Comparison of the Effects of Perineural and Intraperitoneal Ozone Therapy on Nerve Healing in an Experimental Sciatic Nerve Injury Model
Abstract
1. Introduction
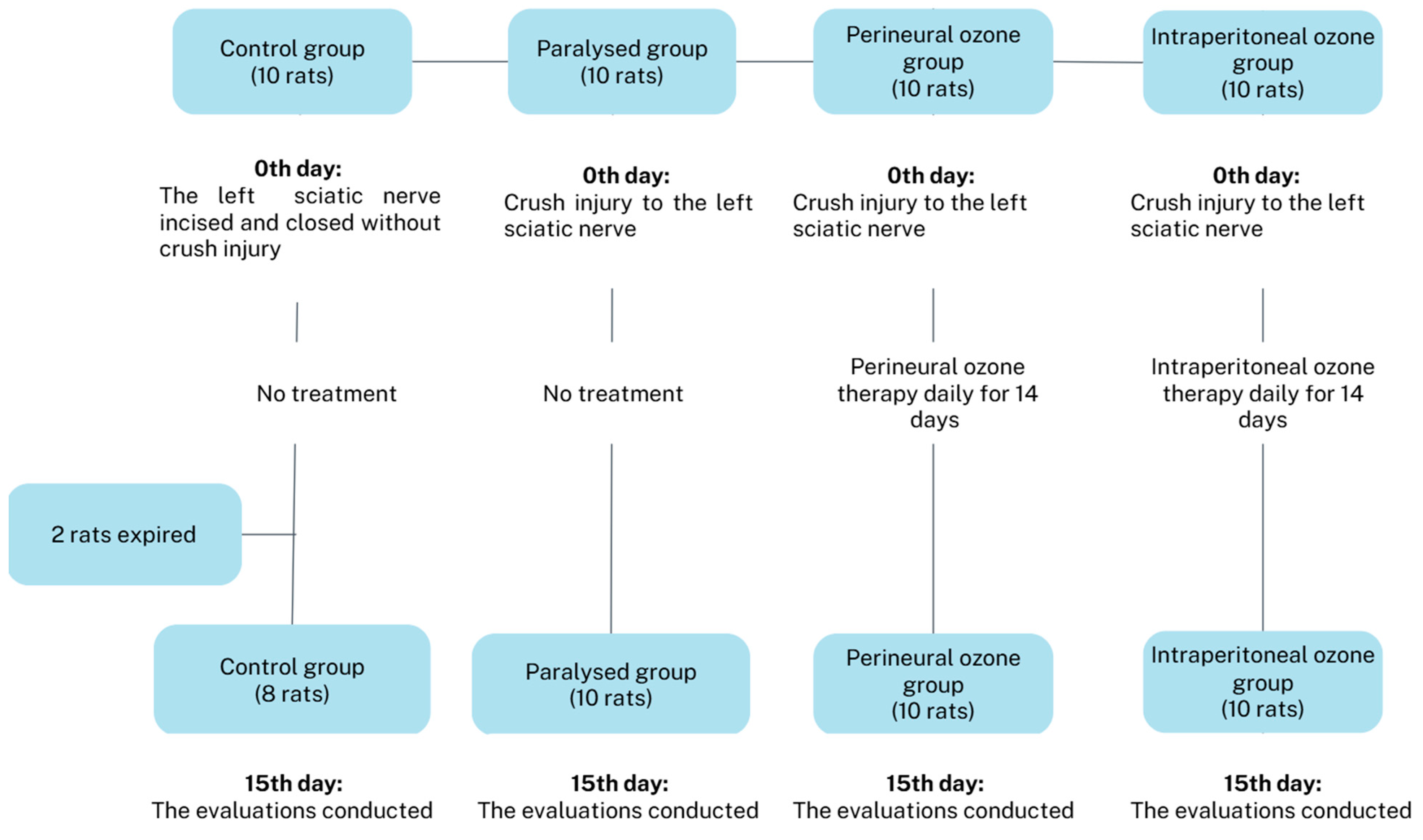
2. Materials and Methods
2.1. Animals
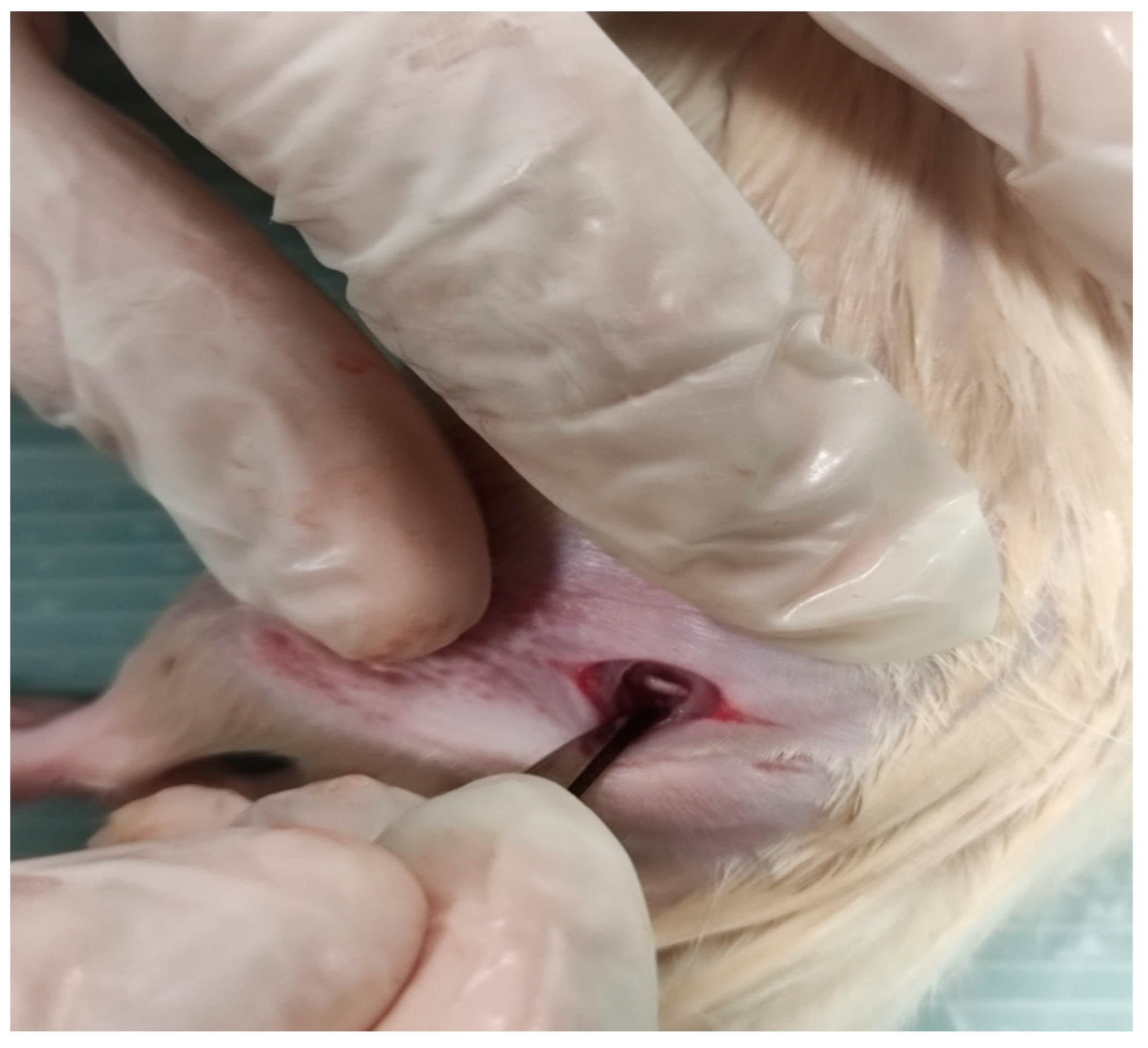
2.2. Surgery
2.3. Ozone Treatment
2.4. Functional Assessment
2.5. Microscopic Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geuna, S. The sciatic nerve injury model in pre-clinical research. J. Neurosci. Methods 2015, 243, 39–46. [Google Scholar] [CrossRef]
- Noorafshan, A.; Omidi, A.; Karbalay-Doust, S. Curcumin protects the dorsal root ganglion and sciatic nerve after crush in rat. Pathol. Pract. 2011, 207, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Dagum, A.B. Peripheral nerve regeneration, repair, and grafting. J. Hand Ther. 1998, 11, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Glickman, L.T.; Mackinnon, S.E. Sensory recovery following digital replantation. Microsurgery 1990, 11, 236–242. [Google Scholar] [CrossRef]
- Ozbay, I.; Ital, I.; Kucur, C.; Akcılar, R.; Deger, A.; Aktas, S.; Oghan, F. Effects of ozone therapy on facial nerve regeneration. Braz. J. Otorhinolaryngol. 2017, 83, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Chen, H.; Lu, C.; Wang, B.; Zhang, Y.; He, X.; Yu, B. Effects of ozone on sciatic nerve in rat. Interv. Neuroradiol. 2011, 17, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Ogut, E.; Yildirim, F.B.; Sarikcioglu, L.; Aydin, M.A.; Demir, N. Neuroprotective effects of ozone therapy after sciatic nerve cut injury. Kurume Med. J. 2018, 65, 137–144. [Google Scholar] [CrossRef]
- Özler, M.; Öter, Ș.; Korkmaz, A. The use of ozone gas for medical purposes. Türk Sİlahlı Kuvvetlerİ Koruyucu Hekİmlİk Bültenİ 2009, 8, 69–74. [Google Scholar]
- Lin, Y.; Hsien, H. Characteristics transformation of humic acid during ozonation and biofiltration treatment processes. Water Environ. Res. 2011, 83, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Babuccu, O. Ozone therapy: Myth and fact. Turkish J. Plast. Surg. 2011, 19, 105–112. [Google Scholar]
- Kızılay, Z.; Kahraman Çetin, N.; Aksel, M.; Abas, B.İ.; Aktaş, S.; Erken, H.A.; Topçu, A.; Yılmaz, A.; Yenisey, C. Ozone partially decreases axonal and myelin damage in an experimental sciatic nerve injury model. J. Investig. Surg. 2019, 32, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Pan, C.; Chen, L.; Hu, L.; Wang, C.; Han, Y.; Yang, Y.; Cheng, Z.; Liu, W.-T. AMPK activation by peri-sciatic nerve administration of ozone attenuates CCI-induced neuropathic pain in rats. J. Mol. Cell Biol. 2017, 9, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, O.; Tezcan, A.H.; Adali, Y.; Yıldırım, C.H.; Aksoy, O.; Yagmurdur, H.; Bilge, A. Effect of ozone and methylprednisolone treatment following crush type sciatic nerve injury. Acta Cir. Bras. 2016, 31, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Somay, H.; Emon, S.T.; Uslu, S.; Orakdogen, M.; Meric, Z.C.; Ince, U.; Hakan, T. The histological effects of ozone therapy on sciatic nerve crush injury in rats. World Neurosurg. 2017, 105, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Abdelsameea, A.A.; Kabil, S.L. Mitigation of cisplatin-induced peripheral neuropathy by canagliflozin in rats. Naunyn. Schmiedebergs. Arch. Pharmacol. 2018, 391, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Baptista, A.F.; Gomes, J.R.S.; Oliveira, J.T.; Santos, S.M.G.; Vannier-Santos, M.A.; Martinez, A.M.B. High-and low-frequency transcutaneous electrical nerve stimulation delay sciatic nerve regeneration after crush lesion in the mouse. J. Peripher. Nerv. Syst. 2008, 13, 71–80. [Google Scholar] [CrossRef]
- Coban, Y.K.; Ciralik, H.; Kurutas, E.B. Ischemic preconditioning reduces the severity of ischemia-reperfusion injury of peripheral nerve in rats. J. Brachial Plex. Peripher. Nerve Inj. 2006, 1, e3–e7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yüce, S.; Gökçe, E.C.; Iskdemir, A.; Koç, E.R.; Cemil, D.B.; Gökçe, A.; Sargon, M.F. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann. Plast. Surg. 2015, 74, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Aldinucci, C. Biochemical modifications induced in human blood by oxygenation-ozonation. J. Biochem. Mol. Toxicol. 2006, 20, 133–138. [Google Scholar] [CrossRef]
- Güçlü, A.; Erken, H.A.; Erken, G.; Dodurga, Y.; Yay, A.; Özçoban, Ö.; Şimşek, H.; Akçılar, A.; Koçak, F.E. The effects of ozone therapy on caspase pathways, TNF-α, and HIF-1α in diabetic nephropathy. Int. Urol. Nephrol. 2016, 48, 441–450. [Google Scholar] [CrossRef]
- Bonetti, M.; Ottaviani, G.M.; Simonetti, L.; Pellicanò, G.; Bonetti, F.; Muto, M. Treatment of Low-Back Pain with Oxygen-Ozone Therapy. In Hernia Updates and Approaches; IntechOpen: Zagreb, Croatia, 2023; ISBN 1837696632. [Google Scholar]
- Fex Svennigsen, Å.; Dahlin, L.B. Repair of the peripheral nerve—Remyelination that works. Brain Sci. 2013, 3, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Pušnik, L.; Serša, I.; Umek, N.; Cvetko, E.; Snoj, Ž. Correlation between diffusion tensor indices and fascicular morphometric parameters of peripheral nerve. Front. Physiol. 2023, 14, 1070227. [Google Scholar] [CrossRef] [PubMed]
- Manzanera Esteve, I.V.; Farinas, A.F.; Pollins, A.C.; Nussenbaum, M.E.; Cardwell, N.L.; Kang, H.; Does, M.D.; Thayer, W.P.; Dortch, R.D. Probabilistic assessment of nerve regeneration with diffusion MRI in rat models of peripheral nerve trauma. Sci. Rep. 2019, 9, 19686. [Google Scholar] [CrossRef] [PubMed]
- Snoj, Ž.; Pušnik, L.; Cvetko, E.; Burica Matičič, U.; Jengojan, S.A.; Omejec, G. Sciatic nerve fascicle differentiation on high-resolution ultrasound with histological verification: An ex vivo study. Muscle Nerve 2024, 70, 265–272. [Google Scholar] [CrossRef] [PubMed]
 ), myelinated axon structures observed in degenerated vacuolar structure (
), myelinated axon structures observed in degenerated vacuolar structure ( ), vascular congestion (
), vascular congestion ( ), vascular wall thickening (
), vascular wall thickening ( ), and proliferating Schwann cells (
), and proliferating Schwann cells ( ). Light microscopy examination of the sciatic nerve specimens taken from the control group after hematoxylin–eosin staining showed a normal histological structure characterized by intact myelinated axons and vascular structures. In the paralyzed group, the light microscopy examination revealed degenerated vacuolar structures, vascular congestion, vascular wall thickening, and inflammation in myelinated axon structures. In the perineural ozone group, near normal myelinated axon structures as well as decreased vascular wall thickening and proliferated Schwann cells were observed in some areas. In the intraperitoneal ozone group, although a small number of vascular congestion was observed in some areas, it revealed a histologic structure close to normal in general evaluation.
). Light microscopy examination of the sciatic nerve specimens taken from the control group after hematoxylin–eosin staining showed a normal histological structure characterized by intact myelinated axons and vascular structures. In the paralyzed group, the light microscopy examination revealed degenerated vacuolar structures, vascular congestion, vascular wall thickening, and inflammation in myelinated axon structures. In the perineural ozone group, near normal myelinated axon structures as well as decreased vascular wall thickening and proliferated Schwann cells were observed in some areas. In the intraperitoneal ozone group, although a small number of vascular congestion was observed in some areas, it revealed a histologic structure close to normal in general evaluation.
 ), myelinated axon structures observed in degenerated vacuolar structure (
), myelinated axon structures observed in degenerated vacuolar structure ( ), vascular congestion (
), vascular congestion ( ), vascular wall thickening (
), vascular wall thickening ( ), and proliferating Schwann cells (
), and proliferating Schwann cells ( ). Light microscopy examination of the sciatic nerve specimens taken from the control group after hematoxylin–eosin staining showed a normal histological structure characterized by intact myelinated axons and vascular structures. In the paralyzed group, the light microscopy examination revealed degenerated vacuolar structures, vascular congestion, vascular wall thickening, and inflammation in myelinated axon structures. In the perineural ozone group, near normal myelinated axon structures as well as decreased vascular wall thickening and proliferated Schwann cells were observed in some areas. In the intraperitoneal ozone group, although a small number of vascular congestion was observed in some areas, it revealed a histologic structure close to normal in general evaluation.
). Light microscopy examination of the sciatic nerve specimens taken from the control group after hematoxylin–eosin staining showed a normal histological structure characterized by intact myelinated axons and vascular structures. In the paralyzed group, the light microscopy examination revealed degenerated vacuolar structures, vascular congestion, vascular wall thickening, and inflammation in myelinated axon structures. In the perineural ozone group, near normal myelinated axon structures as well as decreased vascular wall thickening and proliferated Schwann cells were observed in some areas. In the intraperitoneal ozone group, although a small number of vascular congestion was observed in some areas, it revealed a histologic structure close to normal in general evaluation.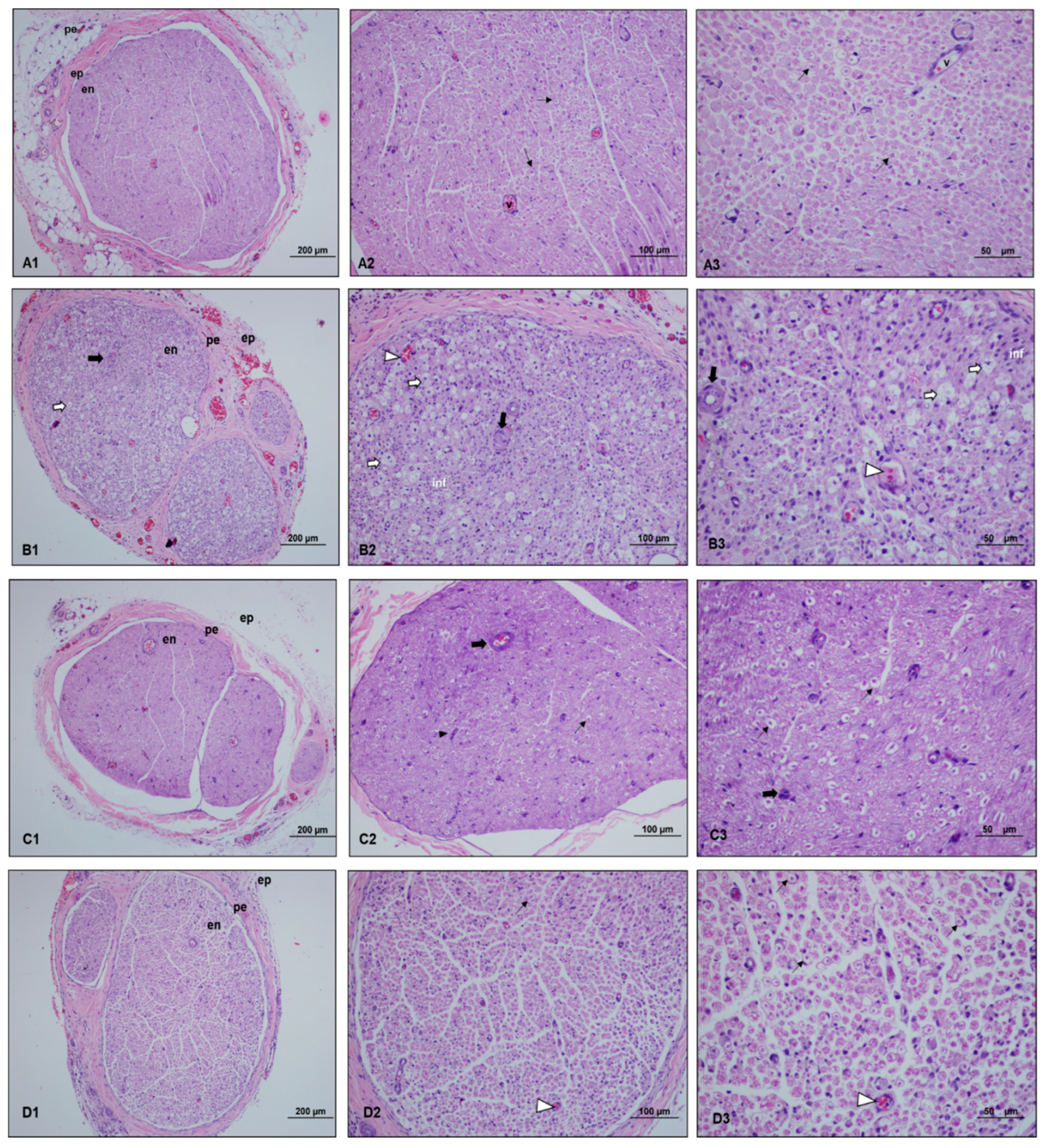
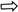 ), myelinated axon structures observed in degenerated vacuolar structure (
), myelinated axon structures observed in degenerated vacuolar structure ( ), normal myelinated axon structures (
), normal myelinated axon structures ( ), and proliferating Schwann cells (
), and proliferating Schwann cells ( ). The light microscopy examination of toluidine blue-stained sciatic nerve specimens revealed a normal histological structure characterized by mast cells, myelinated axon structures, and vascular formations in the control group. In the paralyzed group, mast cells, degenerated vacuolar structures in myelinated axon structures, and few proliferating Schwann cells were detected. In the perineural and intraperitoneal ozone groups, mast cells and myelinated axon structures, reduced degenerated myelinated axon structures, and proliferating Schwann cells were detected in some areas.
). The light microscopy examination of toluidine blue-stained sciatic nerve specimens revealed a normal histological structure characterized by mast cells, myelinated axon structures, and vascular formations in the control group. In the paralyzed group, mast cells, degenerated vacuolar structures in myelinated axon structures, and few proliferating Schwann cells were detected. In the perineural and intraperitoneal ozone groups, mast cells and myelinated axon structures, reduced degenerated myelinated axon structures, and proliferating Schwann cells were detected in some areas.
 ), myelinated axon structures observed in degenerated vacuolar structure (
), myelinated axon structures observed in degenerated vacuolar structure ( ), normal myelinated axon structures (
), normal myelinated axon structures ( ), and proliferating Schwann cells (
), and proliferating Schwann cells ( ). The light microscopy examination of toluidine blue-stained sciatic nerve specimens revealed a normal histological structure characterized by mast cells, myelinated axon structures, and vascular formations in the control group. In the paralyzed group, mast cells, degenerated vacuolar structures in myelinated axon structures, and few proliferating Schwann cells were detected. In the perineural and intraperitoneal ozone groups, mast cells and myelinated axon structures, reduced degenerated myelinated axon structures, and proliferating Schwann cells were detected in some areas.
). The light microscopy examination of toluidine blue-stained sciatic nerve specimens revealed a normal histological structure characterized by mast cells, myelinated axon structures, and vascular formations in the control group. In the paralyzed group, mast cells, degenerated vacuolar structures in myelinated axon structures, and few proliferating Schwann cells were detected. In the perineural and intraperitoneal ozone groups, mast cells and myelinated axon structures, reduced degenerated myelinated axon structures, and proliferating Schwann cells were detected in some areas.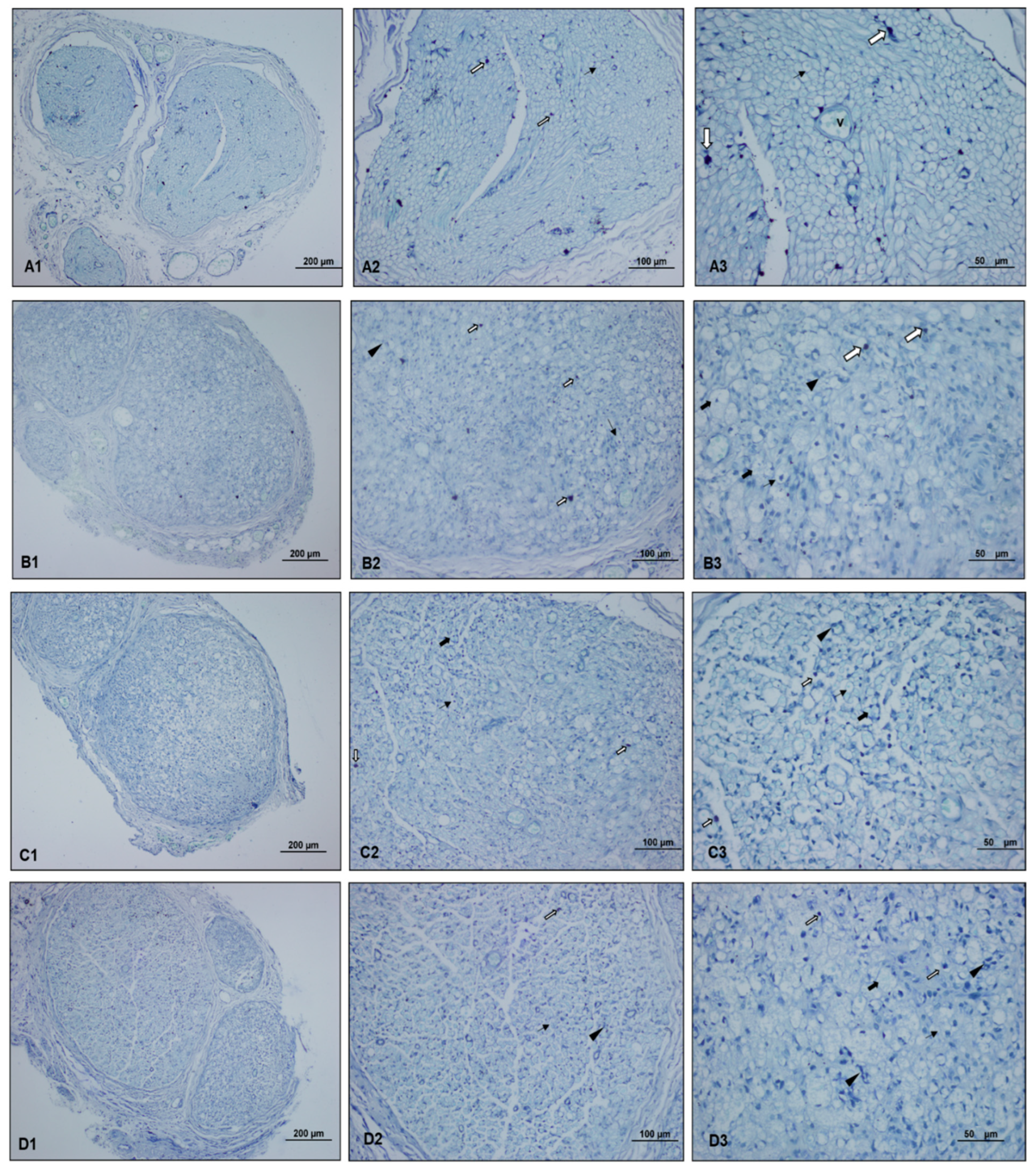
 ). The light microscopy examination of the specimens subjected to TUNEL staining revealed minimal positive staining in the control group, advanced positive staining in the paralyzed group, and moderate positive staining in both ozone groups.
). The light microscopy examination of the specimens subjected to TUNEL staining revealed minimal positive staining in the control group, advanced positive staining in the paralyzed group, and moderate positive staining in both ozone groups.
 ). The light microscopy examination of the specimens subjected to TUNEL staining revealed minimal positive staining in the control group, advanced positive staining in the paralyzed group, and moderate positive staining in both ozone groups.
). The light microscopy examination of the specimens subjected to TUNEL staining revealed minimal positive staining in the control group, advanced positive staining in the paralyzed group, and moderate positive staining in both ozone groups.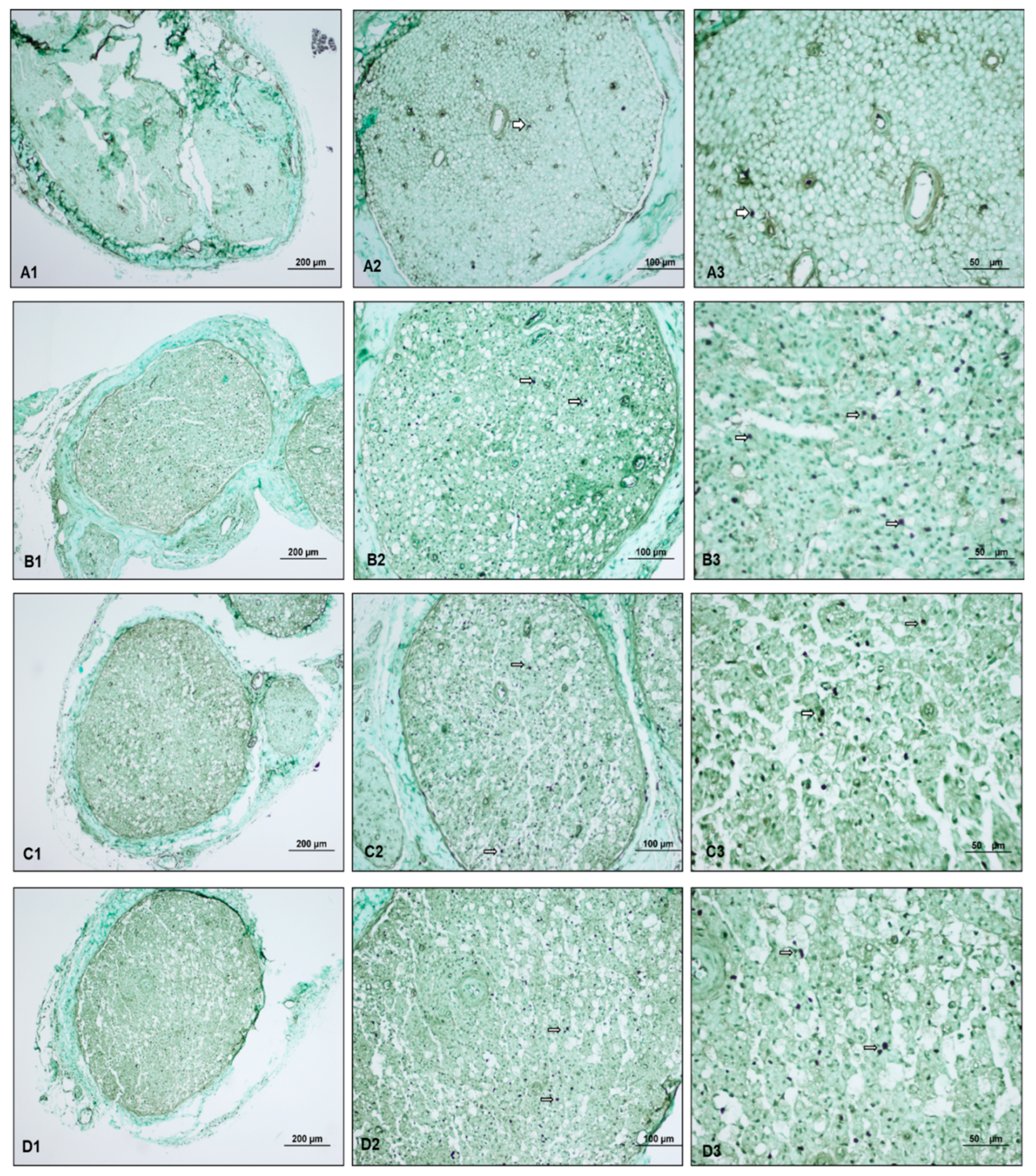
| Variables | Control Group (n = 8) | Paralyzed Group (n = 10) | Perineural Ozone Group (n = 10) | Intraperitoneal Ozone Group (n = 10) | p Value | Post hoc Dunn’s Test |
|---|---|---|---|---|---|---|
| Pinch test score (pre-treatment) | 3 (3–3) | 0 (0–1.25) | 0 (0–0) | 0 (0–1) | <0.001 | 1–2, 1–3, 1–4 |
| Pinch test score (post-treatment) | 3 (3–3) | 0 (0–1.25) | 2.5 (1.75–3) | 2.5 (2–3) | 0.001 | 1–2, 2–3, 2–4 |
| p * value | 0.607 | 0.867 | <0.001 | 0.003 | ||
| Rotarod | 12.5 (5–30.25) | 7 (0–11.5) | 8 (0–15.5) | 9 (5.25–13.5) | 0.402 | NS |
| Control Group (n = 8) | Paralyzed Group (n = 10) | Perineural Ozone Group (n = 10) | İntraperitoneal Ozone Group (n = 10) | p-Value | Post hoc Dunn’s Test | |
|---|---|---|---|---|---|---|
| Myelin Degeneration | 0 (0–0) | 3 (2.75–3) | 1 (0–1) | 0.5 (0–1) | <0.001 | 1–2, 1–3, 2–3, 2–4 |
| Vascular Congestion | 0 (0–0.75) | 3 (2–3) | 1 (0–1) | 1 (0–1) | <0.001 | 1–2, 2–3, 2–4 |
| Vascular Wall Thickness | 0 (0–0) | 3 (3–3) | 1 (1–1.25) | 0 (0–0.25) | <0.001 | 1–2, 1–3, 2–3, 2–4, 3–4 |
| Inflammation | 0 (0–0) | 2 (1–2) | 0 (0–1) | 0 (0–0.25) | <0.001 | 1–2, 2–3, 2–4 |
| Schwann Cell Proliferation | 0 (0–0) | 1 (1–1.25) | 2 (2–3) | 1 (0–1) | <0.001 | 1–2, 1–3, 2–3, 3–4 |
| Toluidine Blue | 0.5 (0–1) | 2 (2–3) | 1.5 (1–2) | 1 (1–2) | <0.001 | 1–2, 1–3, 2–3, 2–4 |
| TUNEL | 0 (0–0) | 3 (2–3) | 1.5 (1–2) | 1 (1–1.25) | <0.001 | 1–2, 1–3, 1–4, 2–3, 2–4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayık, B.; Ortadeveci, A.; Bakılan, F.; Dönmez, D.B.; Öz, S.; Bal, C.; Özden, H.; Armağan, O. Comparison of the Effects of Perineural and Intraperitoneal Ozone Therapy on Nerve Healing in an Experimental Sciatic Nerve Injury Model. Medicina 2024, 60, 2097. https://doi.org/10.3390/medicina60122097
Ayık B, Ortadeveci A, Bakılan F, Dönmez DB, Öz S, Bal C, Özden H, Armağan O. Comparison of the Effects of Perineural and Intraperitoneal Ozone Therapy on Nerve Healing in an Experimental Sciatic Nerve Injury Model. Medicina. 2024; 60(12):2097. https://doi.org/10.3390/medicina60122097
Chicago/Turabian StyleAyık, Burcu, Abdullah Ortadeveci, Fulya Bakılan, Dilek Burukoğlu Dönmez, Semih Öz, Cengiz Bal, Hilmi Özden, and Onur Armağan. 2024. "Comparison of the Effects of Perineural and Intraperitoneal Ozone Therapy on Nerve Healing in an Experimental Sciatic Nerve Injury Model" Medicina 60, no. 12: 2097. https://doi.org/10.3390/medicina60122097
APA StyleAyık, B., Ortadeveci, A., Bakılan, F., Dönmez, D. B., Öz, S., Bal, C., Özden, H., & Armağan, O. (2024). Comparison of the Effects of Perineural and Intraperitoneal Ozone Therapy on Nerve Healing in an Experimental Sciatic Nerve Injury Model. Medicina, 60(12), 2097. https://doi.org/10.3390/medicina60122097






