed on 7 January 2024).
- Che, L.; Paliogiannis, P.; Cigliano, A.; Pilo, M.G.; Chen, X.; Calvisi, D.F. Pathogenetic, prognostic, and therapeutic role of fatty acid synthase in human hepatocellular carcinoma. Front. Oncol. 2019, 9, 1412. [Google Scholar] [CrossRef]
- Cronin, K.A.; Scott, S.; Firth, A.U.; Sung, H.; Henley, S.J.; Sherman, R.L.; Siegel, R.L.; Anderson, R.N.; Kohler, B.A.; Benard, V.B.; et al. Annual report to the nation on the status of cancer, part 1: National cancer statistics. Cancer 2022, 128, 4251–4284. [Google Scholar] [CrossRef]
- Gao, Y.X.; Yang, T.W.; Yin, J.M.; Yang, P.X.; Kou, B.X.; Chai, M.Y.; Liu, X.N.; Chen, D.X. Progress and prospects of biomarkers in primary liver cancer. Int. J. Oncol. 2020, 57, 54–66. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, Z.; Li, K.; Liu, L. The association between systemic immune-inflammation index and chronic obstructive pulmonary disease in adults aged 40 years and above in the United States: A cross-sectional study based on the NHANES 2013-2020. Front. Med. 2023, 10, 1270368. [Google Scholar] [CrossRef]
- Gutta, L.; Shankarappa, M.; Ahmed, T. NLR and PLR ratios—Accessible and affordable predictors of disease severity in COPD. J. Assoc. Physicians India. 2022, 70, 11–12. [Google Scholar]
- Zhang, X.; Zhang, T.; Wu, C.; Zhou, Y.; Chen, Z.; Xu, R. The association between inflammatory biomarkers and carotid artery plaque in normal-weight and metabolically healthy Chinese adults: A cross-sectional study. Hypertens. Res. 2023, 46, 330–338. [Google Scholar] [CrossRef]
- Shahrabi, S.; Saki, N.; Safa, M.; Pezeshki, S.M.S. Complete blood count test in rheumatology: Not just a screening test. Clin. Lab. 2023, 69, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Pinna, A.; Porcu, T.; D’Amico-Ricci, G.; Dore, S.; Boscia, F.; Paliogiannis, P.; Carru, C.; Zinellu, A. Complete blood cell count-derived inflammation biomarkers in men with age-related macular degeneration. Ocul. Immunol. Inflamm. 2019, 27, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Qiu, F.; Fan, W.; Wei, S. Associations of complete blood cell count-derived inflammatory biomarkers with asthma and mortality in adults: A population-based study. Front. Immunol. 2023, 14, 1205687. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Yetkin, N.A.; Tutar, N.; Türe, Z.; Oymak, F.S.; Gülmez, İ. The role of sequentially monitored laboratory values and inflammatory biomarkers in assessing the severity of COVID-19. Cureus 2024, 16, e51458. [Google Scholar] [CrossRef] [PubMed]
- Niu, R.; Yan, Z.; Wang, Y.; Li, Y.; Feng, W.; Liu, J.; Wang, L. Study of clinical traits and systemic immune inflammation index assessments in patients with endogenous endophthalmitis over the last ten years. BMC Ophthalmol. 2024, 24, 11. [Google Scholar] [CrossRef]
- Dong, F.C.; Tan, W.T.; Wang, X.B.; Zheng, X.; Huang, Y.; Li, B.L.; Meng, Z.J.; Gao, Y.H.; Qian, Z.P.; Liu, F.; et al. The neutrophil-to-lymphocyte ratio represents a systemic inflammation marker and reflects the relationship with 90-day mortality in non-cirrhotic chronic severe hepatitis. J. Dig. Dis. 2022, 23, 587–596. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Mangoni, A.A.; Cangemi, M.; Fois, A.G.; Carru, C.; Zinellu, A. Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID-19): A systematic review and meta-analysis. Clin. Exp. Med. 2021, 21, 343–354. [Google Scholar] [CrossRef]
- Wu, D.; Qin, H. Diagnostic and prognostic values of immunocyte ratios in patients with sepsis in the intensive care unit. J. Infect. Dev. Ctries. 2023, 17, 1362–1372. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Deidda, S.; Maslyankov, S.; Paycheva, T.; Farag, A.; Mashhour, A.; Misiakos, E.; Papakonstantinou, D.; Mik, M.; Losinska, J.; et al. Blood cell count indexes as predictors of anastomotic leakage in elective colorectal surgery: A multicenter study on 1432 patients. World J. Surg. Oncol. 2020, 18, 89. [Google Scholar] [CrossRef]
- Ha, S.C.; Tsai, Y.H.; Koh, C.C.; Hong, S.G.; Chen, Y.; Yao, C.L. Blood biomarkers to distinguish complicated and uncomplicated appendicitis in pediatric patients. J. Formos. Med. Assoc. 2024, in press. [CrossRef]
- Xiao, Z.; Wang, X.; Chen, X.; Zhou, J.; Zhu, H.; Zhang, J.; Deng, W. Prognostic role of preoperative inflammatory markers in postoperative patients with colorectal cancer. Front. Oncol. 2023, 13, 1064343. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Ginesu, G.C.; Tanda, C.; Feo, C.F.; Fancellu, A.; Fois, A.G.; Mangoni, A.A.; Sotgia, S.; Carru, C.; Porcu, A.; et al. Inflammatory cell indexes as preoperative predictors of hospital stay in open elective thoracic surgery. ANZ J. Surg. 2018, 88, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, J.; Chen, Y.; Zhang, Q.; Liu, Y.; Jia, H. Preoperative inflammation and nutrition-based comprehensive biomarker for predicting prognosis in resectable colorectal cancer. Front. Oncol. 2023, 13, 1279487. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Y.; Zhang, X.; Zhang, J.G.; Zhou, W.J.; Chen, Q.Y.; Chen, Y.Y.; Yan, W.H.; Lin, A. Pre-operative neutrophil-to-lymphocyte ratio is an independent prognostic factor in patients with gastric cancer. Int. Immunopharmacol. 2022, 113, 109371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Ma, W.; Li, J. An elevated neutrophil-to-lymphocyte ratio predicts poor prognosis in patients with liver cancer after interventional treatments. Biomed. Res. Int. 2022, 2022, 6141317. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Scognamillo, F.; Bellomo, M.; Pittalis, M.L.; Pisano, I.P.; Karlighiotis, A.; Corrado, B.; Sotgiu, G.; Attene, F. Neutrophil to lymphocyte ratio as a predictor of thyroid papillary carcinoma. Acta Med. Mediterr. 2015, 31, 371–375. [Google Scholar]
- Arora, R.; Alam, F.; Zaka-Ur-Rab, A.; Maheshwari, V.; Alam, K.; Hasan, M. Peripheral neutrophil to lymphocyte ratio (NLR), a cogent clinical adjunct for Ki-67 in breast cancer. J. Egypt. Natl. Cancer Inst. 2023, 35, 43. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhou, G.; Zhou, L.; Wang, D.; Xiong, S.; Liu, C.; Zhang, G. Diagnostic roles of neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, C-reactive protein, and cancer antigen 125 for ovarian cancer. J. Int. Med. Res. 2023, 51, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.D.; Ahn, S.J.; Kim, Y.C.; Oh, I.J.; Nam, T.K.; Jeong, J.U.; Song, J.Y. Blood lymphocytes as a prognostic factor for stage III non-small cell lung cancer with concurrent chemoradiation. Chonnam. Med. J. 2024, 60, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Karanadze, N.A.; Begrambekova, Y.L.; Borisov, E.N.; Orlova, Y.A. Red cell distribution width as a predictor of impaired exercise capacity in patients with heart failure. Kardiologiia 2022, 62, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Zinellu, A.; Mangoni, A.A.; Capobianco, G.; Dessole, S.; Cherchi, P.L.; Carru, C. Red blood cell distribution width in pregnancy: A systematic review. Biochem. Med. 2018, 28, 030502. [Google Scholar] [CrossRef]
- Bilgin, B.; Sendur, M.A.N.; Hizal, M.; Dede, D.S.; Akinci, M.B.; Kandil, S.U.; Yaman, S.; Yalçin, A.; Kiliç, M.; Yalçin, B. Prognostic effect of red cell distribution width-to-platelet ratio in colorectal cancer according to tumor stage and localization. J. Cancer Res. Ther. 2019, 15, 54–60. [Google Scholar] [CrossRef]
- Zhao, W.; Shen, X.; Hua, Q.; Yang, L.; Zhou, R.; Zhou, C.; Xu, P. Red cell distribution width-a potential prognostic indicator for colorectal cancer patients after radical resection in China. J. Gastrointest. Oncol. 2023, 14, 1746–1758. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, T.; Zhang, R.; Zhu, X.; Wu, X.; Tan, S.; Jian, Z. Predicting colorectal cancer risk: A novel approach using anemia and blood test markers. Front. Oncol. 2024, 14, 1347058. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Y.; Yang, X.B.; Wang, W.Q.; Bai, Y.; Long, J.Y.; Lin, J.Z.; Xiong, J.P.; Zheng, Y.C.; He, X.D.; Zhao, H.T.; et al. Prognostic impact of the red cell distribution width in esophageal cancer patients: A systematic review and meta-analysis. World. J. Gastroenterol. 2018, 24, 2120–2129. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, S.; Wu, J.Z.; Song, Q. Clinical and prognostic significance of perioperative change in red cell distribution width in patients with esophageal squamous cell carcinoma. BMC Cancer 2023, 23, 319. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, M.; Zhang, J. Preoperative hemoglobin-to-red cell distribution width ratio as a prognostic factor in pulmonary large cell neuroendocrine carcinoma: A retrospective cohort study. Ann. Transl. Med. 2022, 10, 42. [Google Scholar] [CrossRef]
- Zhan, Z.; Fei, Z.; Xu, B.; Zhong, D.; Dong, D. Prognostic significance of red cell distribution width in advanced non-small cell lung cancer patients. Clin. Lab. 2021, 67, 995. [Google Scholar] [CrossRef]
- Akturk, O.M.; Yildirim, D.; Cakir, M.; Vardar, Y.M.; Erozgen, F.; Akinci, M. Is there a threshold for red cell distribution width to predict malignancy in breast masses? Niger J. Clin. Pract. 2022, 25, 349–353. [Google Scholar] [CrossRef]
- Simões, R.; Ferreira, A.C.; Silva, L.M.; Sabino, A.P.; Carvalho, M.D.G.; Gomes, K.B. Evaluation of the RDW Index (Red Cell Distribution Width) in women with breast cancer treated with doxorubicin in a one-year follow-up study. Diagnostics 2023, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.L.; Zhou, X.; Peng, D. Red blood cell distribution width has a prognostic value for gastric cancer patients after gastrectomy: A pooling-up analysis. Medicine 2023, 102, e35554. [Google Scholar] [CrossRef] [PubMed]
- Ngadikun; Pradjatmo, H.; Nugroho, K.A.; Pasala, M.; Prasetyastuti. Analysis of erythrocyte sedimentation rate order in epithelial ovarian cancer. J. Cancer 2023, 14, 2173–2180. [Google Scholar] [CrossRef] [PubMed]
- Aktas, G.; Sit, M.; Karagoz, I.; Erkus, E.; Ozer, B.; Kocak, M.Z.; Yaman, S.; Keyif, F.; Altinordu, R.; Erkol, H.; et al. Could red cell distribution width be a marker of thyroid cancer? J. Coll. Physicians Surg. Pak. 2017, 27, 556–558. [Google Scholar] [PubMed]
- Wang, F.; Liang, J.; Yang, F.; Liu, F.; Han, S.; Xing, N. Preoperative red cell distribution width is associated with postoperative lymphovascular invasion in prostate cancer patients treated with radical prostatectomy: A retrospective study. Front. Endocrinol. 2022, 13, 1020655. [Google Scholar] [CrossRef] [PubMed]
- Eoh, K.J.; Lee, T.K.; Nam, E.J.; Kim, S.W.; Kim, Y.T. Clinical relevance of red blood cell distribution width (RDW) in endometrial cancer: A retrospective single-center experience from Korea. Cancers 2023, 15, 3984. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Sun, Y.; Wang, Q.; Han, Z.; Huang, Y.; Liu, X.; Ding, C.; Hu, C.; Qin, Q.; Deng, A. Red blood cell distribution width is a potential prognostic index for liver disease. Clin. Chem. Lab. Med. 2013, 51, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.; Zishu, G.; Shihe, G.; Yufeng, G.; Qiang, Z. Changes in red blood cell distribution width is associated with liver function parameters and prognosis in patients with chronic HBV liver disease. Clin. Lab. 2016, 62, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Wei, H.; Zhu, X.; Li, S.; Qin, X. Increased red cell distribution width is strong inflammatory marker of liver diseases in a Guangxi population. Clin. Lab. 2017, 63, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Sun, B.; Zhang, S.; Pan, Y.; Fang, J. RDW-SD is superior to RDW-CV in reflecting liver fibrosis stage in patients with chronic hepatitis B. Infect. Drug. Resist. 2023, 16, 6881–6891. [Google Scholar] [CrossRef] [PubMed]
- Kalairajan, S.; Kavitha, K.K.; Govindaraj, P. Red cell distribution width in chronic liver disease: An observational study. Cureus. 2023, 15, e40158. [Google Scholar] [CrossRef]
- Lippi, G.; Cervellin, G.; Sanchis-Gomar, F. Red blood cell distribution width: A marker of anisocytosis potentially associated with atrial fibrillation. World J. Cardiol. 2019, 11, 292–304. [Google Scholar] [CrossRef]
- Danese, E.; Lippi, G.; Montagnana, M. Red blood cell distribution width and cardiovascular diseases. J. Thorac. Dis. 2015, 7, E402–E411. [Google Scholar] [CrossRef]
- Brenner, H.; Altenhofen, L.; Katalinic, A.; Lansdorp-Vogelaar, I.; Hoffmeister, M. Sojourn time of preclinical colorectal cancer by sex and age: Estimates from the German national screening colonoscopy database. Am. J. Epidemiol. 2011, 174, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Smirne, C.; Grossi, G.; Pinato, D.J.; Burlone, M.E.; Mauri, F.A.; Januszewski, A.; Oldani, A.; Minisini, R.; Sharma, R.; Pirisi, M. Evaluation of the red cell distribution width as a biomarker of early mortality in hepatocellular carcinoma. Dig. Liver Dis. 2015, 47, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.T.; Tang, Q.Q.; Qin, B.D.; Ma, N.; Wang, L.L.; Zhou, L.; Zhong, R.Q. Elevated red blood cell distribution width is associated with liver function tests in patients with primary hepatocellular carcinoma. Clin. Hemorheol. Microcirc. 2016, 64, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Howell, J.; Pinato, D.J.; Ramaswami, R.; Arizumi, T.; Ferrari, C.; Gibbin, A.; Burlone, M.E.; Guaschino, G.; Toniutto, P.; Black, J.; et al. Integration of the cancer-related inflammatory response as a stratifying biomarker of survival in hepatocellular carcinoma treated with sorafenib. Oncotarget 2017, 8, 36161–36170. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.S.; Fu, X.L.; Zhao, W.; Kong, L.B. Red cell distribution width as a prognostic factor in patients with hepatocellular carcinoma. Clin. Lab. 2020, 66, 1237. [Google Scholar] [CrossRef] [PubMed]
- Golriz, M.; Ramouz, A.; Ali-Hasan-Al-Saegh, S.; Shafiei, S.; Aminizadeh, E.; Hammad, A.; Mieth, M.; Rupp, C.; Springfeld, C.; Hoffmann, K.; et al. Prognostic value of red blood cell distribution width (RDW) in the recurrence of hepatocellular carcinoma following curative resection. J. Hepatocell. Carcinoma. 2022, 9, 1137–1147. [Google Scholar] [CrossRef]
- Tan, M.; Liu, B.; You, R.; Huang, Q.; Lin, L.; Cai, D.; Yang, R.; Li, D.; Huang, H. Red blood cell distribution width as a potential valuable survival predictor in hepatitis B virus-related hepatocellular carcinoma. Int. J. Med. Sci. 2023, 20, 976–984. [Google Scholar] [CrossRef]
- Goyal, H.; Hu, Z.D. Prognostic value of red blood cell distribution width in hepatocellular carcinoma. Ann. Transl. Med. 2017, 5, 271. [Google Scholar] [CrossRef]

| Total (n = 104) | Survivors (n = 53) | Non-Survivors (n = 51) | p-Value | |
|---|---|---|---|---|
| Age (years) | 70 (63–78) | 70 (64–75) | 69 (62–79) | 0.65 |
| Gender (F/M) | 19/85 | 7/46 | 12/39 | 0.18 |
| BMI | 25.0 (23.3–27.6) | 25.1 (23.4–27.5) | 24.7 (23.1–27.8) | 0.96 |
| Charlson Comorbidity Index | 7.6 ± 1.7 | 7.6 ± 1.8 | 7.7 ± 1.6 | 0.66 |
| Child–Pugh score (A/B/C) | 90/14/0 | 50/3/0 | 40/11/0 | 0.02 |
| BCLC stage (0/A/B/C) | 9/47/42/3 | 5/28/19/1 | 4/19/23/2 | 0.51 |
| Lesion number (single/multiple) | 72/32 | 43/10 | 29/22 | 0.008 |
| Lesion size (<5 cm/≥5 cm) | 72/32 | 37/16 | 35/16 | 0.90 |
| Extrahepatic metastasis (no/yes) | 102/2 | 52/1 | 50/1 | 0.98 |
| Viral hepatitis | 20/83 | 9/43 | 11/40 | 0.59 |
| Treatment (medical/ablation/surgical/multiple) | 5/66/31/2 | 0/32/20/1 | 5/34/11/1 | 0.054 |
| MCV (fL) | 87.7 ± 10.7 | 89.1 ± 8.9 | 86.3 ± 12.3 | 0.19 |
| RDW (%) | 14.5 (13.3–16.0 | 13.7 (13.1–14.8) | 15.1 (14.2–16.6) | 0.0001 |
| PLT (×109/L) | 129 (88–194) | 129 (93–189) | 130 (86–201) | 0.62 |
| MPV (fL) | 9.0 (8.1–10.1) | 9.0 (8.1–10.4) | 9.0 (8.0–9.9) | 0.44 |
| WBC (×109/L) | 5.5 (4.0–7.7) | 5.2 (4.1–6.7) | 5.9 (3.9–8.1) | 0.47 |
| Neutrophils (×109/L) | 3.1 (2.2–4.5) | 3.0 (2.2–4.1) | 3.1 (2.4–4.9) | 0.44 |
| Lymphocytes (×109/L) | 1.40 (1.00–2.10) | 1.40 (1.10–2.02) | 1.40 (0.82–2.08) | 0.44 |
| Monocytes (×109/L) | 0.40 (0.30–0.60) | 0.40 (0.30–0.50) | 0.40 (0.30–0.65) | 0.44 |
| CRP (mg/dL) | 0.68 (0.22–3.53) | 0.42 (0.19–3.98) | 1.25 (0.44–2.87) | 0.07 |
| ESR (mm/h) | 28.5 (13.4–48.0) | 28.5 (11.0–40.0) | 26.5 (13.0–53.0) | 0.65 |
| Albumin (g/dL) | 3.5 (3.1–3.8) | 3.5 (3.1–4.0) | 3.4 (3.0–3.8) | 0.15 |
| Total bilirubin (mg/dL) | 1.03 (0.70–1.50) | 0.90 (0.70–1.30) | 1.20 (0.71–1.85) | 0.04 |
| ALT (U/L) | 48 (28–79) | 50 (30–92) | 47 (28–70) | 0.37 |
| AST (U/L) | 54 (32–87) | 50 (31–90) | 54 (33–82) | 0.95 |
| γ-GT (U/L) | 85 (52–137) | 81 (48–133) | 88 (53–154) | 0.37 |
| ALP (U/L) | 102 (73–145) | 87 (69–127) | 111 (82–156) | 0.02 |
| LDH (U/L) | 209 (175–263) | 205 (178–276) | 215 (172–240) | 0.64 |
| INR | 1.12 (1.06–1.23) | 1.11 (1.07–1.19) | 1.16 (1.05–1.27) | 0.24 |
| Fibrinogen (mg/dL) | 275 ± 83 | 277 ± 80 | 273 ± 87 | 0.84 |
| Creatinine (mg/dL) | 0.83 (0.74–1.01) | 0.83 (0.77–1.02) | 0.82 (0.72–1.01) | 0.51 |
| Urea (mg/dL) | 34 (26–43) | 35 (26–43) | 32 (26–43) | 0.92 |
| AFP (ng/mL) | 10.7 (4.7–47.8) | 9.7 (4.7–23.7) | 13.5 (4.7–86.1) | 0.31 |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.0114 | 0.9736 to 1.0507 | 0.5595 |
| Gender | 2.0220 | 0.7254 to 5.6363 | 0.1783 |
| BMI | 1.0076 | 0.8611 to 1.1790 | 0.9248 |
| Charlson Comorbidity Index | 1.0363 | 0.8252 to 1.3014 | 0.7589 |
| Child–Pugh score | 4.5833 | 1.1970 to 17.5500 | 0.0263 |
| BCLC stage | 1.4614 | 0.8182 to 2.6102 | 0.1998 |
| Lesion number | 3.2621 | 1.3483 to 7.8922 | 0.0087 |
| Lesion size | 1.0571 | 0.4596 to 2.4315 | 0.8960 |
| Extrahepatic metastasis | 1.0400 | 0.0633 to 17.0838 | 0.9781 |
| Viral hepatitis | 0.7611 | 0.2855 to 2.0290 | 0.5853 |
| Treatment | 0.4474 | 0.2185 to 0.9159 | 0.0278 |
| MCV | 0.9757 | 0.9403 to 1.0124 | 0.1912 |
| RDW | 1.5727 | 1.2244 to 2.0200 | 0.0004 |
| PLT | 0.9995 | 0.9946 to 1.0045 | 0.8499 |
| MPV | 0.8775 | 0.6768 to 1.1378 | 0.3241 |
| WBC | 1.0372 | 0.8986 to 1.1972 | 0.6175 |
| Neutrophils | 1.0126 | 0.8600 to 1.1921 | 0.8809 |
| Lymphocytes | 0.8716 | 0.5620 to 1.3517 | 0.5392 |
| Monocytes | 2.8277 | 0.5483 to 14.5839 | 0.2143 |
| CRP | 0.9683 | 0.8474 to 1.1063 | 0.6353 |
| ESR | 1.0050 | 0.9866 to 1.0238 | 0.5975 |
| Albumin | 0.5296 | 0.2777 to 1.0100 | 0.0536 |
| Total bilirubin | 2.2813 | 1.1290 to 4.6097 | 0.0216 |
| ALT | 0.9973 | 0.9918 to 1.0029 | 0.3416 |
| AST | 1.0001 | 0.9937 to 1.0065 | 0.9837 |
| γ-GT | 1.0015 | 0.9980 to 1.0049 | 0.4079 |
| ALP | 1.0099 | 1.0014 to 1.0185 | 0.0228 |
| LDH | 0.9978 | 0.9933 to 1.0023 | 0.3311 |
| INR | 6.0213 | 0.4508 to 80.4309 | 0.1746 |
| Fibrinogen | 0.9995 | 0.9944 to 1.0046 | 0.8423 |
| Creatinine | 0.6328 | 0.1556 to 2.5732 | 0.5225 |
| Urea | 1.0108 | 0.9834 to 1.0389 | 0.4436 |
| AFP | 1.0001 | 0.9999 to 1.0003 | 0.2938 |
| Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Child–Pugh score | 2.0049 | 0.2791 to 14.4038 | 0.4893 |
| Lesion number | 2.6633 | 0.9644 to 7.3551 | 0.0587 |
| Treatment | 0.5366 | 0.2265 to 1.2712 | 0.1572 |
| RDW | 1.3877 | 1.0643 to 1.8093 | 0.0155 |
| Albumin | 1.0818 | 0.4146 to 2.8227 | 0.8723 |
| Total bilirubin | 1.3584 | 0.7867 to 2.3455 | 0.2717 |
| ALP | 1.0068 | 0.9978 to 1.0159 | 0.0555 |
| Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Age | 1.0081 | 0.9805 to 1.0365 | 0.5679 |
| Gender | 1.5989 | 0.8365 to 3.0559 | 0.1556 |
| BMI | 1.0001 | 0.8823 to 1.1336 | 0.9990 |
| Charlson Comorbidity Index | 1.0288 | 0.8757 to 1.2087 | 0.7299 |
| Child–Pugh score | 2.3517 | 1.1984 to 4.6146 | 0.0129 |
| BCLC stage | 1.4584 | 0.9417 to 2.2587 | 0.0909 |
| Lesion number | 2.1653 | 1.2406 to 3.7789 | 0.065 |
| Lesion size | 1.0462 | 0.5789 to 1.8909 | 0.8810 |
| Extrahepatic metastasis | 1.0441 | 0.1438 to 7.5834 | 0.9660 |
| Viral hepatitis | 0.7452 | 0.3822 to 1.4529 | 0.3879 |
| Treatment | 0.5267 | 0.3049 to 0.9097 | 0.0215 |
| MCV | 0.9798 | 0.9535 to 1.0068 | 0.1413 |
| RDW | 1.3932 | 1.2375 to 1.5686 | <0.0001 |
| PLT | 0.9997 | 0.9958 to 1.0036 | 0.8826 |
| MPV | 0.9070 | 0.7468 to 1.1016 | 0.3251 |
| WBC | 1.0232 | 0.9246 to 1.1324 | 0.6574 |
| Neutrophils | 1.0132 | 0.9055 to 1.1337 | 0.8192 |
| Lymphocytes | 0.8509 | 0.5986 to 1.2095 | 0.3681 |
| Monocytes | 1.7069 | 0.5764 to 5.0550 | 0.3344 |
| CRP | 0.9847 | 0.8967 to 1.0813 | 0.7468 |
| ESR | 1.0043 | 0.9911 to 1.0175 | 0.5269 |
| Albumin | 0.6433 | 0.4150 to 0.9970 | 0.0485 |
| Total bilirubin | 1.2558 | 1.1101 to 1.4207 | 0.0003 |
| ALT | 0.9978 | 0.9933 to 1.0022 | 0.3275 |
| AST | 0.9993 | 0.9947 to 1.0039 | 0.7559 |
| γ-GT | 1.0016 | 0.9994 to 1.0038 | 0.1619 |
| ALP | 1.0061 | 1.0015 to 1.0108 | 0.0095 |
| LDH | 0.9978 | 0.9941 to 1.0016 | 0.2635 |
| INR | 3.6363 | 0.6510 to 20.3116 | 0.1413 |
| Fibrinogen | 0.9992 | 0.9953 to 1.0032 | 0.7014 |
| Creatinine | 0.7547 | 0.2623 to 2.1714 | 0.6017 |
| Urea | 1.0086 | 0.9902 to 1.0272 | 0.3632 |
| AFP | 1.0000 | 1.0000 to 1.0001 | 0.3559 |
| Hazard Ratio | 95% CI | p-Value | |
|---|---|---|---|
| Child–Pugh score | 0.7474 | 0.2310 to 2.4178 | 0.6269 |
| BCLC stage | 1.2826 | 0.7455 to 2.2066 | 0.3687 |
| Lesion number | 1.4834 | 0.7648 to 2.8773 | 0.2433 |
| Treatment | 0.6985 | 0.3824 to 1.2758 | 0.2430 |
| RDW | 1.2524 | 1.0786 to 1.4542 | 0.0032 |
| Albumin | 0.7691 | 0.3999 to 1.4794 | 0.4316 |
| Total bilirubin | 1.2121 | 1.0155 to 1.4468 | 0.0331 |
| ALP | 1.0032 | 0.9975 to 1.0090 | 0.2762 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidili, G.; Zinellu, A.; Mangoni, A.A.; Arru, M.; De Murtas, V.; Cuccuru, E.; Fancellu, A.; Paliogiannis, P. Red Cell Distribution Width as a Predictor of Survival in Patients with Hepatocellular Carcinoma. Medicina 2024, 60, 391. https://doi.org/10.3390/medicina60030391
Vidili G, Zinellu A, Mangoni AA, Arru M, De Murtas V, Cuccuru E, Fancellu A, Paliogiannis P. Red Cell Distribution Width as a Predictor of Survival in Patients with Hepatocellular Carcinoma. Medicina. 2024; 60(3):391. https://doi.org/10.3390/medicina60030391
Chicago/Turabian StyleVidili, Gianpaolo, Angelo Zinellu, Arduino Aleksander Mangoni, Marco Arru, Valentina De Murtas, Elena Cuccuru, Alessandro Fancellu, and Panagiotis Paliogiannis. 2024. "Red Cell Distribution Width as a Predictor of Survival in Patients with Hepatocellular Carcinoma" Medicina 60, no. 3: 391. https://doi.org/10.3390/medicina60030391
APA StyleVidili, G., Zinellu, A., Mangoni, A. A., Arru, M., De Murtas, V., Cuccuru, E., Fancellu, A., & Paliogiannis, P. (2024). Red Cell Distribution Width as a Predictor of Survival in Patients with Hepatocellular Carcinoma. Medicina, 60(3), 391. https://doi.org/10.3390/medicina60030391









