1. Introduction
Streptococcus sinensis (
S. sinensis), a member of the
Streptococcus mitis group, has emerged as an intriguing bacterium in the realm of clinical microbiology since its initial characterization in 2001 by Collins et al. [
1]. This novel species has attracted significant attention due to its unique microbiological features, clinical significance, and prevalence in human microbial communities.
S. sinensis is a Gram-positive, non-motile, catalase-negative, facultatively anaerobic bacterium belonging to the
phylum Firmicutes, characterized by partial hemolysis, indicative of its ability to degrade hemoglobin [
2,
3].
S. sinensis diagnosis has been implemented through 16S rRNA or MALDI-TOF Mass Spectrometry [
4,
5]. Furthermore, whole-genome sequencing has provided insights into the genomic architecture of
S. sinensis, revealing its genetic composition, metabolic pathways, and potential virulence factor [
6].
While closely related to other
viridans Streptococci,
S. sinensis exhibits several distinctive features that set it apart within the
Streptococcus genus, including its biochemical profile, antigenic properties, and susceptibility to antimicrobial agents [
6].
Phenotypically, S. sinensis demonstrates unique fermentation patterns for carbohydrates and distinctive enzyme activities, contributing to its differentiation from phylogenetically related species. Additionally, S. sinensis possesses surface antigens and adhesins that mediate its interaction with host tissues and immune evasion strategies. Studies have identified surface proteins involved in adhesion to epithelial cells and extracellular matrix components, potentially influencing its colonization and pathogenicity. Antimicrobial susceptibility testing has revealed varying resistance profiles among clinical isolates of S. sinensis, highlighting the importance of accurate susceptibility testing to guide antimicrobial therapy. While generally susceptible to beta-lactam antibiotics, resistance to other antibiotic classes, such as macrolides and fluoroquinolones, has been reported, necessitating vigilant surveillance of antimicrobial resistance patterns.
S. sinensis is primarily found as a commensal organism in the oropharynx and upper respiratory tract of humans. Epidemiological studies have documented its prevalence in healthy individuals as well as in patients with underlying medical conditions. While the exact prevalence varies across populations and geographical regions,
S. sinensis is commonly isolated from respiratory specimens, saliva, dental plaques, and nasopharyngeal swabs. Moreover,
S. sinensis has been implicated in various clinical infections, including endocarditis, bacteremia, pneumonia, and deep-seated abscesses, underscoring its pathogenic potential. The prevalence of
S. sinensis in clinical settings highlights the need for accurate species identification and characterization to facilitate targeted therapeutic interventions and infection control measures, and to date, very few cases have been reported [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16].
We herein describe the first case of endocarditis, defined as per Duke criteria [
17], by
S. sinensis reported in Italy. Through a systematic literature review, we conduct an analysis of the clinical characteristics and outcomes associated with
S. sinensis endocarditis.
2. Case Presentation
A 75-year-old Italian gentleman was hospitalized because of a 4-week history of exertional dyspnoea, profuse asthenia, progressive loss of appetite and weight loss of approximately 10 Kg, and an episode of loss of consciousness. He had no history of travels or anything relevant to disclose in his recent medical history. His past medical history revealed acute promyelocytic leukemia successfully treated in 2004, aortoiliac bypass surgery for an infrarenal abdominal aortic aneurysm in 2007, and radiotherapy in 2014 for prostate adenocarcinoma. He was also suffering from dyslipidemia on treatment with rosuvastatin 10 mg/day, depression on treatment with sertraline 50 mg/day, diabetes on treatment with empagliflozin 10 mg/day, and hyperthyroidism on treatment with methimazole 5 mg/day. At admission, his temperature was 38.5 °C, his pulse was 75 beats per minute with extrasystoles, his blood pressure was 130/60 mmHg, his respiratory rate was 15 breaths per minute, and his oxygen saturation was 99% in room air. The blood tests showed a normal white blood count with anemia (hemoglobin = 85 g/L), for which the patient received a blood transfusion. The plasma C-reactive (CPR) protein level was 39 mg/L (normal values < 0.5 mg/L), and the procalcitonin level was within the normal range. There was a slight impairment in kidney function, with an eGFR of 48 mL/min and a creatinine level of 110 mmol/L. The liver function tests were normal. In addition, two sets of blood cultures were performed during the febrile episode.
The physical examination revealed a systolic murmur 3/4 Levine prevalent in the mesocardium, raising the suspicion of infectious endocarditis, despite that the patient had no history of recent dental procedures, rheumatic heart disease, or other conditions that could predispose him to the ongoing condition. No cutaneous signs of infectious endocarditis, such as Janeway lesions, Osler nodes, or splinter hemorrhages beneath the fingernails, were detected, and the rest of the clinical assessment was unremarkable. Chest X-ray was negative, as was a pulmonary angio-CT scan performed for a mild elevation of d-dimer (3699 mcg/L, normal values <700) to exclude pulmonary embolism.
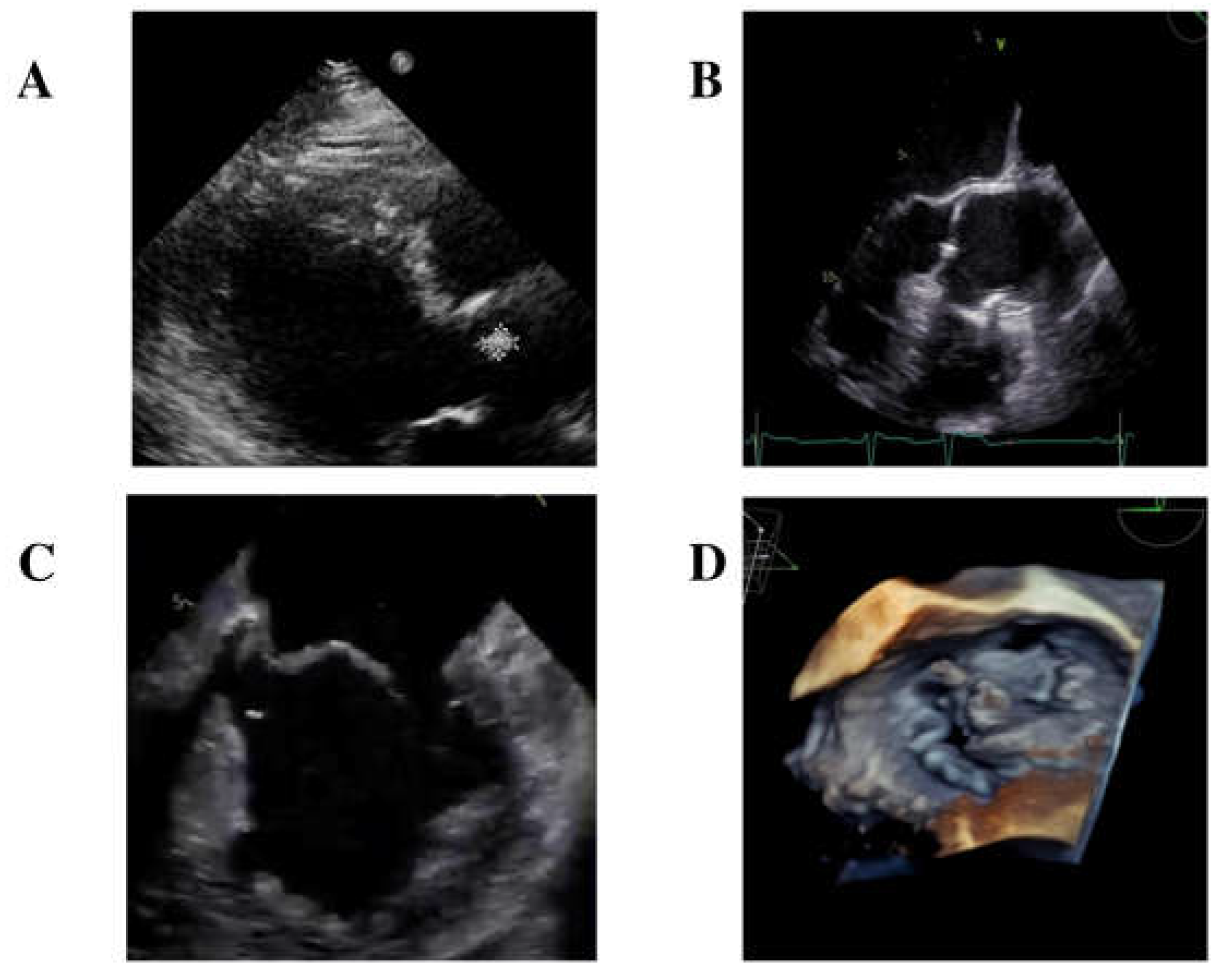
A transthoracic echocardiogram identified a small isoechoic mass (0.7 mm × 10 mm) on the right cusp of the aortic valve (
Figure 1A) and diagnosed mitral valve dysfunction with severe regurgitation, requiring the initiation of diuretic therapy.
Furthermore, an empiric antibiotic treatment with intravenous ceftriaxone 2 gr/day was started. In the meanwhile, blood cultures came back positive for Gram-positive rods, and MALDI-TOF identified
S. sinensis. Further diagnostic evaluations included a transesophageal echocardiogram (TEE), which confirmed the presence of the isoechoic mass measuring 0.7 mm × 0.7 mm on the right cusp of the aortic valve (
Figure 1B). Additionally, TEE identified another long (length = 12.5 mm) mobile iso-echogenic mass on the medial scallop of the posterior mitral leaflet (P2), with a flail of P2 due to rupture of the chordae tendineae (
Figure 1C,D).
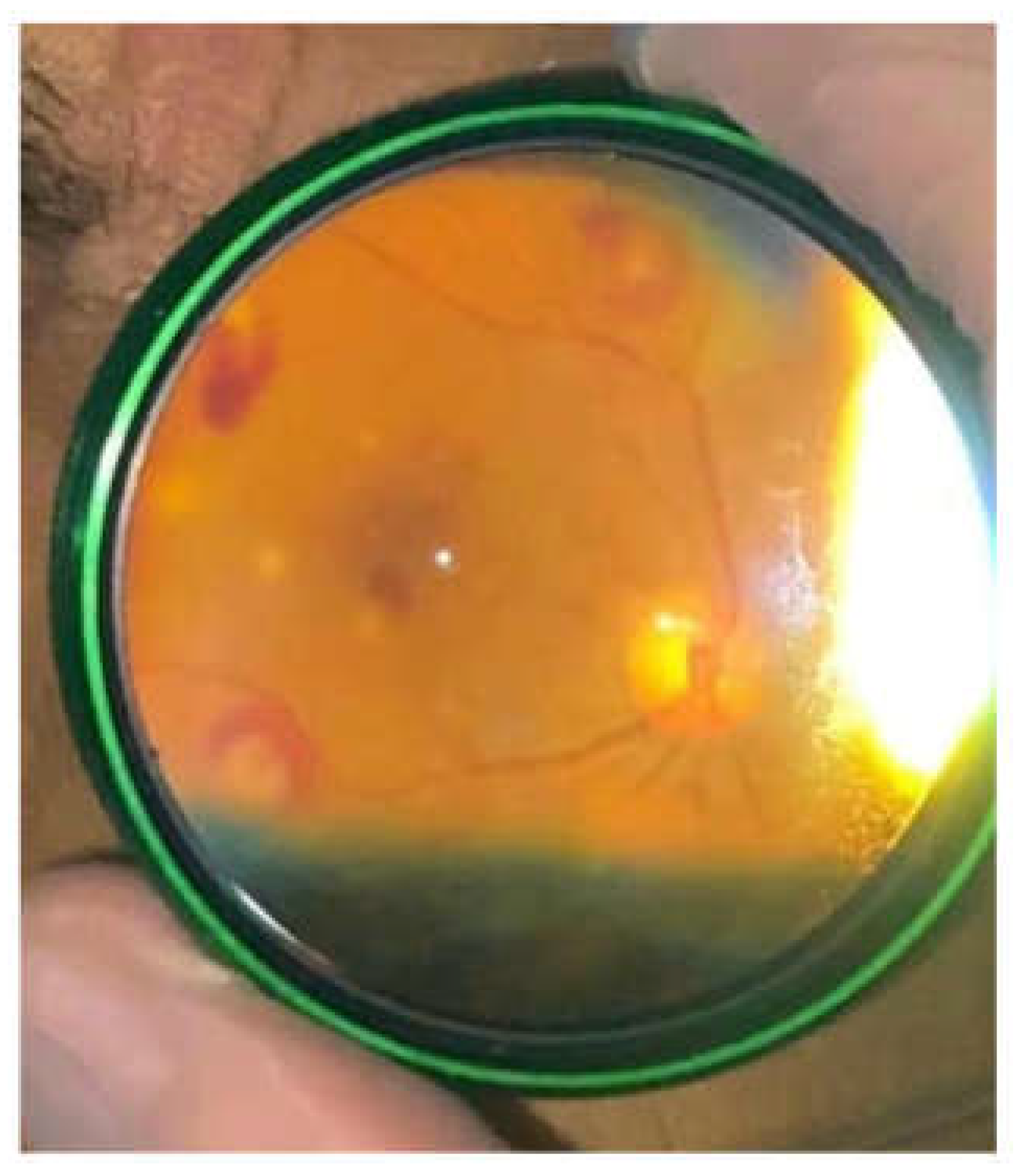
A bedside ophthalmic examination conducted during the hospital stay revealed multiple Roth dots (oval and superficial retinal hemorrhages, with pale center) in the left eye and white retinal infiltrates at the posterior pole, consistent with infectious embolization (
Figure 2). The case was hence defined as certain
S. sinensis endocarditis, according to Duke criteria-ISCVID 2023, with the presence of both major and minor criteria.
CT scans of the brain and abdomen were both negative for embolization. According to the antimicrobial susceptibility testing results, antibiotic therapy with ceftriaxone was continued for a total of 6 weeks. Follow-up blood cultures performed 3 days after the start of the antibiotic confirmed the clearance of the bacteremia. The patient’s condition gradually improved as well as the inflammatory biomarkers. He then underwent a cardiac surgery procedure, which included a successful mitral prosthetic valve replacement. The intervention was complicated by mild bleeding, which required mediastinal revision surgery, but the post-operative course was normal and with a favourable evolution. The patient was discharged home eleven weeks after admission, and follow-up cardiac US performed one month and three months after discharge was negative. To date, the patient is in good clinical condition.
3. Systematic Literature Review: Methodology and Results
The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines were followed to perform the review. The Embase/MEDLINE database was screened backward from 15 July 2024. The search was conducted by using the words “Endocarditis” or “heart infections” AND “Streptococcus sinensis” OR “S. sinensis”. Two reviewers (MM and VS) independently screened the titles and abstracts to deem eligibility for full-text assessment. No language or geographical restrictions were applied. We included all papers that included cases published as full articles/case reports/systematic reviews about S. sinensis endocarditis. From each paper, we extracted, whenever available, information about demographics (gender, age, country), comorbidities, risk factors, symptom onset, diagnosis, treatment, and clinical outcome.
We described the first case of infectious endocarditis by
S. sinensis ever reported in Italy. The other 12 cases reported in the literature are described in
Table 1. Cases reported, including ours, are mostly male (7/13, 53%) and with an age range from 19 to 75 years, with our case being the oldest ever reported.
S. sinensis was isolated for the first time in 2002 in a 42-year-old Chinese woman diagnosed with endocarditis [
7]. It is worth mentioning that most cases have primarily been observed in Southeast Asia, particularly in the Hong Kong region [
7,
13]. However, there have been more recent reports of cases in Europe [
8,
9,
10,
11,
12]. Out of the 12 clinical cases documented in the literature, 8 people were from Asia, 1 was from Europe, and in 3 cases, the origin was not available. Additionally, while in most cases, a travel history was reported, in our case, there was no mention of travel. This underscores the significance of determining the travel history of those with
S. sinensis infection to identify geographical sources for this pathogen, highlighting its potential as a new emerging infectious agent, but at the same time, it is worth considering the oral source as the main driver. The median time interval between symptoms onset and infectious endocarditis diagnosis in eight out of nine individuals was 10 weeks (range: 1–36 weeks), categorizing them as late-diagnosed infectious endocarditis cases (diagnosis occurring more than 1 month after initial symptoms), accounting for approximately 45% of all bacterial IE cases [
18]. As already stated by Zhang et al. in their review [
15], previous
S. sinensis clinical cases lack in-depth characterizations, and it is not possible to speculate much about prognostic factors and their correlation with treatment outcomes. Most cases described had predisposing risk factors for developing infectious endocarditis, such as rheumatic heart disease, dental procedures, or valve alterations (either congenital or degenerative). The median antibiotic treatment length was 6 weeks and was mainly based on combination therapy of penicillin or ceftriaxone with gentamycin, while monotherapy with ceftriaxone was used only in two cases. Most patients underwent cardiac surgery either due to significant valve dysfunction or the size of vegetations, except for one patient who was planned for intervention but could not undergo it due to the severity of clinical presentation and personal beliefs, and three for whom this information is not available. Embolization phenomena were reported in 7 cases (53.8%), primarily affecting the central nervous system and eye.
Unfortunately, one case resulted in a fatality due to central nervous system embolization complications. The overall mortality rate was 7.7% (1/13), which is relatively lower compared to other pathogens like
Enterococci (15–25%),
Staphylococcus aureus (25–47%),
Pseudomonas aeruginosa, and fungal species (50%) [
19].
4. Discussion
The clinical significance of S. sinensis infection relies on its potential to cause severe infections, particularly in immunocompromised individuals or those with predisposing risk factors.
To date, the most common clinical manifestations of
S. sinensis infections include bloodstream infections and endocarditis [
13]. Both require prompt diagnosis and aggressive management, including surgery, to avoid further dissemination of the infection, which often causes central nervous system dissemination. In addition, timely identification of the causative organism and assessment of antimicrobial susceptibility are crucial for enhancing patient outcomes.
S. sinensis, along with
Streptococcus cristatus, is classified into the “cristatus clade” of the
S. mitis group of the streptococci genus, according to the new phylogenetic classification [
5]. Despite its microbiological features, the treatment of
S. sinensis endocarditis does not differ much from cases caused by other strains from the same group. The oral cavity seems to be the natural reservoir of this bacterium, similar to other
viridans Streptococci. It was detected in 22% of saliva samples from 100 healthy volunteers, and it was probably the apparent source of the infection in patients with infective endocarditis [
19]. Indeed, in our cases, the history of travel, which has been mentioned in other cases as a major risk factor, is lacking, making it more probable that the etiology came from the oral cavity. However, global concerns are emerging since most cases were detected in Asia, but the number of cases in Europe is rising. Currently,
S. sinensis is very sensitive to penicillin, ceftriaxone, cefepime, clindamycin, erythromycin, ofloxacin, tetracycline, and vancomycin [
7,
20,
21]. However, the ability of
S. sinensis to form biofilms on host surfaces further exacerbates its pathogenic potential, rendering it more resistant to host immune defenses and antimicrobial agents, particularly in endocarditis cases [
22]. In fact, most of the reported cases underwent surgery due to severe native valve disruption. All cases reported involved native valves, but the latest recently published for which the bioprosthetic aortic valve was involved, posing further challenges in clinical management [
16].
S. sinensis has been implicated in polymicrobial infections, where it coexists with other pathogenic or commensal microorganisms. Coexisting pathogens complicate further clinical management and the selection of appropriate antimicrobial therapy.
5. Conclusions
In conclusion, S. sinensis represents a fascinating microorganism with distinctive microbiological features, clinical significance, and prevalence in human microbial communities. Continued research into its epidemiology, pathogenesis, antimicrobial resistance, and host interactions is essential for enhancing our understanding of this emerging pathogen and improving clinical management strategies. By elucidating the complex interplay between S. sinensis and the host environment, we can develop more effective preventive measures and therapeutic interventions to mitigate the impact of S. sinensis-associated infections on human health.
Author Contributions
Conceptualization, M.M.; methodology, M.M.; software, V.S.; formal analysis, V.S.; data curation, M.T.S., F.L. and V.P.; writing—original draft preparation and reviewing and editing, V.P., G.G., A.L., M.M. and I.C.; supervision, P.S. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Our Ethics Committee (Padua University Hospital) approved the retrospective data collection (Approval Number: n. AOP2552, Approval Date: 20 October 2023).
Informed Consent Statement
The patient gave his written informed consent for the publication of information and images.
Data Availability Statement
All data used to generate this manuscript are herein reported.
Acknowledgments
We thank our patient for giving his consent to share his clinical history and images.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Facklam, R. What happened to the streptococci: Overview of taxonomic and nomenclature changes. Clin. Microbiol. Rev. 2002, 15, 613–630. [Google Scholar] [CrossRef]
- Doern, C.D.; Burnham, C.A. It’s not easy being green: The viridans group streptococci, with a focus on pediatric clinical manifestations. J. Clin. Microbiol. 2010, 48, 3829–3835. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tan, T.K.; Paterson, I.C.; Mutha, N.V.; Siow, C.C.; Tan, S.Y.; Old, L.A.; Jakubovics, N.S.; Choo, S.W. StreptoBase: An Oral Streptococcus mitis Group Genomic Resource and Analysis Platform. PLoS ONE 2016, 11, e0151908. [Google Scholar] [CrossRef] [PubMed]
- Angeletti, S.; Dicuonzo, G.; Avola, A.; Crea, F.; Dedej, E.; Vailati, F.; Farina, C.; De Florio, L. Viridans Group Streptococci clinical isolates: MALDI-TOF mass spectrometry versus gene sequence-based identification. PLoS ONE 2015, 10, e0120502. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Scholz, C.F.P.; Kilian, M. Re-evaluation of the taxonomy of the Mitis group of the genus Streptococcus based on whole genome phylogenetic analyses, and proposed reclassification of Streptococcus dentisani as Streptococcus oralis subsp. dentisani comb. nov., Streptococcus tigurinus as Streptococcus oralis subsp. tigurinus comb. nov., and Streptococcus oligofermentans as a later synonym of Streptococcus cristatus. Int. J. Syst. Evol. Microbiol. 2016, 66, 4803–4820. [Google Scholar]
- Woo, P.C.; Teng, J.L.; Lau, S.K.; Yuen, K.Y. Clinical, phenotypic, and genotypic evidence for Streptococcus sinensis as the common ancestor of anginosus and mitis groups of streptococci. Med. Hypotheses 2006, 66, 345–351. [Google Scholar] [CrossRef]
- Woo, P.C.; Tam, D.M.; Leung, K.W.; Lau, S.K.; Teng, J.L.; Wong, M.K.; Yuen, K.Y. Streptococcus sinensis sp. nov., a novel species isolated from a patient with infective endocarditis. J. Clin. Microbiol. 2002, 40, 805–810. [Google Scholar] [CrossRef]
- Woo, P.C.; Teng, J.L.L.; Leung, K.W.; Lau, S.K.P.; Tse, H.; Wong, B.H.L.; Yuen, K.Y. Streptococcus sinensis may react with Lancefield group F antiserum. J. Clin. Med. Microbiol. 2024, 53, 1083–1088. [Google Scholar] [CrossRef]
- Uckay, I.; Rohner, P.; Bolivar, I.; Ninet, B.; Djordjevic, M.; Nobre, V.; Garzoni, C.; Schrenzel, J. Streptococcus sinensis endocarditis outside Hong Kong. Emerg. Infect. Dis. 2007, 13, 1250–1252. [Google Scholar] [CrossRef]
- Faibis, F.; Mihaila, L.; Perna, S.; Lefort, J.F.; Demachy, M.C.; Le Fleche-Mateos, A.; Bouvet, A. Streptococcus sinensis: An emerging agent of infective endocarditis. J. Med. Microbiol. 2008, 57 Pt 4, 528–531. [Google Scholar] [CrossRef]
- Seta, V.; Teicher, E.; Fortineau, N.; Ladouceur, M.; Lambotte, O. Infective endocarditis caused by Streptococcus sinensis. Med. Mal. Infect. 2015, 45, 56–57. [Google Scholar] [CrossRef] [PubMed]
- San Francisco, A.; Tomlinson, J.S.; Walters, S.; Curtis, S.; James, R. Lesson of the month 2, When steroids stop working—Infective endocarditis, the great mimicker. Clin. Med. 2019, 19, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Goret, J.; Baudinet, T.; Camou, F.; Issa, N.; Gaillard, P.; Wirth, G.; Greib, C.; Barandon, L.; Megraud, F.; Bebear, C.; et al. Identification of Streptococcus sinensis from a patient with endocarditis using MALDI-TOF mass spectrometry, 16S rDNA- and sodA-based phylogeny. J. Microbiol. Immunol. Infect. 2019, 52, 507–509. [Google Scholar] [CrossRef]
- van Ommen, A.; Slavenburg, S.; Diepersloot, R.; de Vries Feyens, C.A. Fatal outcome of first case of Streptococcus sinensis in infective endocarditis in the Netherlands: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Zhan, Y.; Tang, R.; Wang, H.; Qin, T.; Lu, Z. Case report: Infective endocarditis caused by Streptococcus sinensis: The first case in mainland China and literature review. Front. Cardiovasc. Med. 2022, 9, 935725. [Google Scholar] [CrossRef]
- Pan, Y.; Qian, J.; Wang, G.; Zhao, H. Infective Endocarditis Caused by Streptococcus sinensis in a patient with Bioprosthetic Aortica Valve: A case report and literature review. Infect. Drug Res. 2024, 17, 2957–2964. [Google Scholar] [CrossRef]
- Fowler, V.G.; Durack, D.T.; Selton-Suty, C.; Athan, E.; Bayer, A.S.; Chamis, A.L.; Dahl, A.; Dibernardo, L.; Durante-Mangoni, E.; Duval, X.; et al. The 2023 Duke-ISCVID Criteria for Infective Endocarditis: Updating the Modified Duke Criteria. Clin. Infect. Dis. 2023, 77, 518–526. [Google Scholar] [CrossRef]
- Bennani, G.; Zahri, S.; Habbal, R. Time interval between first symptoms and diagnosis of infective endocarditis: Characteristics, microorganisms and impact on prognosis. Arch. Cardiovasc. Dis. 2024, 117, S81–S82. [Google Scholar] [CrossRef]
- Mylonakis, E.; Calderwood, S.B. Infective endocarditis in adults. N. Engl. J. Med. 2001, 345, 1318–1330. [Google Scholar] [CrossRef]
- Woo, P.C.; Teng, J.L.; Tsang, S.N.; Tse, C.W.; Lau, S.K.; Yuen, K.Y. The oral cavity as a natural reservoir for Streptococcus sinensis. Clin. Microbiol. Infect. 2008, 14, 1075–1079. [Google Scholar] [CrossRef]
- Singh, N.; Poggensee, L.; Huang, Y.; Evans, C.T.; Suda, K.J.; Bulman, Z.P. Antibiotic susceptibility patterns of viridans group streptococci isolates in the United States from 2010 to 2020. JAC Antimicrob. Resist. 2022, 4, dlac049. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Verma, S.; Bauer, R.; Kumari, M.; Dua, M.; Johri, A.K.; Yadav, V.; Spellerberg, B. Deciphering Streptococcal Biofilms. Microorganisms 2020, 8, 1835. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).