MTHFR Gene Polymorphisms and DNA Methylation in Idiopathic Spontaneous Preterm Birth
Abstract
1. Introduction
2. Materials and Methods
2.1. The Subjects
2.2. DNA Extraction and Genotype Analysis
2.3. Bisulfite Treatment and DNA Methylation Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Preterm Birth. Internet 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 1 March 2024).
- Gupta, R.; Froeb, K. Preterm Birth: Two Startling Trends, One Call to Action. J. Perinat. Neonatal Nurs. 2020, 34, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, E.; Maitra, A. Spontaneous preterm birth: The underpinnings in the maternal and fetal genomes. NPJ Genom. Med. 2021, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Mead, E.C.; Wang, C.A.; Phung, J.; Fu, J.Y.; Williams, S.M.; Merialdi, M.; Jacobsson, B.; Lye, S.; Menon, R.; Pennell, C.E. The Role of Genetics in Preterm Birth. Reprod. Sci. 2023, 30, 3410–3427. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Griggs, K.M.; Hrelic, D.A.; Williams, N.; McEwen-Campbell, M.; Cypher, R. Preterm Labor and Birth. MCN Am. J. Matern./Child Nurs. 2020, 45, 328–337. [Google Scholar] [CrossRef]
- Pennell, C.E.; Jacobsson, B.; Williams, S.M.; Buus, R.M.; Muglia, L.J.; Dolan, S.M.; Morken, N.-H.; Ozcelik, H.; Lye, S.J.; Relton, C. Genetic epidemiologic studies of preterm birth: Guidelines for research. Am. J. Obstet. Gynecol. 2007, 196, 107–118. [Google Scholar] [CrossRef]
- Hong, X.; Sherwood, B.; Ladd-Acosta, C.; Peng, S.; Ji, H.; Hao, K.; Burd, I.; Bartell, T.R.; Wang, G.; Tsai, H.-J.; et al. Genome-wide DNA methylation associations with spontaneous preterm birth in US blacks: Findings in maternal and cord blood samples. Epigenetics 2018, 13, 163–172. [Google Scholar] [CrossRef]
- Tan, Q.; Li, S.; Frost, M.; Nygaard, M.; Soerensen, M.; Larsen, M.; Christensen, K.; Christiansen, L. Epigenetic signature of preterm birth in adult twins. Clin. Epigenetics 2018, 10, 87. [Google Scholar] [CrossRef]
- Liew, S.C.; Gupta, E.D. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: Epidemiology, metabolism and the associated diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, P.; Geng, X.; Liu, Z.; Cui, L.; Gao, Z.; Jiang, B.; Yang, L. Genetic polymorphism of MTHFR C677T with preterm birth and low birth weight susceptibility: A meta-analysis. Arch. Gynecol. Obstet. 2017, 295, 1105–1118. [Google Scholar] [CrossRef]
- Tiwari, D.; Bose, P.D.; Das, S.; Das, C.R.; Datta, R.; Bose, S. MTHFR (C677T) polymorphism and PR (PROGINS) mutation as genetic factors for preterm delivery, fetal death and low birth weight: A Northeast Indian population based study. Meta Gene 2015, 3, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Friso, S.; Choi, S.-W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R.; et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5611. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.J.; Mandaviya, P.R.; Dib, M.J.; Uitterlinden, A.G.; Van Meurs, J.; Heil, S.G.; Andrew, T.; Ahmadi, K.R.; Nash, A.J.; Mandaviya, P.R.; et al. Interaction between plasma homocysteine and the MTHFR c.677C > T polymorphism is associated with site-specific changes in DNA methylation in humans. FASEB J. 2019, 33, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Villicaña, S.; Bell, J.T. Genetic impacts on DNA methylation: Research findings and future perspectives. Genome Biol. 2021, 22, 127. [Google Scholar] [CrossRef]
- Barišić, A.; Stanković, A.; Stojković, L.; Pereza, N.; Ostojić, S.; Peterlin, A.; Peterlin, B.; Vraneković, J. Maternal LINE-1 DNA Methylation in Early Spontaneous Preterm Birth. Biol. Res. Nurs. 2022, 24, 85–93. [Google Scholar] [CrossRef]
- Rajeevan, H.; Osier, M.V.; Cheung, K.H.; Deng, H.; Druskin, L.; Heinzen, R.; Kidd, J.R.; Stein, S.; Pakstis, A.J.; Tosches, N.P.; et al. ALFRED: The ALelle FREquency Database. Update. Nucleic Acids Res. 2003, 31, 270–271. [Google Scholar] [CrossRef]
- Babić Božović, I.; Vraneković, J.; Starčević Čizmarević, N.; Mahulja-Stamenković, V.; Prpić, I.; Brajenović-Milić, B. MTHFR C677T and A1298C polymorphisms as a risk factor for congenital heart defects in Down syndrome. Pediatr. Int. 2011, 53, 546–550. [Google Scholar] [CrossRef]
- Friedman, G.; Goldschmidt, N.; Friedlander, Y.; Ben-Yehuda, A.; Selhub, J.; Babaey, S.; Mendel, M.; Kidron, M.; Bar-On, H. A Common Mutation A1298C in Human Methylenetetrahydrofolate Reductase Gene: Association with Plasma Total Homocysteine and Folate Concentrations. J. Nutr. 1999, 129, 1656–1661. [Google Scholar] [CrossRef]
- Weisenberger, D.J.; Campan, M.; Long, T.I.; Kim, M.; Woods, C.; Fiala, E.; Ehrlich, M.; Laird, P.W. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005, 33, 6823–6836. [Google Scholar] [CrossRef]
- Nan, Y.; Li, H. Original Article MTHFR genetic polymorphism increases the risk of preterm delivery. Int. J. Clin. Exp. Pathol. 2015, 8, 7397. [Google Scholar] [PubMed]
- Hwang, I.W.; Kang, Y.D.; Kwon, B.N.; Hong, J.H.; Han, S.H.; Kim, J.S.; Park, J.W.; Jin, H.J. Genetic variations of MTHFR gene and their association with preterm birth in Korean women. Medicina 2017, 53, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of hyperhomocysteinemia and its pathological significance. Arch. Pharm. Res. 2018, 41, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Djafarian, K.; Moradi, S.; Shab-Bidar, S. C667T and A1298C polymorphisms of methylenetetrahydrofolate reductase gene and susceptibility to myocardial infarction: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 217, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, R.; Yu, J. New Understanding of the Relevant Role of LINE-1 Retrotransposition in Human Disease and Immune Modulation. Front. Cell Dev. Biol. 2020, 8, 657. [Google Scholar] [CrossRef]
- Parets, S.E.; Conneely, K.N.; Kilaru, V.; Menon, R.; Smith, A.K. DNA methylation provides insight into intergenerational risk for preterm birth in African Americans. Epigenetics 2015, 10, 784–792. [Google Scholar] [CrossRef]
- Burris, H.H.; Rifas-Shiman, S.L.; Baccarelli, A.; Tarantini, L.; Boeke, C.E.; Kleinman, K.; Litonjua, A.A.; Rich-Edwards, J.W.; Gillman, M.W. Associations of long interspersed nuclear element-1 DNA methylation with preterm birth in a prospective cohort study. J. Dev. Orig. Health Dis. 2012, 3, 173–181. [Google Scholar] [CrossRef]
- Kaye, A.D.; Jeha, G.M.; Pham, A.D.; Fuller, M.C.; Lerner, Z.I.; Sibley, G.T.; Cornett, E.M.; Urits, I.; Viswanath, O.; Kevil, C.G. Folic Acid Supplementation in Patients with Elevated Homocysteine Levels. Adv. Ther. 2020, 37, 4149–4164. [Google Scholar] [CrossRef]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X. A Novel Review of Homocysteine and Pregnancy Complications. BioMed Res. Int. 2021, 2021, 6652231. [Google Scholar] [CrossRef]
- Bošković, A.; Ćuk, A.; Mandrapa, V.; Šimić, A.D.; Cvetković, I.; Vlaho, M.O.; Krešić, T.; Tomić, T.; Tomić, V. Association of MTHFR polymorphism, folic acid, and vitamin B12 with serum homocysteine levels in pregnant women. Biomol. Biomed. 2024, 24, 138–143. [Google Scholar] [CrossRef]
- Salehi-Pourmehr, H.; Mohamad-Alizadeh, S.; Malakouti, J.; Farshbaf-Khalili, A. Association of the folic acid consumption and its serum levels with preeclampsia in pregnant women. Iran J. Nurs. Midwifery Res. 2012, 17, 461–466. [Google Scholar] [PubMed]
| Maternal Characteristics | SPTB Group | Control Group | p |
|---|---|---|---|
| Maternal age (years) | Median (range) | Median (range) | |
| 30 (17–42) | 31 (20–42) | 0.375 | |
| Gestational age at delivery (weeks) | Mean ± SD | Mean ± SD | |
| 30.7 ± 3.0 | 39.5 ± 1.0 | <0.001 * | |
| Smoking before pregnancy | N (%) | N (%) | |
| Yes | 16 (32%) | 9 (20%) | 0.185 |
| No | 34 (68%) | 36 (80%) | |
| Smoking during pregnancy | N (%) | N (%) | |
| Yes | 7 (14%) | 5 (11%) | 0.672 |
| No | 43 (86%) | 40 (89%) | |
| Previous PTB | N (%) | N (%) | |
| Yes | 5 (10%) | 1 (2%) | 0.119 |
| No | 45 (90%) | 44 (98%) | |
| Familial PTB | N (%) | N (%) | |
| Yes | 16 (32%) | 1 (2%) | <0.001 ** |
| No | 34 (68%) | 44 (98%) | |
| Newborn characteristics | Patients | Control subjects | p |
| Newborn weight (g) | Mean ± SD | Mean ± SD | |
| 1764.1 ± 556.1 | 3475.1 ± 340.6 | <0.001 *** | |
| SPTB Group/N (%) | Control Group/N (%) | χ2 | p | ||
|---|---|---|---|---|---|
| MTHFR C677T | |||||
| Genotype | CC | 21 (42.0) | 18 (36.0) | ||
| CT | 23 (46.0) | 27 (54.0) | |||
| TT | 6 (12.0) | 5 (10.0) | 0.64 | 0.726 | |
| Allele | C | 65 (65.0) | 63 (63.0) | ||
| T | 35 (35.0) | 37 (37.0) | 0.09 | 0.768 | |
| MTHFR A1298C | |||||
| Genotype | AA | 21 (42.0) | 22 (44.0) | ||
| AC | 23 (46.0) | 25 (50.0) | |||
| CC | 6 (12.0) | 3 (6.0) | 1.11 | 0.575 | |
| Allele | A | 65 (65.0) | 69 (69.0) | ||
| C | 35 (35.0) | 31 (31.0) | 0.36 | 0.547 | |
| Genetic Models | ISPTB Group | Control Group | OR | 95% CI | p |
|---|---|---|---|---|---|
| MTHFR C677T | |||||
| CT+TT/CC | 29/21 | 32/18 | 0.78 | 0.35–1.74 | 0.539 |
| TT/CT+CC | 6/44 | 5/45 | 1.28 | 0.35–4.32 | 0.749 |
| TT/CC | 6/21 | 5/18 | 1.03 | 0.27–3.94 | 0.967 |
| CT/CC | 23/21 | 27/18 | 0.73 | 0.31–1.69 | 0.463 |
| MTHFR A1298C | |||||
| AC+CC/AA | 29/21 | 28/22 | 1.09 | 0.49–2.40 | 0.840 |
| CC/AC+AA | 6/44 | 3/47 | 2.14 | 0.50–9.07 | 0.303 |
| CC/AA | 6/21 | 3/22 | 2.10 | 0.46–9.48 | 0.337 |
| AC/AA | 23/21 | 25/22 | 0.96 | 0.42–2.20 | 0.930 |
| Maternal/Newborn Characteristics | MTHFR C677T | MTHFR A1298C | ||
|---|---|---|---|---|
| χ2 | p | χ2 | p | |
| Gestational age at delivery | 5.15 | 0.272 | 4.42 | 0.352 |
| Smoking before pregnancy | 3.02 | 0.221 | 1.30 | 0.522 |
| Smoking during pregnancy | 2.47 | 0.291 | 2.47 | 0.291 |
| Previous PTB | 0.91 | 0.635 | 0.35 | 0.842 |
| Familial PTB | 0.05 | 0.976 | 1.28 | 0.527 |
| t | p | t | p | |
| Maternal age | −0.17 | 0.863 | −1.77 | 0.089 |
| Newborn weight | −1.18 | 0.246 | −0.28 | 0.783 |
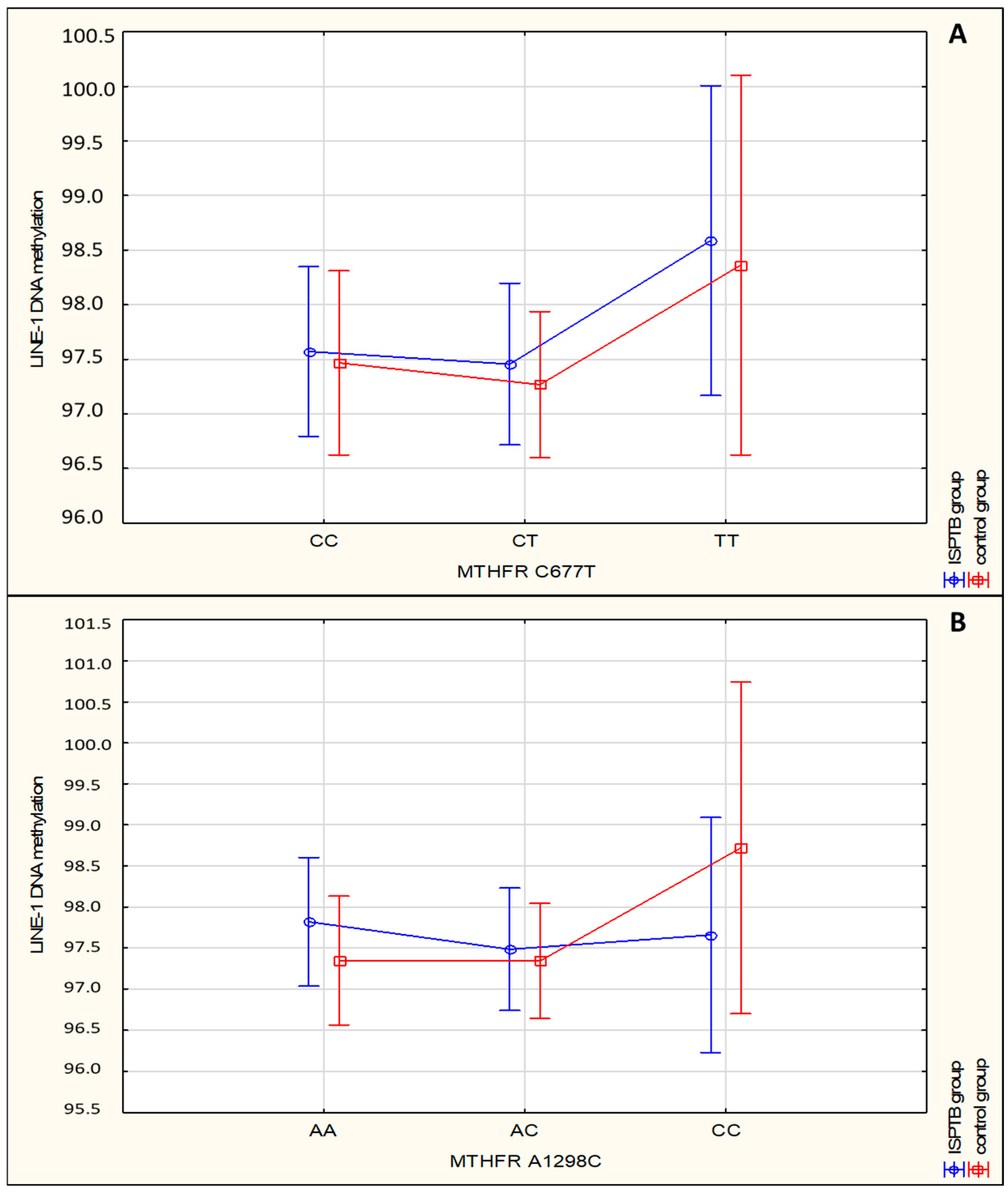
| LINE-1 DNA Methylation | SPTB Group/Mean (SD) | Control Group/Mean (SD) | χ2 | p | |
|---|---|---|---|---|---|
| MTHFR C677T | |||||
| CC | 97.571 (1.210) | 97.468 (2.055) | |||
| CT | 97.456 (1.712) | 97.267 (2.035) | |||
| TT | 98.588 (0.821) | 98.364 (1.635) | 2.14 | 0.344 | |
| MTHFR A1298C | |||||
| Genotype | AA | 97.819 (1.373) | 97.347 (1.789) | ||
| AC | 97.484 (1.591) | 97.341 (2.177) | |||
| CC | 97.659 (1.329) | 98.719 (1.944) | 0.40 | 0.818 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dević Pavlić, S.; Šverko, R.; Barišić, A.; Mladenić, T.; Vraneković, J.; Stanković, A.; Peterlin, A.; Peterlin, B.; Ostojić, S.; Pereza, N. MTHFR Gene Polymorphisms and DNA Methylation in Idiopathic Spontaneous Preterm Birth. Medicina 2024, 60, 2028. https://doi.org/10.3390/medicina60122028
Dević Pavlić S, Šverko R, Barišić A, Mladenić T, Vraneković J, Stanković A, Peterlin A, Peterlin B, Ostojić S, Pereza N. MTHFR Gene Polymorphisms and DNA Methylation in Idiopathic Spontaneous Preterm Birth. Medicina. 2024; 60(12):2028. https://doi.org/10.3390/medicina60122028
Chicago/Turabian StyleDević Pavlić, Sanja, Roberta Šverko, Anita Barišić, Tea Mladenić, Jadranka Vraneković, Aleksandra Stanković, Ana Peterlin, Borut Peterlin, Saša Ostojić, and Nina Pereza. 2024. "MTHFR Gene Polymorphisms and DNA Methylation in Idiopathic Spontaneous Preterm Birth" Medicina 60, no. 12: 2028. https://doi.org/10.3390/medicina60122028
APA StyleDević Pavlić, S., Šverko, R., Barišić, A., Mladenić, T., Vraneković, J., Stanković, A., Peterlin, A., Peterlin, B., Ostojić, S., & Pereza, N. (2024). MTHFR Gene Polymorphisms and DNA Methylation in Idiopathic Spontaneous Preterm Birth. Medicina, 60(12), 2028. https://doi.org/10.3390/medicina60122028







