C-Reactive Protein-to-Serum Chloride Ratio: A Novel Marker of All-Cause Mortality in Maintenance Haemodialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Haemodialysis Technique
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Haemodialysis Adequacy
3.3. Bioimpedance-Related Parameters
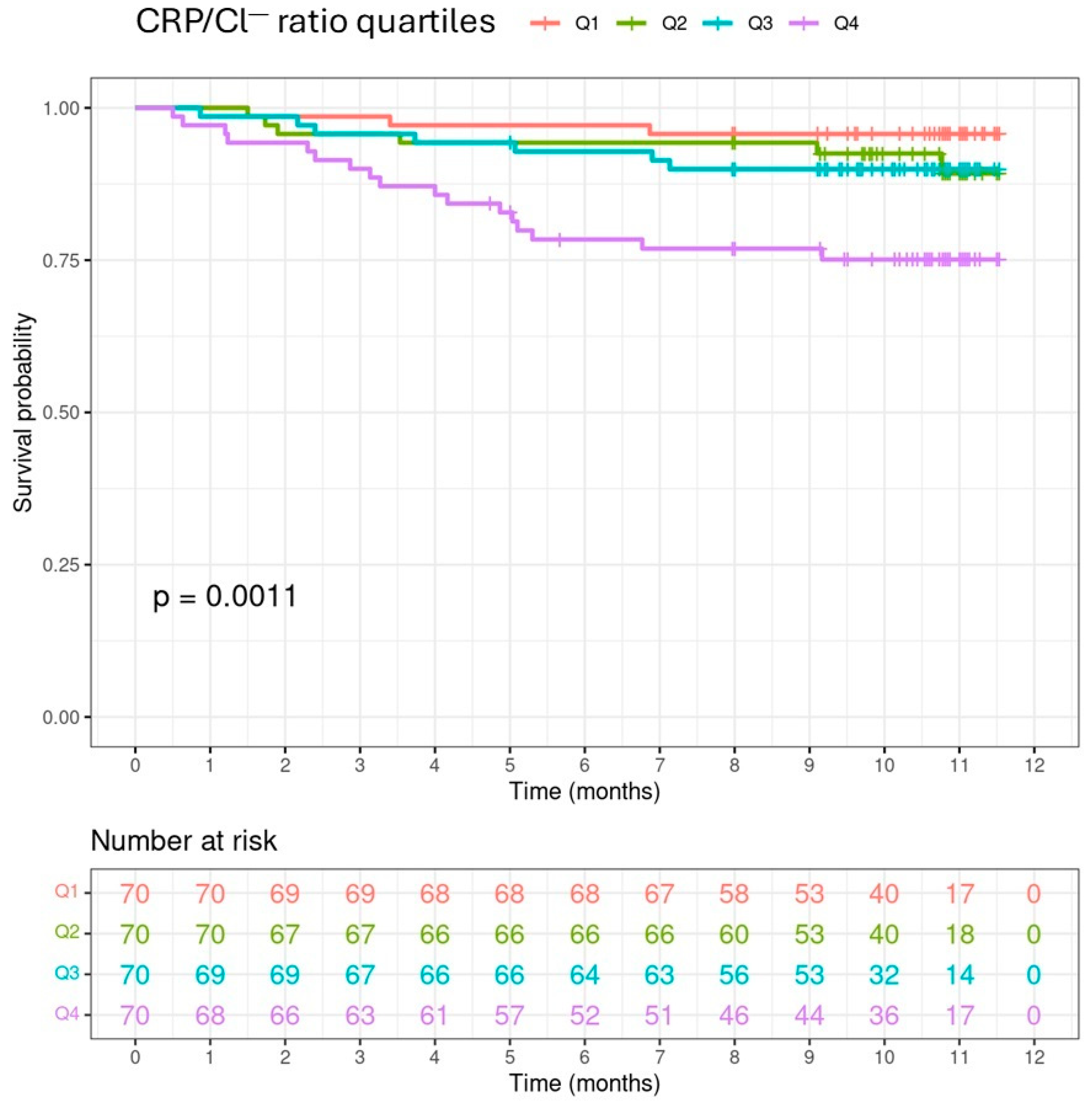
3.4. CRP/Cl− Ratio and Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubota, K.; Sakaguchi, Y.; Hamano, T.; Oka, T.; Yamaguchi, S.; Shimada, K.; Matsumoto, A.; Hashimoto, N.; Mori, D.; Matsui, I.; et al. Prognostic value of hypochloremia versus hyponatremia among patients with chronic kidney disease-a retrospective cohort study. Nephrol. Dial. Transplant. 2020, 35, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Mandai, S.; Kanda, E.; Iimori, S.; Naito, S.; Noda, Y.; Kikuchi, H.; Akazawa, M.; Oi, K.; Toda, T.; Sohara, E.; et al. Association of serum chloride level with mortality and cardiovascular events in chronic kidney disease: The CKD-ROUTE study. Clin. Exp. Nephrol. 2017, 21, 104–111. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, X.; Zhan, X.; Feng, X.; Wang, N.; Peng, F.; Wen, Y.; Wu, X. Serum Chloride and Mortality in patients on continuous ambulatory peritoneal dialysis: A multi-center retrospective study. EClinicalMedicine 2021, 41, 101133. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Xu, Y.; Wu, K.; Lu, X.; Qiu, Y.; Yang, X.; Liu, Q.; Mao, H. Association between serum chloride levels with mortality in incident peritoneal dialysis patients. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 624–631. [Google Scholar] [CrossRef]
- Valga, F.; Monzon, T.; Vega-Diaz, N.; Santana, A.; Moscol, G.; Ruiz-Santana, S.; Rodriguez-Perez, J.C. Serum chloride as a marker of cardiovascular and all-cause mortality in chronic hemodialysis patients: 5-Year follow-up study. Nefrologia 2023, 43 (Suppl. S2), 47–56. [Google Scholar] [CrossRef]
- McCallum, L.; Jeemon, P.; Hastie, C.E.; Patel, R.K.; Williamson, C.; Redzuan, A.M.; Dawson, J.; Sloan, W.; Muir, S.; Morrison, D.; et al. Serum chloride is an independent predictor of mortality in hypertensive patients. Hypertension 2013, 62, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Kazory, A.; Ronco, C. Emergence of Chloride as an Overlooked Cardiorenal Connector in Heart Failure. Blood Purif. 2020, 49, 219–221. [Google Scholar] [CrossRef]
- Grodin, J.L.; Simon, J.; Hachamovitch, R.; Wu, Y.; Jackson, G.; Halkar, M.; Starling, R.C.; Testani, J.M.; Tang, W.H. Prognostic Role of Serum Chloride Levels in Acute Decompensated Heart Failure. J. Am. Coll. Cardiol. 2015, 66, 659–666. [Google Scholar] [CrossRef]
- Pfortmueller, C.A.; Uehlinger, D.; von Haehling, S.; Schefold, J.C. Serum chloride levels in critical illness-the hidden story. Intensive Care Med. Exp. 2018, 6, 10. [Google Scholar] [CrossRef]
- Kazory, A.; Costanzo, M.R. The dynamic relationship between serum chloride and cardiorenal syndrome. Rev. Cardiovasc. Med. 2020, 21, 25–29. [Google Scholar] [CrossRef]
- Rivera, F.B.; Alfonso, P.; Golbin, J.M.; Lo, K.; Lerma, E.; Volgman, A.S.; Kazory, A. The Role of Serum Chloride in Acute and Chronic Heart Failure: A Narrative Review. Cardiorenal Med. 2021, 11, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, J.S.; Rao, V.; Ter Maaten, J.M.; Laur, O.; Brisco, M.A.; Perry Wilson, F.; Grodin, J.L.; Assefa, M.; Samuel Broughton, J.; Planavsky, N.J.; et al. Hypochloremia and Diuretic Resistance in Heart Failure: Mechanistic Insights. Circ. Heart Fail. 2016, 9, 3180. [Google Scholar] [CrossRef] [PubMed]
- Valga, F.; Monzón, T.; Vega-Diaz, N.; Rodriguez-Perez, J.C.; Ruiz-Santana, S. Inflammation and hemodialysis adequacy: Are C-reactive protein levels influenced by the dose of dialysis? Nefrologia 2022, 42, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhao, S. Risk factors for mortality in patients undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 238, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Furusho, T.; Sohara, E.; Mandai, S.; Kikuchi, H.; Takahashi, N.; Fujimaru, T.; Hashimoto, H.; Arai, Y.; Ando, F.; Zeniya, M.; et al. Renal TNFα activates the WNK phosphorylation cascade and contributes to salt-sensitive hypertension in chronic kidney disease. Kidney Int. 2020, 97, 713–727. [Google Scholar] [CrossRef]
- López-Gómez, J. Bioimpedancia. Nefrología al Día. 2012. Available online: https://www.nefrologiaaldia.org/98 (accessed on 1 September 2024).
- Eyre, S.; Stenberg, J.; Wallengren, O.; Keane, D.; Avesani, C.M.; Bosaeus, I.; Clyne, N.; Heimbürger, O.; Indurain, A.; Johansson, A.C.; et al. Bioimpedance analysis in patients with chronic kidney disease. J. Ren. Care 2023, 49, 147–157. [Google Scholar] [CrossRef]
- Keber, G.; Hojs, R.; Dvoršak, B.; Bevc, S.; Vodošek Hojs, N.; Petreski, T.; Ekart, R. Assessment of volume status with bioimpendance prior to hemodialysis and its importance for predicting survival in hemodialysis patients. Clin. Nephrol. 2021, 96, 68–73. [Google Scholar] [CrossRef]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef]
- Rattanasompattikul, M.; Feroze, U.; Molnar, M.Z.; Dukkipati, R.; Kovesdy, C.P.; Nissenson, A.R.; Norris, K.C.; Kopple, J.D.; Kalantar-Zadeh, K. Charlson comorbidity score is a strong predictor of mortality in hemodialysis patients. Int. Urol. Nephrol. 2012, 44, 1813–1823. [Google Scholar] [CrossRef]
- Pérez-García, R.; García Maset, R.; Gonzalez Parra, E.; Solozábal Campos, C.; Ramírez Chamond, R.; Martín-Rabadán, P.; Sobrino Pérez, P.E.; Gallego Pereira, O.; Dominguez, J.; de la Cueva Matute, E.; et al. Guideline for dialysate quality of Spanish Society of Nephrology (second edition, 2015). Nefrologia 2016, 36, e1–e52. [Google Scholar] [CrossRef][Green Version]
- Mickey, R.M.; Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; He, L.; Du, Y.; Liu, J.; Peng, L.; Yang, M.; Sun, S.; Liu, J.; Li, J.; Cao, J.; et al. Macrophage WNK1 senses intracellular hypo-chlorine to regulate vulnerability to sepsis attack during hypochloremia. Int. Immunopharmacol. 2024, 139, 112721. [Google Scholar] [CrossRef] [PubMed]
- Romejko, K.; Szamotulska, K.; Rymarz, A.; Tomasz, R.; Niemczyk, S. The association of appendicular skeletal muscle mass with anthropometric, body composition, nutritional, inflammatory, and metabolic variables in non-dialysis-dependent chronic kidney disease men. Front. Med. 2024, 11, 1380026. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Zarowitz, B.J.; Hyzy, R.; Eichenhorn, M.; Peterson, E.L.; Popovich, J., Jr. Bioelectrical impedance assessment of nutritional status in critically ill patients. Am. J. Clin. Nutr. 1993, 57, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Pérez-García, R.; Jaldo, M.; Alcázar, R.; de Sequera, P.; Albalate, M.; Puerta, M.; Ortega, M.; Ruiz, M.C.; Corchete, E. El Kt/V alto, a diferencia del Kt, se asocia a mayor mortalidad: Importancia de la V baja. Nefrología 2019, 39, 58–66. [Google Scholar] [CrossRef]
- Caravaca, F.; Martínez del Viejo, C.; Villa, J.; Martínez Gallardo, R.; Ferreira, F. Hydration status assessment by multi-frequency bioimpedance in patients with advanced chronic kidney disease. Nefrologia 2011, 31, 537–544. [Google Scholar] [CrossRef]
| Characteristics | Total N = 281 | Q1 N = 70 | Q2 N = 70 | Q3 N = 71 | Q4 N = 70 | p-Value |
|---|---|---|---|---|---|---|
| Female, n (%) | 89 (31.8) | 22 (31.4) | 22 (31.4) | 27 (38.6) | 18 (25.7) | 0.4517 |
| Age (years) | 70 (59–77) | 70 (60–76) | 73 (62–78) | 68 (58–76) | 70 (58–77) | 0.4616 |
| Diabetic nephropathy, n (%) | 81 (28.9) | 24 (34.3) | 17 (24.3) | 18 (25.7) | 22 (31.4) | 0.5363 |
| Death, n (%) | 33 (11.8) | 3 (4.3) | 6 (8.6) | 7 (10.0) | 17 (24.3) | 0.0033 |
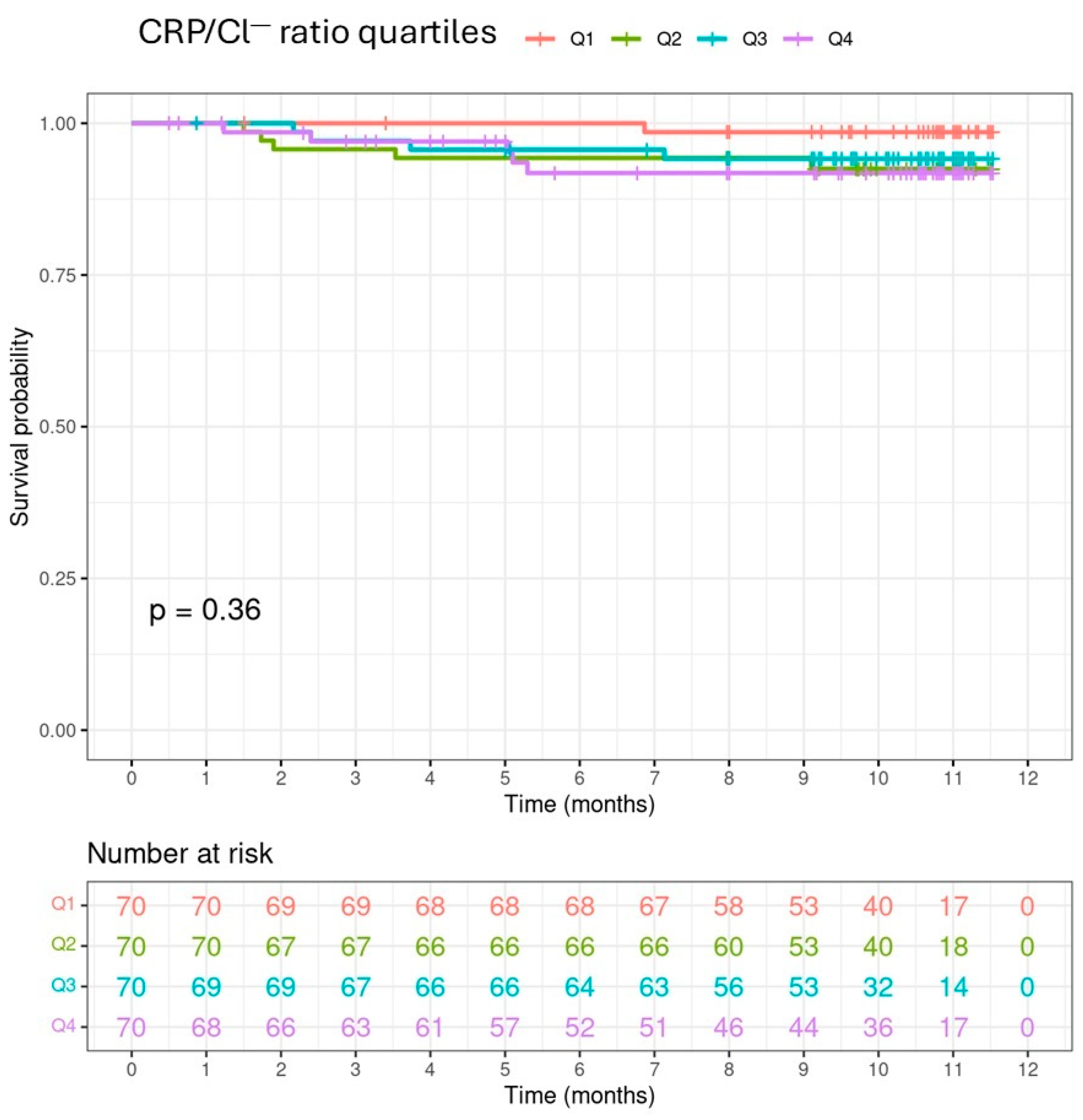
| Cause of death (cardiovascular), n (%) | 5 (45.5) | 1 (33.3) | 5 (83.3) | 4 (57.1) | 5 (29.4) | 0.1668 |
| Technique (OL-HDF), n (%) | 119 (42.5) | 33 (47.1) | 28 (40.0) | 31 (44.3) | 27 (38.6) | 0.7351 |
| Vascular access (AVF), n (%) | 65 (58.9) | 39 (55.7) | 38 (54.3) | 45 (64.3) | 43 (61.4) | 0.5965 |
| MCI | 9.00 (6.75–11.00) | 9.00 (6.00–11.00) | 9.00 (8.00–11.00) | 9.00 (5.50–11.00) | 9.00 (6.00–12.00) | 0.2075 |
| Dialysis vintage (days) | 1135 (652–1863) | 846 (578–1370) | 1156 (568–1901) | 1424 (861–2111) | 1282 (702–2246) | 0.0114 |
| Diuretics (yes), n (%) | 124 (44.3) | 33 (47.1) | 27 (38.6) | 32 (45.7) | 32 (45.7) | 0.7429 |
| BMI (kg/m2) | 25.90 (22.75–30.13) | 25.90 (22.35–29.30) | 25.75 (22.65–30.10) | 26.15 (22.68–30.73) | 26.00 (23.50–29.45) | 0.9035 |
| BSA (m2) | 1.85 (1.68–2.00) | 1.83 (1.66–1.99) | 1.79 (1.67–1.93) | 1.87 (1.72–2.03) | 1.87 (1.72–2.02) | 0.2874 |
| Sodium (mEq/L) | 138 (136–140) | 139 (137–140) | 138 (137–141) | 138 (136–140) | 138 (136–139) | 0.0857 |
| Potassium (mEq/L), mean ± SD | 5.01 ± 0.87 | 5.24 ± 0.85 | 4.89 ± 0.90 | 4.92 ± 0.81 | 5.00 ± 0.89 | 0.1353 |
| Anion Gap (mmol/L), mean ± SD | 22.84 ± 3.50 | 22.47 ± 3.48 | 22.73 ± 4.03 | 23.22 ± 2.85 | 22.95 ± 3.60 | 0.3040 |
| HCO3− (mEq/L) | 20.35 (18.80–21.80) | 20.45 (19.30–22.00) | 20.70 (18.83–21.98) | 20.10 (18.42–21.20) | 20.35 (18.80–21.58) | 0.5882 |
| Albumin (g/dL) | 3.90 (3.60–4.10) | 4.00 (3.82–4.20) | 3.90 (3.70–4.20) | 3.80 (3.62–4.00) | 3.70 (3.40–3.98) | <0.0001 |
| Characteristics | Total N = 281 | NO N = 247 | YES N = 34 | p-Value |
|---|---|---|---|---|
| Female, n (%) | 89 (31.7) | 77 (31.2) | 12 (35.3) | 0.6947 |
| Age (years) | 70 (59–77) | 70 (58–76) | 77 (71–81) | 0.0006 |
| MCI | 9(7–11) | 9(6–11) | 11(9–12) | 0.0004 |
| Diabetic nephropathy, n (%) | 82 (29.2) | 70 (28.3) | 12 (35.3) | 0.4226 |
| Technique (OL-HDF), n (%) | 120 (42.7) | 113 (45.7) | 7 (20.6) | 0.0055 |
| Vascular access (AVF), n (%) | 166 (59.1) | 148 (59.9) | 18 (52.9) | 0.4613 |
| Dialysis vintage (days) | 1135 (652–1864) | 1136 (652–1869) | 1088 (669–1799) | 0.5204 |
| Diuretics (yes), n (%) | 124 (44.1) | 107 (43.3) | 17 (50.0) | 0.4679 |
| BMI (kg/m2) | 26(22.8–30.2) | 26.3 (22.9–30.6) | 24(21.65–26.95) | 0.011 |
| CRP/Cl− ratio | 0.04 (0.02–0.12) | 0.04 (0.02–0.10) | 0.12 (0.04–0.19) | 0.0005 |
| BSA (m2) | 1.85 (1.68–2) | 1.86 (1.72–2.02) | 1.75 (1.59–1.87) | 0.0036 |
| IDWG (L) | 2.10 (1.37–2.8) | 2.20 (1.5–2.9) | 1.65 (0.72–2.32) | 0.0029 |
| Hypochloremia, n (%) | 72(25.6) | 60 (24.3) | 12 (35.3) | 0.2083 |
| High CRP, n (%) | 139(49.5) | 114 (46.2) | 25 (73.5) | 0.0032 |
| Potassium (mEq/L), mean ± SD | 5.01 ± 0.87 | 5.03 ± 0.88 | 4.84 ± 0.73 | 0.1564 |
| Anion gap (mmol/L), mean ± SD | 22.84 ± 3.50 | 22.91 ± 3.47 | 22.36 ± 3.70 | 0.4240 |
| Sodium (mEq/L) | 138 (136–140) | 138 (137–140) | 138 (135–139) | 0.4770 |
| HCO3− (mEq/L) | 20.40 (18.80–21.80) | 20.40 (18.80–21.75) | 20.30 (19.40–21.90) | 0.5705 |
| Albumin (g/dL) | 3.90 (3.60–4.10) | 3.90 (3.70–4.10) | 3.70 (3.32–3.80) | <0.0001 |
| Characteristics | Total N = 281 | Q1 N = 70 | Q2 N = 70 | Q3 N = 71 | Q4 N = 70 | p-Value |
|---|---|---|---|---|---|---|
| Kt (L) | 56 (52; 62) | 54 (50; 60) | 56 (51; 61) | 56 (52; 62) | 58 (54; 64) | 0.0405 |
| KtBSA (L), mean ± SD | 50.55 ± 4.09 | 50.18 ± 4.13 | 49.99 ± 3.93 | 50.96 ± 3.65 | 51.09 ± 4.57 | 0.0912 |
| ΔKt (L), mean ± SD | 6.04 ± 7.85 | 4.77 ± 8.38 | 5.94 ± 8.37 | 5.78 ± 6.78 | 7.66 ± 7.66 | 0.0424 |
| Ultrafiltration rate (mL/kg/h) | 8.20 (6.06–10.45) | 8.57 (5.88–11.06) | 8.51 (6.15–10.74) | 7.97 (6.40–9.85) | 7.76 (5.41–10.19) | 0.5375 |
| Negative fluid balance per session (L) | 2.30 (1.50–2.90) | 2.30 (1.23–2.80) | 2.20 (1.60–2.90) | 2.25 (1.50–2.90) | 2.35 (1.33–2.98) | 0.9866 |
| Kt/V (BIA) | 1.44 (1.25–1.64) | 1.40 (1.19–1.57) | 1.48 (1.28–1.72) | 1.44 (1.25–1.60) | 1.47 (1.28–1.66) | 0.2374 |
| Kt/V (W) | 1.49 (1.31–1.69) | 1.46 (1.29–1.65) | 1.53 (1.30–1.73) | 1.48 (1.32–1.67) | 1.48 (1.34–1.69) | 0.7661 |
| Kt/V (HW) | 1.44 (1.26–1.62) | 1.43 (1.24–1.62) | 1.47 (1.24–1.66) | 1.42 (1.28–1.57) | 1.45 (1.29–1.64) | 0.8613 |
| IDWG (L) | 2.10 (1.35–2.80) | 2.20 (1.30–3.00) | 2.00 (1.50–2.60) | 2.20 (1.33–3.00) | 2.10 (1.30–2.70) | 0.7103 |
| Characteristics | Total N = 281 | NO N = 247 | YES N = 34 | p-Value |
|---|---|---|---|---|
| PhA (°) | 4.80 (4.20–5.50) | 4.90 (4.30–5.60) | 4.15 (3.70–5.07) | 0.0014 |
| Xc (Ω) | 44 (36–54) | 45 (37–54) | 38 (33–53) | 0.1398 |
| Rz (Ω), mean ± SD | 535.15 ± 100.20 | 532.33 ± 98.51 | 555.66 ± 111.14 | 0.2517 |
| Nae:Ke | 1.13 (1.00–1.39) | 1.11 (0.99–1.36) | 1.32 (1.05–1.67) | 0.0043 |
| FFM (kg) | 52 (45; 59) | 52 (46; 59) | 46 (42; 55) | 0.0154 |
| ECW (L) | 20.20 (17.60–22.70) | 20.30 (17.60–22.75) | 19.55 (17.45–22.55) | 0.5991 |
| ICW (L) | 18.80 (15.40–22.20) | 19.10 (15.80–22.40) | 16.60 (13.07–19.43) | 0.0027 |
| TBW (L) | 39 (34–44) | 39 (35–44) | 35 (31–42) | 0.0223 |
| TBW (W) (L) | 38 (33–43) | 38 (34–43) | 34 (30–39) | 0.0045 |
| TBW (HW) (L) | 40 (34–44) | 40 (35–44) | 38 (29–42) | 0.0215 |
| ECW/ICW | 1.08 (0.93–1.27) | 1.05 (0.91–1.23) | 1.27 (1.02–1.46) | 0.0016 |
| BCM (kg) | 24.60 (20.10–29.40) | 24.90 (20.60–29.95) | 21.90 (16.97–25.52) | 0.0014 |
| FM (kg) | 21.40 (15.00–28.50) | 22.40 (15.20–29.55) | 17.55 (12.25–25.75) | 0.0222 |
| MM (kg) | 31 (26–36) | 32 (26–37) | 27.75 (21.88–32.30) | 0.0021 |
| BMR (kcal) | 1462 (1334–1604) | 1471 (1348–1618) | 1386 (1241–1490) | 0.0014 |
| SMM (kg) | 24.50 (20.20–28.40) | 25.10 (20.65–28.70) | 22.30 (18.62–27.17) | 0.0441 |
| Covariates | Beta (se) | HR | HR CI 95% | p-Value |
|---|---|---|---|---|
| CRP/Cl− | 0.033 (0.012) | 1.033 | [1.009, 1.058] | 0.0068 |
| MCI | 0.190 (0.051) | 1.209 | [1.095, 1.336] | 0.0066 |
| Albumin | −1.465 (0.374) | 0.231 | [0.111, 0.481] | <0.0001 |
| CRP | 0.033 (1.033) | 1.033 | [1.009, 1.058] | 0.0066 |
| Age | 0.046 (0.017) | 1.047 | [1.013, 1.083] | 0.0068 |
| FFM | −0.052 (0.020) | 0.949 | [0.912, 0.988] | 0.0101 |
| TBW | −0.055 (0.025) | 0.947 | [0.901, 0.995] | 0.0318 |
| ICW | −0.124 (0.041) | 0.883 | [0.815, 0.957] | 0.0023 |
| ECW/ICW | 1.389 (0.459) | 4.010 | [1.631, 9.858] | 0.0025 |
| BCM | −0.094 (0.029) | 0.910 | [0.860, 0.964] | 0.0013 |
| FM | −0.036 (0.017) | 0.964 | [0.934, 0.996] | 0.0283 |
| PhA | −0.559 (0.186) | 0.572 | [0.397, 0.822] | 0.0026 |
| Nae:Ke | 0.886 (0.360) | 2.424 | [1.197, 4.911] | 0.0139 |
| MM | −0.079 (0.025) | 0.924 | [0.879, 0.971] | 0.0018 |
| BMR | −0.003 (0.001) | 0.997 | [0.995, 0.999] | 0.0013 |
| BMI | −0.075 (0.034) | 0.928 | [0.869, 0.991] | 0.0264 |
| SMM | −0.054 (0.030) | 0.947 | [0.894, 1.004] | 0.0668 |
| Negative fluid balance per session | −0.342 (0.162) | 0.710 | [0.517, 0.976] | 0.0347 |
| Ultrafiltration rate | −0.001 (0.001) | 0.999 | [0.998, 1.000] | 0.0644 |
| BSA | −2.478 (0.833) | 0.084 | [0.016, 0.429] | 0.0029 |
| TBW (W) | −0.080 (0.028) | 0.923 | [0.875, 0.975] | 0.0039 |
| TBW (HW) | −0.066 (0.026) | 0.936 | [0.890, 0.985] | 0.0113 |
| KtBSA | −0.133 (0.044) | 0.876 | [0.803, 0.955] | 0.0028 |
| ΔKt | 0.035 (0.021) | 1.036 | [0.994, 1.080] | 0.0964 |
| Kt/V (BIA) | 1.405 (0.568) | 4.076 | [1.339, 12.409] | 0.0134 |
| Kt/V (W) | 1.668 (0.538) | 5.299 | [1.846, 15.213] | 0.0019 |
| Kt/V (WH) | 1.502 (0.556) | 4.492 | [1.509, 13.367] | 0.0069 |
| IDWG | −0.431 (0.156) | 0.650 | [0.479, 0.882] | 0.0057 |
| Gender | 0.744 (0.420) | 2.104 | [0.924, 4.790] | 0.0766 |
| Vascular access | −0.849 (0.396) | 0.428 | [0.197, 0.930] | 0.0320 |
| Covariate | Beta (se) | HR | HR CI95% | p-Value |
|---|---|---|---|---|
| Albumin | −0.720 (0.402) | 0.487 | [0.222; 1.070] | 0.0733 |
| MCI | 0.153 (0.056) | 1.165 | [1.045; 1.300] | 0.0060 |
| BSA | −2.341 (1.008) | 0.096 | [0.013; 0.693] | 0.0202 |
| CRP/Cl− | 0.027 (0.014) | 1.027 | [1.000; 1.055] | 0.0469 |
| IDWG | −0.731 (0.349) | 0.481 | [0.243; 0.955] | 0.0363 |
| ΔKt | 0.037 (0.025) | 1.038 | [0.989; 1.090] | 0.1335 |
| Ultrafiltration rate (mL/kg/h) | 0.002 (0.001) | 1.002 | [0.999; 1.004] | 0.1498 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valga, F.; Monzón, T.; De la Flor, J.C.; Santana-del-Pino, A.; Vega-Díaz, N.; Sanchez-Santana, A.Y.; Antón-Pérez, G.; Ruiz-Santana, S.; Rodríguez-Pérez, J.C.; Perez-Borges, P. C-Reactive Protein-to-Serum Chloride Ratio: A Novel Marker of All-Cause Mortality in Maintenance Haemodialysis Patients. Medicina 2024, 60, 1765. https://doi.org/10.3390/medicina60111765
Valga F, Monzón T, De la Flor JC, Santana-del-Pino A, Vega-Díaz N, Sanchez-Santana AY, Antón-Pérez G, Ruiz-Santana S, Rodríguez-Pérez JC, Perez-Borges P. C-Reactive Protein-to-Serum Chloride Ratio: A Novel Marker of All-Cause Mortality in Maintenance Haemodialysis Patients. Medicina. 2024; 60(11):1765. https://doi.org/10.3390/medicina60111765
Chicago/Turabian StyleValga, Francisco, Tania Monzón, José C. De la Flor, Angelo Santana-del-Pino, Nicanor Vega-Díaz, Ana Yurena Sanchez-Santana, Gloria Antón-Pérez, Sergio Ruiz-Santana, José C. Rodríguez-Pérez, and Patricia Perez-Borges. 2024. "C-Reactive Protein-to-Serum Chloride Ratio: A Novel Marker of All-Cause Mortality in Maintenance Haemodialysis Patients" Medicina 60, no. 11: 1765. https://doi.org/10.3390/medicina60111765
APA StyleValga, F., Monzón, T., De la Flor, J. C., Santana-del-Pino, A., Vega-Díaz, N., Sanchez-Santana, A. Y., Antón-Pérez, G., Ruiz-Santana, S., Rodríguez-Pérez, J. C., & Perez-Borges, P. (2024). C-Reactive Protein-to-Serum Chloride Ratio: A Novel Marker of All-Cause Mortality in Maintenance Haemodialysis Patients. Medicina, 60(11), 1765. https://doi.org/10.3390/medicina60111765







