Integrating Rehabilitation Services into Routine Care of Rheumatoid Arthritis May Reduce the Inflammatory Response: A Hospital-Based Follow-Up Study in Taiwan
Abstract
1. Introduction
2. Materials and Methods
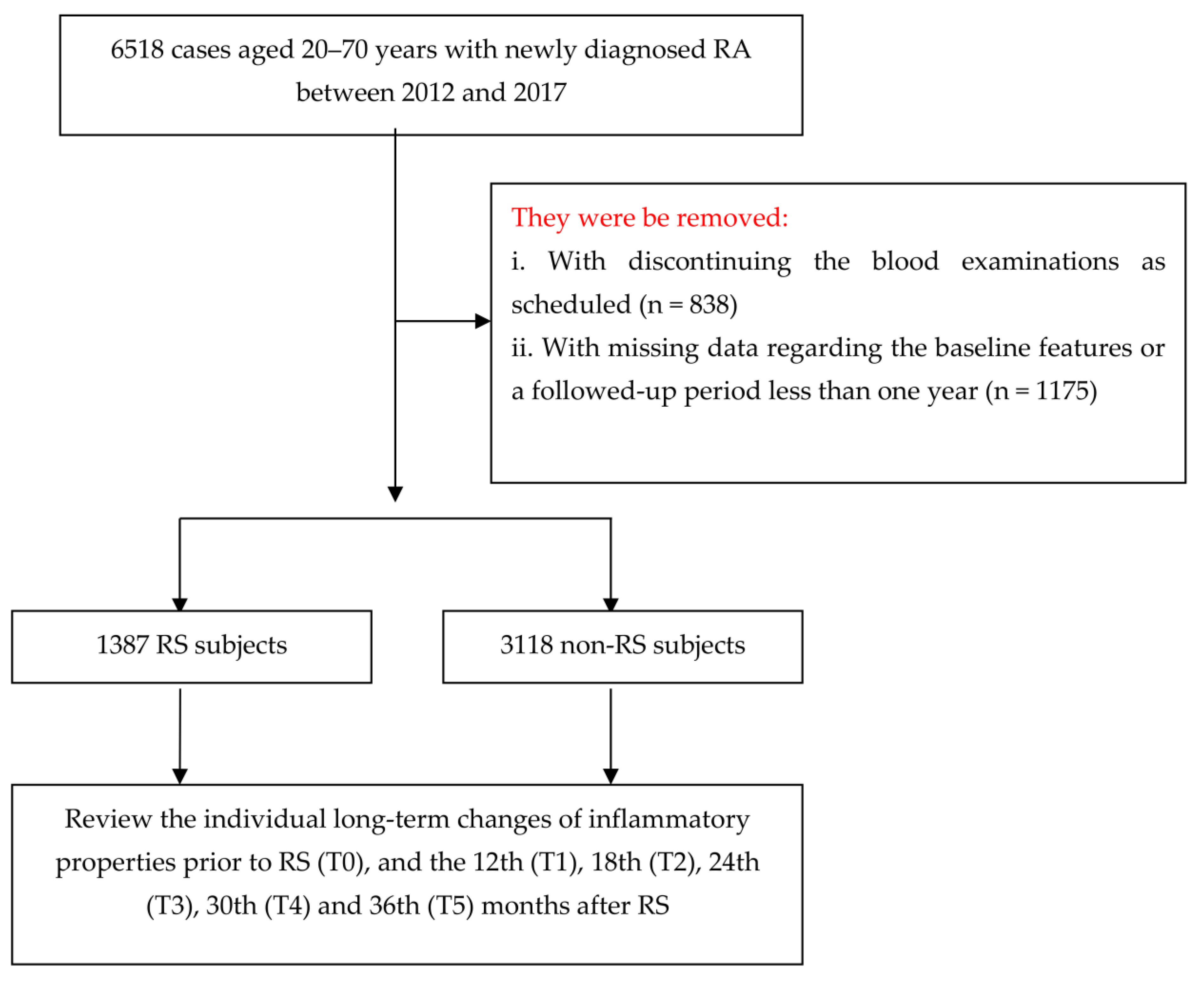
2.1. Research Design and Participants
2.2. Exposure of Rehabilitation Services Usage
2.3. Measurement of Primary Outcomes
2.4. Covariates
2.5. Statistical Modeling
3. Results
3.1. Baseline Characteristics for All Participants
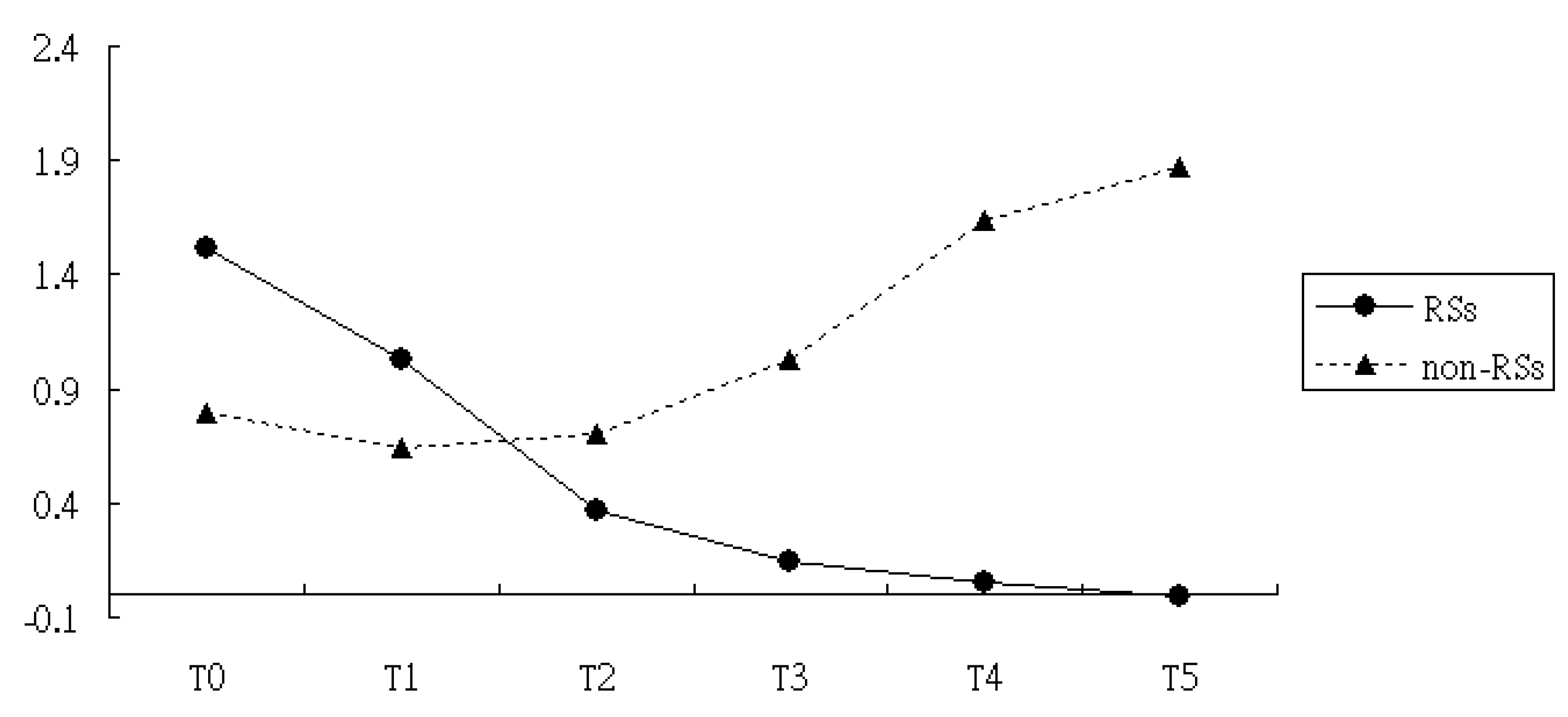
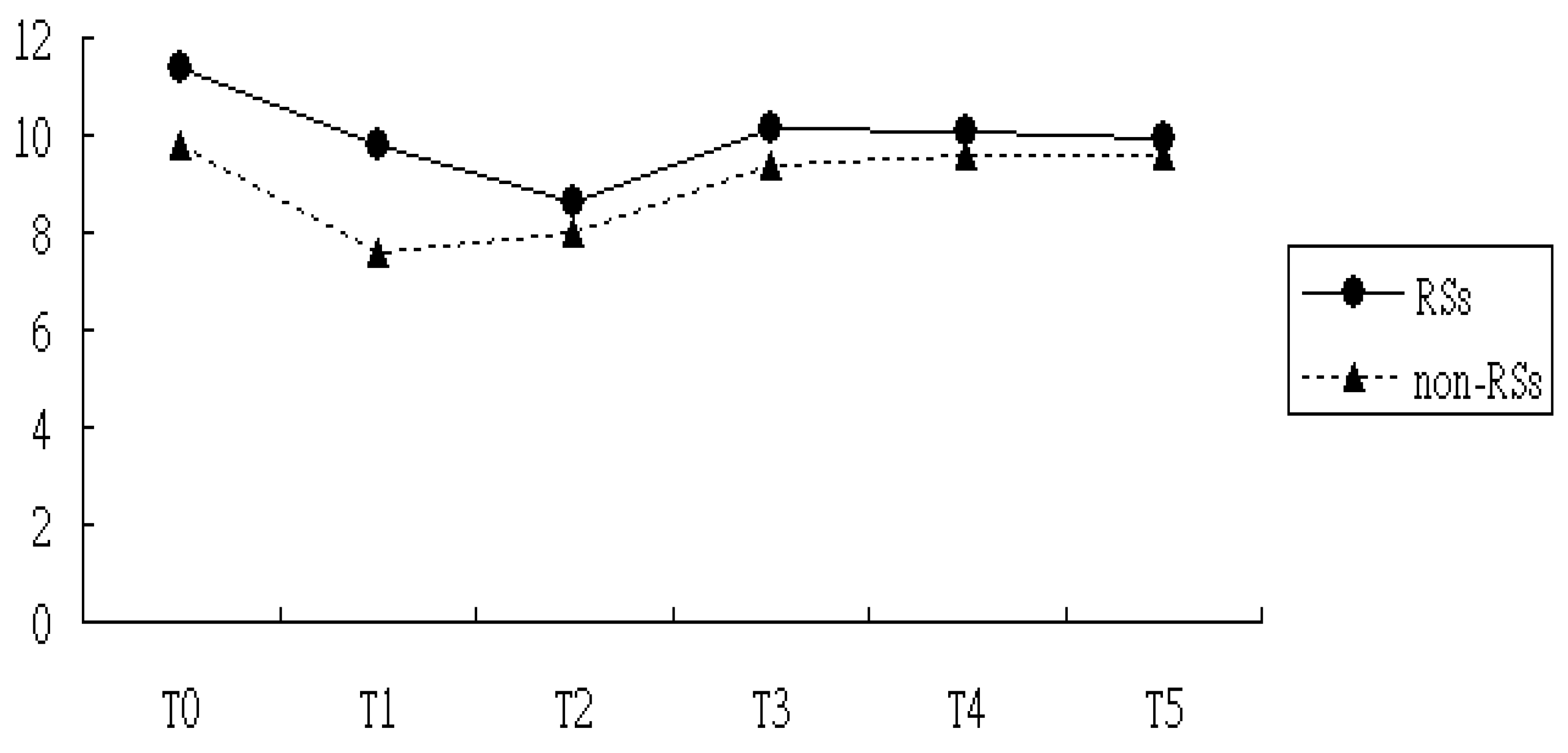
3.2. Comparisons of Levels of CRP and ESR in the RSs Groups Versus Non-RSs Groups
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kirkeskov, L.; Bray, K. Employment of patients with rheumatoid arthritis—A systematic review and meta-analysis. BMC Rheumatol. 2023, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Sokka, T. Work disability in early rheumatoid arthritis Clin. Exp. Rheumatol. 2003, 21, S71–S74. [Google Scholar]
- Chen, C.I.; Wang, L.; Wei, W.; Yuce, H.; Phillips, K. Burden of rheumatoid arthritis among US medicare population: Co-morbidities, health-care resource utilization and costs. Rheumatol. Adv. Pract. 2018, 2, I1–I9. [Google Scholar] [CrossRef] [PubMed]
- Burska, A.; Boissinot, M.; Ponchel, F. Cytokines as biomarkers in rheumatoid arthritis. Mediat. Inflamm. 2014, 2014, 545493. [Google Scholar] [CrossRef]
- Cook, M.J.; Bellou, E.; Bowes, J.; Sergeant, J.C.; O’Neill, T.W.; Barton, A.; Verstappen, S.M.M. The prevalence of co-morbidities and their impact on physical activity in people with inflammatory rheumatic diseases compared with the general population: Results from the UK Biobank. Rheumatology 2018, 57, 2172–2182. [Google Scholar] [CrossRef]
- Pope, J.E.; Choy, E.H. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin. Arthritis Rheum. 2021, 51, 219–229. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, L.G.; McEleney, K.G.; Cochrane, C.; Tan, K.B.; Shukla, P.; Gardiner, P.V.; Small, D.; Zhang, S.D.; Gibson, D.S. Assessment of a dried blood spot C-reactive protein method to identify disease flares in rheumatoid arthritis patients. Sci. Rep. 2020, 10, 21089. [Google Scholar] [CrossRef]
- Goodson, N.J.; Symmons, D.P.; Scott, D.G.; Bunn, D.; Lunt, M.; Silman, A.J. Baseline levels of c-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year follow-up study of a primary care–based inception cohort. Arthritis Rheum. 2005, 52, 2293–2299. [Google Scholar] [CrossRef]
- Al-Qubaeissy, K.Y.; Fatoye, F.A.; Goodwin, P.C.; Yohannes, A.M. The effectiveness of hydrotherapy in the management of rheumatoid arthritis: A systematic review. Musculoskelet. Care 2013, 11, 3–18. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A.; Fatima, N.; Shahzad, S. Role of hydrotherapy in the amelioration of oxidant-antioxidant status in rheumatoid arthritis patients. Int. J. Rheum. Dis. 2018, 21, 1822–1830. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, S.A.; López-Loeza, S.M.; Acosta-Jiménez, S.; Cuevas-Martínez, R.; Pacheco-Silva, C.; Chaparro-Barrera, E.; Pacheco-Tena, C. Low-intensity physical exercise decreases inflammation and joint damage in the preclinical phase of a rheumatoid arthritis murine model. Biomolecules 2023, 13, 488. [Google Scholar] [CrossRef] [PubMed]
- Kohn, L.T.; Corrigan, J.M.; Donaldson, M.S. To Err is Human: Building a Safer Health System; National Academy of Sciences: Washington, DC, USA, 2000. [Google Scholar]
- Lu, M.C.; Livneh, H.; Yen, C.T.; Huang, H.L.; Lin, M.C.; Yen, S.W.; Lai, N.S.; Tsai, T.Y. Association of use of rehabilitation services with development of dementia among patients with rheumatoid arthritis: Analysis of domestic data in Taiwan. Front. Med. 2020, 7, 446. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef]
- Overall, J.E.; Tonidandel, S. Robustness of Generalized Estimating Equation (GEE) tests of significance against misspecification of the error structure model. Biom. J. 2004, 46, 203–213. [Google Scholar] [CrossRef]
- Lu, M.-C.; Guo, H.-R.; Livneh, H.; Lin, M.-C.; Lai, N.-S.; Tsai, T.-Y. The effectiveness of nurse-led case management for patients with rheumatoid arthritis in Taiwan. Int. J. Clin. Pract. 2020, 74, e13443. [Google Scholar] [CrossRef]
- Letnes, J.M.; Nes, B.M.; Wisløff, U. Age-related decline in peak oxygen uptake: Cross-sectional vs. longitudinal findings. A review. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 16, 200171. [Google Scholar] [CrossRef]
- Kelley, G.A.; Kelley, K.S.; Hootman, J.M. Effects of exercise on depression in adults with arthritis: A systematic review with meta-analysis of randomized controlled trials. Arthritis Res. Ther. 2015, 17, 21. [Google Scholar] [CrossRef]
- Qu, Z.; Zhang, B.; Kong, L.; Gong, Y.; Feng, M.; Gao, X.; Wang, D.; Yan, L. Receptor activator of nuclear factor-IoB ligand-mediated osteoclastogenesis signaling pathway and related therapeutic natural compounds. Front. Pharmacol. 2022, 13, 1043975. [Google Scholar] [CrossRef] [PubMed]
- Litao, M.K.; Kamat, D. Erythrocyte sedimentation rate and C-reactive protein: How best to use them in clinical practice. Pediatr. Ann. 2014, 43, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. Chapter 11—Structural and Functional Diversity of Fibrinogen-Related Domains. The Evolution of the Immune System; Malagoli, D., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 275–294. [Google Scholar]
- Harrison, M. Erythrocyte sedimentation rate and C-reactive protein. Aust. Prescr. 2015, 38, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Costenbader, K.H.; Chibnik, L.B.; Schur, P.H. Discordance between erythrocyte sedimentation rate and C-reactive protein measurements: Clinical significance. Clin. Exp. Rheumatol. 2007, 25, 746–749. [Google Scholar]
- Lapiæ, I.; Padoan, A.; Bozzato, D.; Plebani, M. Erythrocyte sedimentation rate and c-reactive protein in acute inflammation. Am. J. Clin. Pathol. 2020, 153, 14–29. [Google Scholar] [CrossRef]
| Variables | All Enrollees (n = 4505) | RSs Group (n = 1387) | Non-RSs Group (n = 3118) | p | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Demographic data | |||||||
| Sex | <0.01 | ||||||
| Female | 3443 | 76.4 | 1109 | 80.0 | 2334 | 74.9 | |
| Male | 1062 | 23.6 | 278 | 20.0 | 784 | 25.1 | |
| Cigarette smoking | 0.001 | ||||||
| Yes | 63 | 1.4 | 31 | 2.2 | 32 | 1.0 | |
| NO | 4442 | 98.6 | 1356 | 97.8 | 3086 | 99.0 | |
| Age (mean ± SD) | 54.2 ± 13.1 | 56.2 ± 12.7 | 53.25 ± 13.1 | <0.01 | |||
| Height | 156.6 ± 7.4 | 156.9 ± 7.6 | 156.3 ± 7.3 | 0.65 | |||
| Weight | 59.9 ± 10.9 | 62.7 ± 11.3 | 57.6 ± 10.0 | 0.01 | |||
| Clinical characteristics | |||||||
| Medication use | 0.06 | ||||||
| Yes | 3090 | 68.6 | 923 | 66.5 | 2167 | 69.5 | |
| No | 1415 | 31.4 | 464 | 33.5 | 951 | 30.5 | |
| CCI (mean ± SD) | 2.56 ± 1.32 | 3.17 ± 1.34 | 2.29 ± 1.30 | <0.01 | |||
| Baseline CRP (mean ± SD) | 1.14 ± 2.91 | 1.63 ± 3.38 | 0.92 ± 2.13 | <0.01 | |||
| Baseline ESP (mean ± SD) | 20.37 ± 11.42 | 21.46 ± 14.10 | 19.86 ± 10.02 | 0.03 | |||
| Variables | CRP | ESR | ||
|---|---|---|---|---|
| Regression Coefficient + | p | Regression Coefficient + | p | |
| Intercept | 0.79 | <0.01 | 9.80 | <0.01 |
| RSs vs. non-RSs | 0.73 | <0.01 | 1.56 | 0.04 |
| T1 vs. T0 | −0.15 | 0.07 | −4.0 | <0.01 |
| T2 vs. T0 | −0.09 | 0.10 | −3.83 | 0.02 |
| T3 vs. T0 | 0.24 | 0.22 | −1.39 | 0.14 |
| T4 vs. T0 | 0.85 | 0.09 | −0.89 | 0.49 |
| T5 vs. T0 | 1.08 | 0.06 | −0.41 | 0.16 |
| Interaction of T1×Group | −0.22 | 0.04 | 1.81 | 0.22 |
| Interaction of T2×Group | −1.06 | 0.01 | 1.09 | 0.18 |
| Interaction of T3×Group | −2.13 | <0.01 | 0.27 | 0.14 |
| Interaction of T4×Group | −3.02 | <0.01 | −0.11 | 0.08 |
| Interaction of T5×Group | −2.72 | <0.01 | −0.20 | 0.07 |
| RSs Usage Pattern | CRP | ESR | ||
|---|---|---|---|---|
| Regression Coefficient | p | Regression Coefficient | p | |
| Non-RSs group | 1 | 1 | ||
| RSs group | −0.54 | <0.01 | 1.33 | 0.52 |
| Low intensity | −0.37 | 0.003 | 1.74 | 0.29 |
| High intensity | −0.69 | <0.01 | 1.26 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-J.; Chen, W.-J.; Livneh, H.; Huang, H.-L.; Lu, M.-C.; Tsai, T.-Y. Integrating Rehabilitation Services into Routine Care of Rheumatoid Arthritis May Reduce the Inflammatory Response: A Hospital-Based Follow-Up Study in Taiwan. Medicina 2024, 60, 1938. https://doi.org/10.3390/medicina60121938
Huang H-J, Chen W-J, Livneh H, Huang H-L, Lu M-C, Tsai T-Y. Integrating Rehabilitation Services into Routine Care of Rheumatoid Arthritis May Reduce the Inflammatory Response: A Hospital-Based Follow-Up Study in Taiwan. Medicina. 2024; 60(12):1938. https://doi.org/10.3390/medicina60121938
Chicago/Turabian StyleHuang, Hui-Ju, Wei-Jen Chen, Hanoch Livneh, Hua-Lung Huang, Ming-Chi Lu, and Tzung-Yi Tsai. 2024. "Integrating Rehabilitation Services into Routine Care of Rheumatoid Arthritis May Reduce the Inflammatory Response: A Hospital-Based Follow-Up Study in Taiwan" Medicina 60, no. 12: 1938. https://doi.org/10.3390/medicina60121938
APA StyleHuang, H.-J., Chen, W.-J., Livneh, H., Huang, H.-L., Lu, M.-C., & Tsai, T.-Y. (2024). Integrating Rehabilitation Services into Routine Care of Rheumatoid Arthritis May Reduce the Inflammatory Response: A Hospital-Based Follow-Up Study in Taiwan. Medicina, 60(12), 1938. https://doi.org/10.3390/medicina60121938







